Transcranial Direct Current Stimulation (tDCS) in the Treatment of Youth Depression: Integrating Literature Review Insights in a Pilot Clinical Trial
Abstract
Highlights:
- What are the main findings?
- This study identified transcranial direct stimulation (tDCS) as a promising and feasible treatment modality for youth depression. A systematic review conducted up until 20 November 2024 identified fourteen eligible registered/ published studies in tDCS for youth depression. Among the limited clinical data available, two trials demonstrated substantial symptom improvement. However, recruitment challenges and high risks of bias underscore the need for robust evidence supporting the feasibility of conducting tDCS RCTs in this population.
- This pilot trial demonstrated high session attendance and retention rates with no dropouts or serious adverse events during the five-day, 30 min, 2 mA tDCS protocol.
- Decentralised administration of tDCS required prompting in some cases and may have introduced variability in adherence.
- What are the implications of the main findings?
- tDCS has the potential as a safe and acceptable intervention for youth depression
- First study to review and pilot youth tDCS implementation
- Provides practical insights into translating neuromodulation reviews into a pilot trial
- Although not powered to detect efficacy, it offers a framework for designing future trials
- Emphasizes need to evaluate long-term safety- specifically the absence of unintended outcomes of tDCS.
Abstract
1. Introduction
1.1. Background
1.2. Review
1.2.1. Search Strategy
1.2.2. Eligibility Criteria
1.2.3. Review Results
1.2.4. Clinical Trials
1.2.5. Case Reports
1.2.6. Ongoing Unpublished Clinical Trials
1.3. Integrating Review Insights into a Pilot Trial
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Interventions
2.4. Procedures
2.5. Blinding
2.6. Assessments and Outcomes
2.7. Statistical Analysis
3. Results
3.1. Primary Feasibility Outcomes
3.2. Exploratory Outcomes
3.3. Tolerability and Engagement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Depressive Disorder (Depression) 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 1 April 2025).
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Koretz, D.; Merikangas, K.R.; Rush, A.J.; Walters, E.E.; Wang, P.S. The Epidemiology of Major Depressive Disorder: Results from the National Comorbidity Survey Replication (NCS-R). JAMA J. Am. Med. Assoc. 2003, 289, 3095–3105. [Google Scholar] [CrossRef]
- Paus, T.; Giedd, J.N.; Keshavan, M. Why Do Many Psychiatric Disorders Emerge during Adolescence? Nat. Rev. Neurosci. 2008, 9, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Piao, J.; Huang, Y.; Han, C.; Li, Y.; Xu, Y.; Liu, Y.; He, X. Alarming Changes in the Global Burden of Mental Disorders in Children and Adolescents from 1990 to 2019: A Systematic Analysis for the Global Burden of Disease Study. Eur. Child. Adolesc. Psychiatry 2022, 31, 1827–1845. [Google Scholar] [CrossRef]
- Sverre, K.T.; Nissen, E.R.; Farver-Vestergaard, I.; Johannsen, M.; Zachariae, R. Comparing the Efficacy of Mindfulness-Based Therapy and Cognitive-Behavioral Therapy for Depression in Head-to-Head Randomized Controlled Trials: A Systematic Review and Meta-Analysis of Equivalence. Clin. Psychol. Rev. 2023, 100, 102234. [Google Scholar] [CrossRef]
- Zhou, X.; Teng, T.; Zhang, Y.; Giovane, C.D.; Furukawa, T.A.; Weisz, J.R.; Li, X.; Cuijpers, P.; Coghill, D.; Xiang, Y.; et al. Comparative Efficacy and Acceptability of Antidepressants, Psychotherapies, and Their Combination for Acute Treatment of Children and Adolescents with Depressive Disorder: A Systematic Review and Network Meta-Analysis. Lancet Psychiatry 2020, 7, 581–601. [Google Scholar] [CrossRef] [PubMed]
- Spielmans, G.I.; Spence-Sing, T.; Parry, P. Duty to Warn: Antidepressant Black Box Suicidality Warning Is Empirically Justified. Front. Psychiatry 2020, 11, 18. [Google Scholar] [CrossRef]
- Häge, A.; Weymann, L.; Bliznak, L.; Märker, V.; Mechler, K.; Dittmann, R.W. Non-Adherence to Psychotropic Medication Among Adolescents—A Systematic Review of the Literature. Z. Kinder Jugendpsychiatr. Psychother. 2018, 46, 69–78. [Google Scholar] [CrossRef]
- Luft, M.J.; Lamy, M.; DelBello, M.P.; McNamara, R.K.; Strawn, J.R. Antidepressant-Induced Activation in Children and Adolescents: Risk, Recognition and Management. Curr. Probl. Pediatr. Adolesc. Health Care 2018, 48, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Kozloff, N.; Cheung, A.H.; Schaffer, A.; Cairney, J.; Dewa, C.S.; Veldhuizen, S.; Kurdyak, P.; Levitt, A.J. Bipolar Disorder among Adolescents and Young Adults: Results from an Epidemiological Sample. J. Affect. Disord. 2010, 125, 350–354. [Google Scholar] [CrossRef]
- Lo, H.K.Y.; Tong, C.C.H.Y.; Chan, J.K.N.; Kam, C.T.K.; Wong, C.S.M.; Cheng, C.P.W.; Ho, C.; Leung, B.M.H.; Wong, W.S.H.; Yu, Z.H.S.; et al. Temporal Trends of Antidepressant Utilization Patterns in Children and Adolescents in Hong Kong: A 14-Year Population-Based Study with Joinpoint Regression Analysis. J. Affect. Disord. 2024, 344, 61–68. [Google Scholar] [CrossRef]
- Lee, J.C.; Lewis, C.P.; Daskalakis, Z.J.; Croarkin, P.E. Transcranial Direct Current Stimulation: Considerations for Research in Adolescent Depression. Front. Psychiatry 2017, 8, 91. [Google Scholar] [CrossRef]
- Jog, M.A.; Anderson, C.; Kubicki, A.; Boucher, M.; Leaver, A.; Hellemann, G.; Iacoboni, M.; Woods, R.; Narr, K. Transcranial Direct Current Stimulation (tDCS) in Depression Induces Structural Plasticity. Sci. Rep. 2023, 13, 2841. [Google Scholar] [CrossRef]
- Palm, U.; Kumpf, U.; Behler, N.; Wulf, L.; Kirsch, B.; Wörsching, J.; Keeser, D.; Hasan, A.; Padberg, F. Home Use, Remotely Supervised, and Remotely Controlled Transcranial Direct Current Stimulation: A Systematic Review of the Available Evidence. Neuromodul. Technol. Neural Interface 2018, 21, 323–333. [Google Scholar] [CrossRef]
- Pilloni, G.; Vogel-Eyny, A.; Lustberg, M.; Best, P.; Malik, M.; Walton-Masters, L.; George, A.; Mirza, I.; Zhovtis, L.; Datta, A.; et al. Tolerability and Feasibility of At-Home Remotely Supervised Transcranial Direct Current Stimulation (RS-tDCS): Single-Center Evidence from 6779 Sessions. Brain Stimul. 2022, 15, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Transcranial Direct Current Stimulation—Update 2011. Restor. Neurol. Neurosci. 2011, 29, 463–492. [Google Scholar] [CrