Trends in Hyperinsulinemia and Insulin Resistance Among Nondiabetic US Adults, NHANES, 1999–2018
Abstract
1. Background
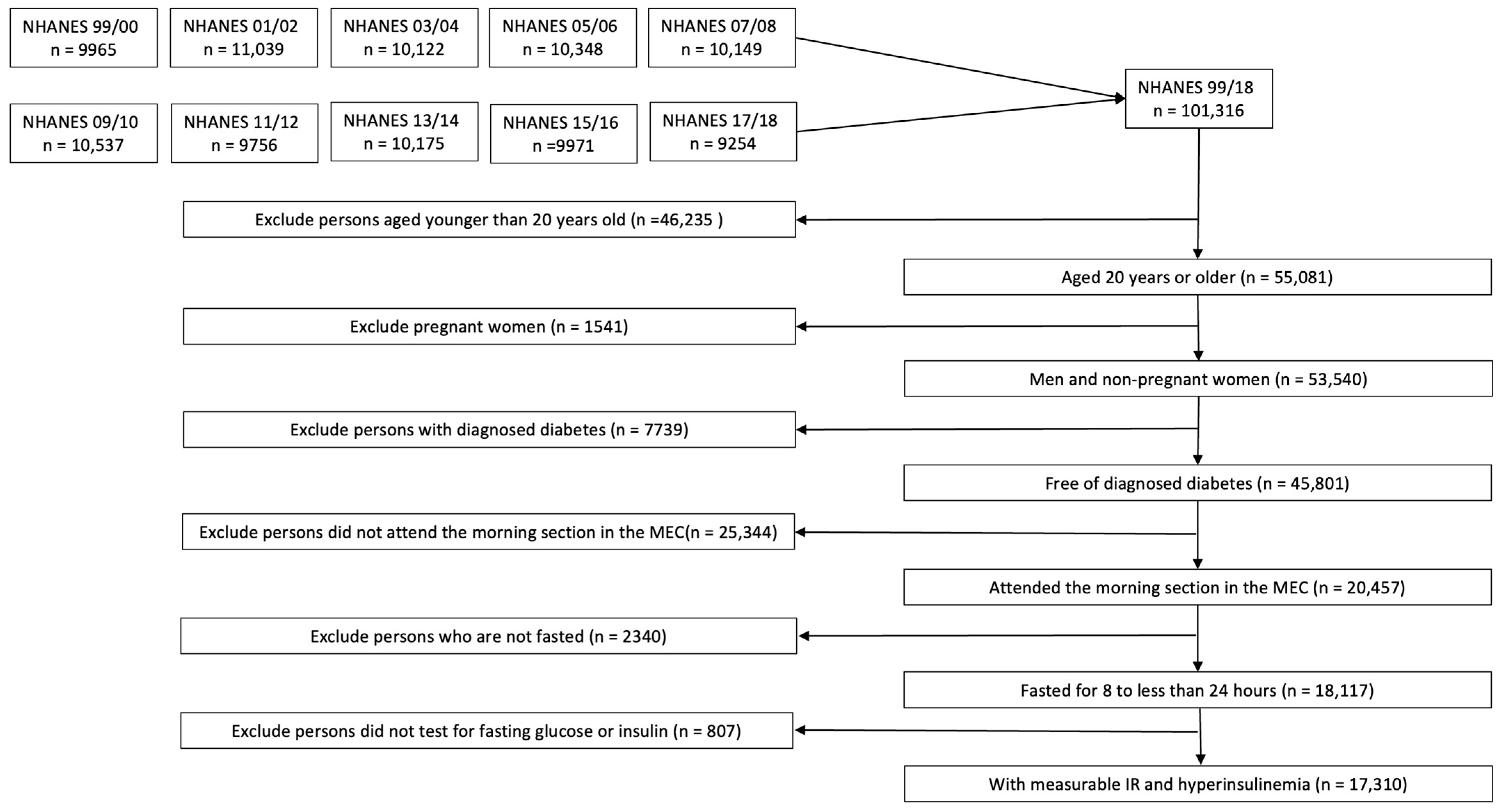
2. Method
2.1. Study Design and Study Population
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Participant Characteristics
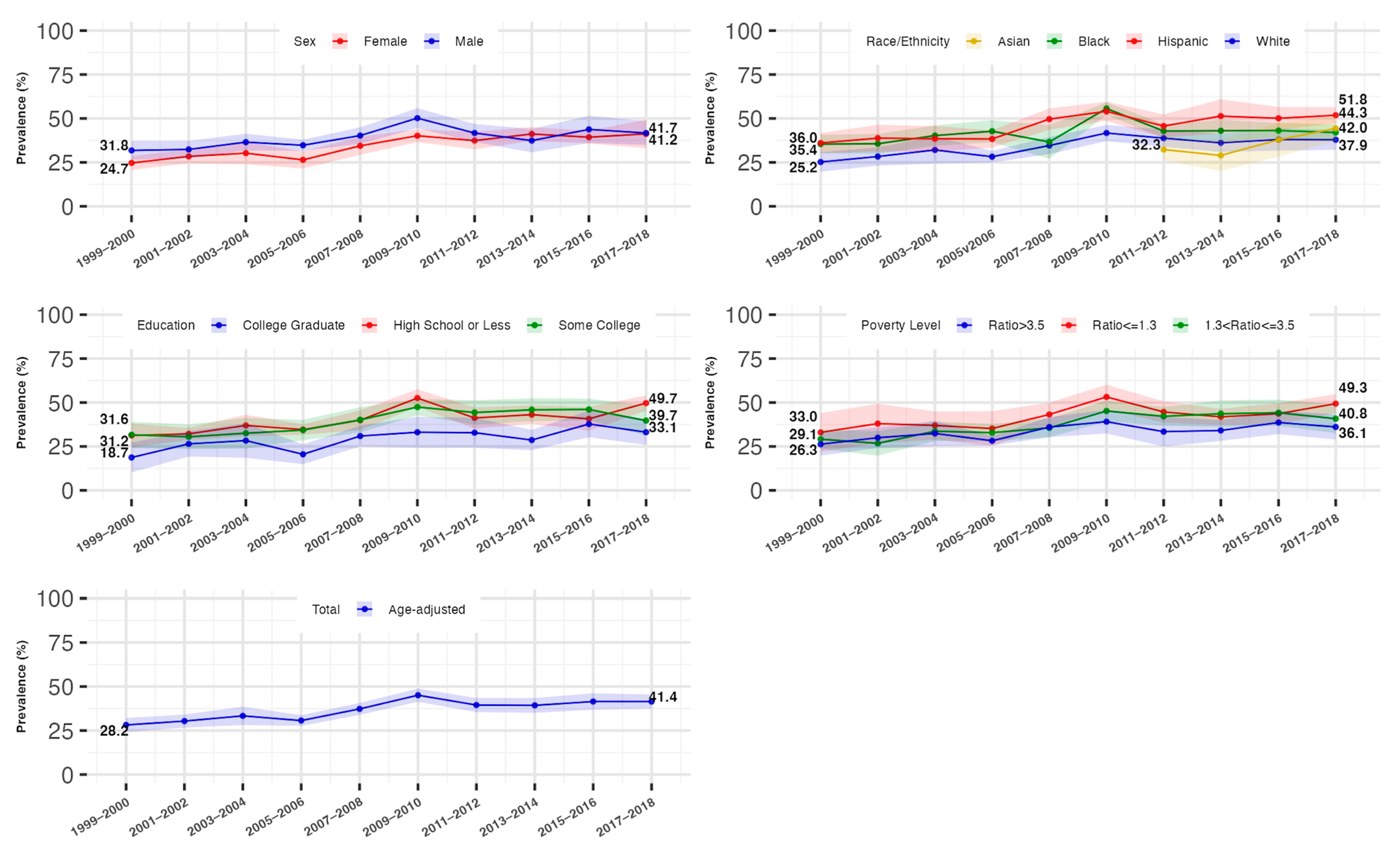
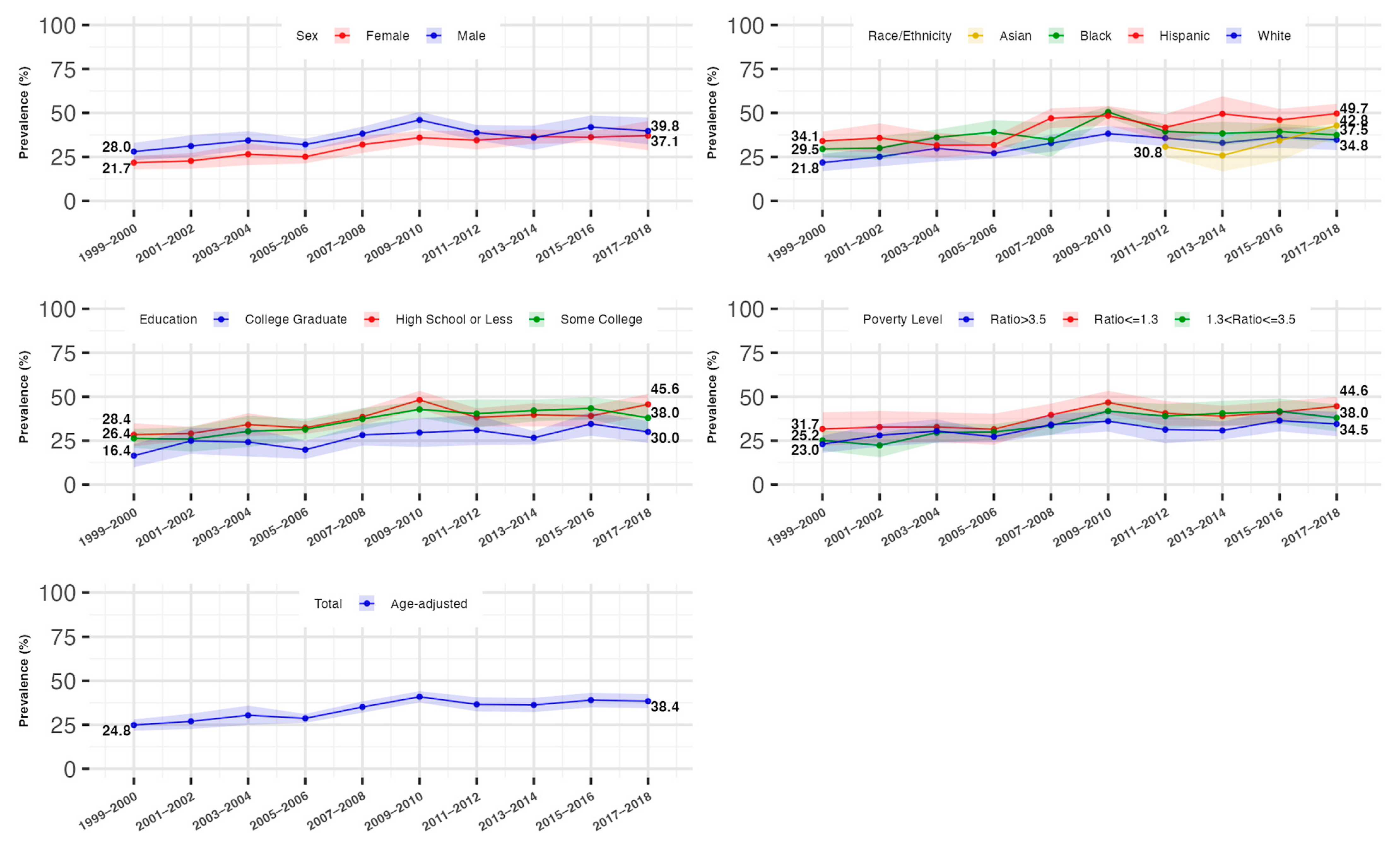
3.2. Overall Trends in the Prevalence of Hyperinsulinemia and IR
3.3. Trends in the Prevalence of Hyperinsulinemia and IR by Gender
3.4. Trends in the Prevalence of Hyperinsulinemia and IR by Race/Ethnicity
3.5. Trends in the Prevalence of Hyperinsulinemia and IR by Socioeconomic Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Shanik, M.H.; Xu, Y.; Skrha, J.; Dankner, R.; Zick, Y.; Roth, J. Insulin resistance and hyperinsulinemia: Is hyperinsulinemia the cart or the horse? Diabetes Care 2008, 31 (Suppl. 2), S262–S268. [Google Scholar] [CrossRef]
- Corkey, B.E. Banting lecture 2011: Hyperinsulinemia: Cause or consequence? Diabetes 2012, 61, 4–13. [Google Scholar] [CrossRef]
- Nolan, C.J.; Prentki, M. Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: Time for a conceptual framework shift. Diabetes Vasc. Dis. Res. 2019, 16, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Nolan, C.J.; Ruderman, N.B.; Kahn, S.E.; Pedersen, O.; Prentki, M. Insulin resistance as a physiological defense against metabolic stress: Implications for the management of subsets of type 2 diabetes. Diabetes 2015, 64, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.; DeFronzo, R.A. Insulin Resistance and Hyperinsulinemia: The Egg and the Chicken. J. Clin. Endocrinol. Metab. 2021, 106, e1897–e1899. [Google Scholar] [CrossRef]
- Gluvic, Z.; Zaric, B.; Resanovic, I.; Obradovic, M.; Mitrovic, A.; Radak, D.; Isenovic, E. Link between Metabolic Syndrome and Insulin Resistance. Curr. Vasc. Pharmacol. 2017, 15, 30–39. [Google Scholar] [CrossRef]
- Thomas, D.D.; Corkey, B.E.; Istfan, N.W.; Apovian, C.M. Hyperinsulinemia: An Early Indicator of Metabolic Dysfunction. J. Endocr. Soc. 2019, 3, 1727–1747. [Google Scholar] [CrossRef]
- Janssen, J. Hyperinsulinemia and Its Pivotal Role in Aging, Obesity, Type 2 Diabetes, Cardiovascular Disease and Cancer. Int. J. Mol. Sci. 2021, 22, 7797. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Bousvarou, M.D.; Kostara, C.E.; Papakonstantinou, E.J.; Salamou, E.; Guzman, E. Insulin resistance and cardiovascular disease. J. Int. Med. Res. 2023, 51, 3000605231164548. [Google Scholar] [CrossRef]
- Arcidiacono, B.; Iiritano, S.; Nocera, A.; Possidente, K.; Nevolo, M.T.; Ventura, V.; Foti, D.; Chiefari, E.; Brunetti, A. Insulin Resistance and Cancer Risk: An Overview of the Pathogenetic Mechanisms. Exp. Diabetes Res. 2012, 2012, 789174. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Costea, C.F.; Ciocoiu, M.; Lacatusu, C.M.; Maranduca, M.A.; Ouatu, A.; Floria, M. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J. Diabetes Res. 2020, 2020, 3920196. [Google Scholar] [CrossRef]
- Dunaif, A. Insulin Resistance and the Polycystic Ovary Syndrome: Mechanism and Implications for Pathogenesis. Endocr. Rev. 1997, 18, 774–800. [Google Scholar] [PubMed]
- Sarafidis, P.A. Obesity, insulin resistance and kidney disease risk: Insights into the relationship. Curr. Opin. Nephrol. Hypertens. 2008, 17, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, A.; Mendes, F.; Silva, A.P.; Neves, P.L. Insulin resistance as a predictor of cardiovascular morbidity and end-stage renal disease. J. Diabetes Complicat. 2015, 29, 1098–1104. [Google Scholar] [CrossRef]
- Sędzikowska, A.; Szablewski, L. Insulin and Insulin Resistance in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 9987. [Google Scholar] [CrossRef] [PubMed]
- Gulli, G.; Ferrannini, E.; Stern, M.; Haffner, S.; Defronzo, R.A. The Metabolic Profile of NIDDM Is Fully Established in Glucose-Tolerant Offspring of Two Mexican-American NIDDM Parents. Available online: http://diabetesjournals.org/diabetes/article-pdf/41/12/1575/359351/41-12-1575.pdf (accessed on 28 January 2025).
- Moore, J.X.; Chaudhary, N.; Akinyemiju, T. Metabolic Syndrome Prevalence by Race/Ethnicity and Sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev. Chronic Dis. 2017, 14, E24. [Google Scholar] [CrossRef]
- Hirode, G.; Wong, R.J. Trends in the Prevalence of Metabolic Syndrome in the United States, 2011–2016. JAMA 2020, 323, 2526–2528. [Google Scholar] [CrossRef]
- Menke, A.; Casagrande, S.; Geiss, L.; Cowie, C.C. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988-2012. JAMA 2015, 314, 1021–1029. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Wang, Z.; Bancks, M.P.; Carnethon, M.R.; Greenland, P.; Feng, Y.-Q.; Wang, H.; Zhong, V.W. Trends in Prevalence of Diabetes and Control of Risk Factors in Diabetes Among US Adults, 1999–2018. JAMA 2021, 326, 704–716. [Google Scholar] [CrossRef]
- He, J.; Zhu, Z.; Bundy, J.D.; Dorans, K.S.; Chen, J.; Hamm, L.L. Trends in Cardiovascular Risk Factors in US Adults by Race and Ethnicity and Socioeconomic Status, 1999–2018. JAMA 2021, 326, 1286–1298. [Google Scholar] [CrossRef]
- Cowie, C.C.; Casagrande, S.S.; Menke, A.; Cissell, M.A.; Eberhardt, M.S.; Meigs, J.B.; Gregg, E.W.; Knowler, W.C.; Barrett-Connor, E.; Becker, D.J.; et al. (Eds.) Diabetes in America; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2018. [Google Scholar]
- Li, C.; Ford, E.S.; McGuire, L.C.; Mokdad, A.H.; Little, R.R.; Reaven, G.M. Trends in hyperinsulinemia among nondiabetic adults in the U.S. Diabetes Care 2006, 29, 2396–2402. [Google Scholar] [CrossRef] [PubMed]
- US Burden of Disease Collaborators; Mokdad, A.H.; Ballestros, K.; Echko, M.; Glenn, S.; Olsen, H.E.; Mullany, E.; Lee, A.; Khan, A.R.; Ahmadi, A.; et al. The state of US health, 1990–2016: Burden of diseases, injuries, and risk factors among US states. JAMA 2018, 319, 1444–1472. [Google Scholar] [CrossRef]
- Zipf, G.; Chiappa, M.; Porter, K.S.; Ostchega, Y.; Lewis, B.G.; Dostal, J. National health and nutrition examination survey: Plan and operations, 1999–2010. Vital Health Stat. 1 2013, 56, 1–37. [Google Scholar]
- US Department of Health & Human Services. Poverty Guidelines, Research, and Measurement; US Department of Health & Human Services: Washington, DC, USA. Available online: https://aspe.hhs.gov/topics/poverty-economic-mobility/poverty-guidelines (accessed on 28 January 2025).
- Ogden, C.L.; Carroll, M.D.; Fakhouri, T.H.; Hales, C.M.; Fryar, C.D.; Li, X.; Freedman, D.S. Morbidity and Mortality Weekly Report Prevalence of Obesity Among Youths by Household Income and Education Level of Head of Household-United States 2011–2014. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 186–189. [Google Scholar] [CrossRef] [PubMed]
- NHANES 2003–2004: Plasma Fasting Glucose, Serum C-peptide & Insulin Data Documentation, Codebook, and Frequencies. Available online: https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2003/DataFiles/L10AM_C.htm (accessed on 28 January 2025).
- NHANES 2005–2006: Plasma Fasting Glucose & Insulin Data Documentation, Codebook, and Frequencies. Available online: https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2005/DataFiles/GLU_D.htm (accessed on 28 January 2025).
- NHANES 2011–2012: Plasma Fasting Glucose & Insulin Data Documentation, Codebook, and Frequencies. Available online: https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2011/DataFiles/GLU_G.htm (accessed on 28 January 2025).
- INS_H. Available online: https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2013/DataFiles/INS_H.htm (accessed on 28 January 2025).
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E.; Kiechl, S.; Willeit, J.; Oberhollenzer, F.; Egger, G.; Targher, G.; Alberiche, M.; Bonadonna, R.C.; Muggeo, M. Prevalence of insulin resistance in metabolic disorders: The Bruneck Study. Diabetes 1998, 47, 1643–1649. [Google Scholar] [CrossRef]
- Emoto, M.; Nishizawa, Y.; Maekawa, K.; Hiura, Y.; Kanda, H.; Kawagishi, T.; Shoji, T.; Okuno, Y.; Morii, H. Homeostasis model assessment as a clinical index of insulin resistance in type 2 diabetic patients treated with sulfonylureas. Diabetes Care 1999, 22, 818–822. [Google Scholar] [CrossRef]
- Tahapary, D.L.; Pratisthita, L.B.; Fitri, N.A.; Marcella, C.; Wafa, S.; Kurniawan, F.; Rizka, A.; Tarigan, T.J.E.; Harbuwono, D.S.; Purnamasari, D.; et al. Challenges in the diagnosis of insulin resistance: Focusing on the role of HOMA-IR and Tryglyceride/glucose index. Diabetes Metab. Syndr. 2022, 16, 102581. [Google Scholar] [CrossRef]
- Gastaldelli, A. Measuring and estimating insulin resistance in clinical and research settings. Obesity 2022, 30, 1549–1563. [Google Scholar] [CrossRef]
- Sumner, A.E.; Cowie, C.C. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis 2008, 196, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.L.; Dohrmann, S.M.; Burt, V.L.; Mohadjer, L.K. National health and nutrition examination survey: Sample design, 2011–2014. Vital Health Stat. 2 2014, 162, 1–33. [Google Scholar]
- Curtin, L.R.; Mohadjer, L.K.; Dohrmann, S.M.; Montaquila, J.M.; Kruszan-Moran, D.; Mirel, L.B.; Carroll, M.D.; Hirsch, R.; Schober, S.; Johnson, C.L. The National Health and Nutrition Examination Survey: Sample Design, 1999–2006. Vital Health Stat. 2 2012, 155, 1–39. [Google Scholar]
- Curtin, L.R.; Mohadjer, L.K.; Dohrmann, S.M.; Kruszon-Moran, D.; Mirel, L.B.; Carroll, M.D.; Hirsch, R.; Burt, V.L.; Johnson, C.L. National Health and Nutrition Examination Survey: Sample design, 2007–2010. Vital Health Stat. 2 2013, 160, 1–23. [Google Scholar]
- Chen, T.C.; Clark, J.; Riddles, M.K.; Mohadjer, L.K.; Fakhouri, T.H.I. National Health and Nutrition Examination Survey, 2015–2018: Sample Design and Estimation Procedures. Vital Health Stat. 2 2020, 184, 1–35. [Google Scholar]
- Age and Sex Composition in the United States: 2010. Available online: https://www.census.gov/data/tables/2010/demo/age-and-sex/2010-age-sex-composition.html (accessed on 28 January 2025).
- Ingram, D.D.; Malec, D.J.; Makuc, D.M.; Kruszon-Moran, D.; Gindi, R.M.; Albert, M.; Beresovsky, V.; Hamilton, B.E.; Holmes, J.; Schiller, J.; et al. National Center for Health Statistics Guidelines for Analysis of Trends. Vital Health Stat. 2 2018, 179, 1–71. [Google Scholar]
- Joinpoint Regression Program. Available online: https://surveillance.cancer.gov/joinpoint/ (accessed on 28 January 2025).
- Kim, H.J.; Fay, M.P.; Yu, B.; Barrett, M.J.; Feuer, E.J. Comparability of segmented line regression models. Biometrics 2004, 60, 1005–1014. [Google Scholar] [CrossRef]
- Du, Y.; Liu, B.; Sun, Y.; Snetselaar, L.G.; Wallace, R.B.; Bao, W. Trends in Adherence to the Physical Activity Guidelines for Americans for Aerobic Activity and Time Spent on Sedentary Behavior among US Adults, 2007 to 2016. JAMA Netw Open. 2019, 2, e197597. [Google Scholar] [CrossRef]
- Ciarambino, T.; Crispino, P.; Guarisco, G.; Giordano, M. Gender Differences in Insulin Resistance: New Knowledge and Perspectives. Curr. Issues Mol. Biol. 2023, 45, 7845–7861. [Google Scholar] [CrossRef]
- Gado, M.; Tsaousidou, E.; Bornstein, S.R.; Perakakis, N. Sex-based differences in insulin resistance. J. Endocrinol. 2024, 261, e230245. [Google Scholar] [CrossRef]
- Hasson, B.R.; Apovian, C.; Istfan, N. Racial/Ethnic Differences in Insulin Resistance and Beta Cell Function: Relationship to Racial Disparities in Type 2 Diabetes among African Americans versus Caucasians. Curr. Obes. Rep. 2015, 4, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Armiyaw, L.; Sarcone, C.; Fosam, A.; Muniyappa, R. Increased β-cell responsivity independent of insulin sensitivity in healthy African American adults. J. Clin. Endocrinol. Metab. 2020, 105, e2438. [Google Scholar] [CrossRef]
- Rehm, C.D.; Peñalvo, J.L.; Afshin, A.; Mozaffarian, D. Dietary intake among US Adults, 1999–2012. JAMA 2016, 315, 2542–2553. [Google Scholar] [CrossRef] [PubMed]
- Fuller-Rowell, T.E.; Homandberg, L.K.; Curtis, D.S.; Tsenkova, V.K.; Williams, D.R.; Ryff, C.D. Disparities in insulin resistance between black and white adults in the United States: The role of lifespan stress exposure. Psychoneuroendocrinology 2019, 107, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fakhouri, T.H.I.; Martin, C.B.; Chen, T.C.; Akinbami, L.J.; Ogden, C.L.; Paulose-Ram, R.; Riddles, M.K.; Van de Kerckhove, W.; Roth, S.B.; Clark, J.; et al. An Investigation of Nonresponse Bias and Survey Location Variability in the 2017–2018 National Health and Nutrition Examination Survey. Vital Health Stat. 2 2020, 185, 1–36. [Google Scholar]
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45 (Suppl. 1), S17–S38. [Google Scholar] [CrossRef]
| Characteristics | Overall n = 17,310 | 1999–2000 n = 1461 | 2001–2002 n = 1695 | 2003–2004 n = 1567 | 2005–2006 n = 1521 | 2007–2008 n = 1875 | 2009–2010 n = 2125 | 2011–2012 n = 1768 | 2013–2014 n = 1901 | 2015–2016 n = 1710 | 2017–2018 n = 1687 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age distribution, n * (%) †, years | |||||||||||
| 20–39 | 6381 (41) | 494 (45) | 623 (44) | 552 (42) | 576 (40) | 671 (41) | 774 (40) | 726 (39) | 701 (41) | 647 (41) | 617 (40) |
| 40–59 | 5782 (38) | 455 (35) | 567 (39) | 475 (38) | 506 (40) | 625 (39) | 758 (39) | 578 (38) | 684 (37) | 577 (35) | 557 (35) |
| ≥60 | 5147 (21) | 512 (20) | 505 (17) | 540 (19) | 439 (20) | 579 (20) | 593 (21) | 464 (23) | 516 (23) | 486 (24) | 513 (24) |
| Age, Mean (SD) †, years | 45.3 (0.2) | 44.3 (0.7) | 44.0 (1.0) | 44.8 (0.6) | 45.5 (0.8) | 45.2 (0.6) | 45.4 (0.7) | 46.0 (0.7) | 45.6 (0.7) | 45.6 (0.7) | 46.3 (0.6) |
| Female sex, n * (%) † | 8890 (51) | 737 (51) | 843 (51) | 783 (51) | 709 (49) | 972 (51) | 1149 (51) | 890 (52) | 996 (51) | 903 (52) | 908 (52) |
| Race and Ethnicity, n * (%) † | |||||||||||
| Non-Hispanic White | 7987 (68) | 679 (71) | 944 (73) | 866 (71) | 792 (72) | 924 (70) | 1035 (67) | 712 (67) | 860 (65) | 599 (62) | 576 (61) |
| Non-Hispanic Black | 3241 (11) | 251 (10) | 281 (10) | 287 (11) | 332 (11) | 327 (10) | 344 (11) | 367 (11) | 338 (12) | 336 (12) | 378 (11) |
| Hispanic | 4444 (14) | 496 (16) | 420 (12) | 345 (12) | 328 (11) | 545 (14) | 630 (14) | 369 (15) | 421 (16) | 500 (16) | 390 (17) |
| Non-Hispanic Asian ‡ | 976 (6) | NA | NA | NA | NA | NA | NA | 277 (6) | 237 (5) | 216 (6) | 246 (6) |
| Other § (Including Asian) | 1638 (7) | 35 (4) | 50 (4) | 69 (6) | 69 (6) | 79 (6) | 116 (7) | 320 (8) | 282 (8) | 275 (11) | 343 (11) |
| Other § (Excluding Asian) | 244 (3) | NA | NA | NA | NA | NA | NA | 43 (2) | 45 (2) | 59 (4) | 97 (5) |
| Educational Level, n * (%) † | |||||||||||
| High school or less | 8272 (39) | 857 (48) | 860 (42) | 815 (42) | 736 (40) | 961 (40) | 1053 (39) | 731 (34) | 790 (35) | 742 (36) | 727 (38) |
| Some college or associate’s degree | 5005 (32) | 344 (27) | 489 (34) | 437 (33) | 470 (33) | 491 (30) | 604 (30) | 542 (33) | 583 (32) | 504 (32) | 541 (32) |
| College graduate or higher | 4014 (29) | 257 (25) | 343 (24) | 313 (25) | 312 (27) | 422 (30) | 464 (30) | 495 (33) | 527 (32) | 464 (32) | 417 (31) |
| Frequency Missing | 19 | 3 | 3 | 2 | 3 | 1 | 4 | 0 | 1 | 0 | 2 |
| Poverty–Income Ratio, n * (%) † | |||||||||||
| Ratio ≤ 1.3 | 4538 (20) | 326 (18) | 360 (18) | 392 (20) | 343 (14) | 491 (19) | 637 (22) | 536 (24) | 596 (25) | 460 (21) | 397 (20) |
| 1.3 < Ratio ≤ 3.5 | 6051 (36) | 486 (34) | 618 (35) | 592 (36) | 590 (38) | 661 (33) | 712 (37) | 566 (34) | 593 (33) | 619 (38) | 614 (37) |
| Ratio > 3.5 | 5183 (44) | 450 (47) | 599 (46) | 481 (44) | 521 (48) | 570 (49) | 572 (41) | 516 (42) | 570 (41) | 453 (41) | 451 (43) |
| Frequency Missing | 1538 | 199 | 118 | 102 | 67 | 153 | 204 | 150 | 142 | 178 | 225 |
| Fasting glucose, Mean (SD) †, mmol/L | 5.4 (0.01) | 5.2 (0.03) | 5.3 (0.02) | 5.3 (0.03) | 5.4 (0.03) | 5.5 (0.02) | 5.4 (0.02) | 5.4 (0.02) | 5.4 (0.03) | 5.5 (0.02) | 5.6 (0.02) |
| Fasting insulin **, Median (q1, q3) †, μU/mL | 7.8 (4.7, 13.0) | 6.8 (4.2, 10.9) | 6.7 (4.2, 11.2) | 7.3 (4.4, 12.2) | 6.5 (3.6, 11.9) | 7.8 (4.3, 13.0) | 9.0 (5.2, 15.4) | 8.2 (5.3, 13.7) | 8.2 (5.2, 13.2) | 8.4 (5.4, 14.0) | 8.5 (5.6, 13.6) |
| HOMA-IR, Median (q1, q3) † | 1.9 (1.1, 3.2) | 1.6 (0.9, 2.6) | 1.6 (1.0, 2.7) | 1.7 (1.0, 3.0) | 1.5 (0.8, 3.0) | 1.9 (1.0, 3.2) | 2.1 (1.2, 3.8) | 2.0 (1.2, 3.4) | 2.0 (1.2, 3.2) | 2.1 (1.3, 3.5) | 2.1 (1.4, 3.5) |
| Age-Adjusted Models | |||||
|---|---|---|---|---|---|
| Joinpoint Wave † | OR (95%CI) p-Value | Contrast p-Value ‡ | Interaction p-Value § | ||
| Segment 1 | Segment 2 | ||||
| Overall | 6 | 1.13 (1.09–1.17) <0.001 | 1.00 (0.96–1.04) 0.99 | <0.001 | NA |
| Sex | |||||
| Female | NA | 1.09 (1.06–1.12) <0.001 | NA | 0.062 | |
| Male | 6 | 1.13 (1.08–1.19) <0.001 | 0.96 (0.90–1.03) 0.25 | 0.002 | ref |
| Race/Ethnicity || | |||||
| Non-Hispanic White | 6 | 1.14 (1.08–1.19) <0.001 | 0.99 (0.93–1.05) 0.66 | 0.004 | ref |
| Non-Hispanic Black | 6 | 1.12 (1.07–1.18) <0.001 | 0.93 (0.88–0.99) 0.027 | <0.001 | 0.098 |
| Hispanic | NA | 1.08 (1.05–1.11) <0.001 | NA | 0.67 | |
| Non-Hispanic Asian ¶ | NA | 1.22 (1.08–1.38) 0.003 | NA | 0.005 | |
| Educational level ** | |||||
| High school or less | 6 | 1.14 (1.08–1.20) <0.001 | 1.00 (0.95–1.06) 1.00 | 0.007 | 0.93 |
| Some college or associate’s degree | 7 | 1.14 (1.08–1.20) <0.001 | 0.94 (0.84–1.04) 0.22 | 0.006 | 0.98 |
| College graduate or higher | NA | 1.08 (1.03–1.12) <0.001 | NA | ref | |
| Poverty–Income Ratio †† | |||||
| Ratio ≤ 1.3 | NA | 1.06 (1.02–1.10) 0.002 | NA | 0.66 | |
| 1.3 < Ratio ≤ 3.5 | 8 | 1.12 (1.08–1.17) <0.001 | 0.91 (0.77–1.08) 0.29 | 0.034 | 0.087 |
| Ratio > 3.5 | NA | 1.05 (1.02–1.08) 0.002 | NA | ref | |
| Fully Adjusted ‡‡ Models | |||||
| Joinpoint Wave † | OR (95%CI) p-Value | Contrast p-Value ‡ | Interaction p-Value § | ||
| Segment 1 | Segment 2 | ||||
| Overall | 6 | 1.13 (1.09–1.18) <0.001 | 1.00 (0.95–1.04) 0.84 | <0.001 | NA |
| Sex | |||||
| Female | NA | 1.09 (1.06–1.12) <0.001 | NA | 0.033 | |
| Male | 6 | 1.13 (1.08–1.19) <0.001 | 0.96 (0.90–1.02) 0.191 | 0.001 | ref |
| Race/Ethnicity || | |||||
| Non-Hispanic White | 6 | 1.15 (1.09–1.21) <0.001 | 0.98 (0.93–1.04) 0.58 | 0.002 | ref |
| Non-Hispanic Black | 6 | 1.11 (1.05–1.17) <0.001 | 0.93 (0.87–1.00) 0.040 | 0.002 | 0.023 |
| Hispanic | NA | 1.08 (1.05–1.12) <0.001 | NA | 0.52 | |
| Non-Hispanic Asian ¶ | NA | 1.20 (1.03–1.40) 0.019 | NA | 0.022 | |
| Educational level ** | |||||
| High school or less | 6 | 1.13 (1.07–1.20) <0.001 | 0.99 (0.93–1.05) 0.65 | 0.008 | 0.92 |
| Some college or associate’s degree | 7 | 1.13 (1.07–1.20) <0.001 | 0.95 (0.84–1.06) 0.33 | 0.018 | 0.80 |
| College graduate or higher | NA | 1.07 (1.02–1.11) 0.003 | NA | Ref | |
| Poverty–Income Ratio †† | |||||
| Ratio ≤ 1.3 | NA | 1.06 (1.02–1.10) 0.002 | NA | 0.86 | |
| 1.3 < Ratio ≤ 3.5 | 8 | 0.89 (0.85–0.92) <0.001 | 1.11 (0.94–1.32) 0.23 | 0.023 | 0.113 |
| Ratio > 3.5 | NA | 1.06 (1.03–1.10) <0.001 | NA | ref | |
| Age-Adjusted Models | |||||
|---|---|---|---|---|---|
| Joinpoint Wave † | OR (95%CI) p-Value | Contrast p-Value ‡ | Interaction p-Value § | ||
| Segment 1 | Segment 2 | ||||
| Overall | 6 | 1.14 (1.10–1.18) <0.001 | 1.00 (0.96–1.04) 0.96 | <0.001 | NA |
| Sex | |||||
| Female | NA | 1.09 (1.06–1.12) <0.001 | NA | 0. 101 | |
| Male | 6 | 1.13 (1.08–1.18) <0.001 | 0.98 (0.91–1.04) 0.46 | 0.004 | ref |
| Race/Ethnicity || | |||||
| Non-Hispanic White | 6 | 1.15 (1.09–1.21) <0.001 | 0.98 (0.92–1.04) 0.53 | 0.002 | ref |
| Non-Hispanic Black | 6 | 1.15 (1.10–1.21) <0.001 | 0.92 (0.87–0.98) 0.006 | <0.001 | 0.140 |
| Hispanic | NA | 1.08 (1.05–1.12) <0.001 | NA | 0.55 | |
| Non-Hispanic Asian ¶ | NA | 1.22 (1.08–1.38) 0.002 | NA | 0.005 | |
| Educational level ** | |||||
| High school or less | NA | 1.08 (1.05–1.11) <0.001 | NA | 0.93 | |
| Some college or associate’s degree | 7 | 1.15 (1.09–1.21) <0.001 | 0.95 (0.86–1.06) 0.39 | 0.0116 | 0.63 |
| College graduate or higher | NA | 1.08 (1.04–1.12) <0.001 | NA | ref | |
| Poverty–Income Ratio †† | |||||
| Ratio ≤ 1.3 | NA | 1.06 (1.03–1.10) <0.001 | NA | 0.65 | |
| 1.3 < Ratio ≤ 3.5 | 7 | 1.16 (1.10–1.22) <0.001 | 0.97 (0.87–1.08) 0.55 | 0.0119 | 0.03 |
| Ratio > 3.5 | NA | 1.05 (1.02–1.08) 0.001 | NA | ref | |
| Fully Adjusted ‡‡ Models | |||||
| Joinpoint Wave † | OR (95%CI) p-Value | Contrast p-Value ‡ | Interaction p-Value § | ||
| Segment 1 | Segment 2 | ||||
| Overall | 6 | 1.14 (1.10–1.18) <0.001 | 1.00 (0.96–1.04) 1.00 | <0.001 | NA |
| Sex | |||||
| Female | NA | 1.09 (1.06–1.13) <0.001 | NA | 0.061 | |
| Male | 6 | 1.13 (1.08–1.19) <0.001 | 0.97 (0.91–1.04) 0.41 | 0.003 | ref |
| Race/Ethnicity || | |||||
| Non-Hispanic White | 6 | 1.16 (1.10–1.22) <0.001 | 0.99 (0.93–1.05) 0.62 | 0.001 | ref |
| Non-Hispanic Black | 6 | 1.13 (1.07–1.19) <0.001 | 0.92 (0.86–0.98) 0.010 | <0.001 | 0.026 |
| Hispanic | NA | 1.09 (1.06–1.12) <0.001 | NA | 0.52 | |
| Non-Hispanic Asian ¶ | NA | 1.20 (1.03–1.39) 0.017 | NA | 0.030 | |
| Educational level ** | |||||
| High school or less | NA | 1.07 (1.04–1.10) <0.001 | NA | 0.97 | |
| Some college or associate’s degree | 7 | 1.14 (1.08–1.21) <0.001 | 0.96 (0.86–1.08) 0.53 | 0.030 | 0.58 |
| College graduate or higher | NA | 1.07 (1.03–1.11) <0.001 | NA | ref | |
| Poverty–Income Ratio †† | |||||
| Ratio ≤ 1.3 | NA | 1.06 (1.03–1.10) <0.001 | NA | 0.87 | |
| 1.3 < Ratio ≤ 3.5 | 7 | 1.17 (1.11–1.23) <0.001 | 0.96 (0.86–1.07) 0.44 | 0.007 | 0.050 |
| Ratio > 3.5 | NA | 1.07 (1.03–1.10) <0.001 | NA | ref | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Ke, Y.; Nianogo, R.A. Trends in Hyperinsulinemia and Insulin Resistance Among Nondiabetic US Adults, NHANES, 1999–2018. J. Clin. Med. 2025, 14, 3215. https://doi.org/10.3390/jcm14093215
Wu C, Ke Y, Nianogo RA. Trends in Hyperinsulinemia and Insulin Resistance Among Nondiabetic US Adults, NHANES, 1999–2018. Journal of Clinical Medicine. 2025; 14(9):3215. https://doi.org/10.3390/jcm14093215
Chicago/Turabian StyleWu, Chuyue, Yixun Ke, and Roch A. Nianogo. 2025. "Trends in Hyperinsulinemia and Insulin Resistance Among Nondiabetic US Adults, NHANES, 1999–2018" Journal of Clinical Medicine 14, no. 9: 3215. https://doi.org/10.3390/jcm14093215
APA StyleWu, C., Ke, Y., & Nianogo, R. A. (2025). Trends in Hyperinsulinemia and Insulin Resistance Among Nondiabetic US Adults, NHANES, 1999–2018. Journal of Clinical Medicine, 14(9), 3215. https://doi.org/10.3390/jcm14093215






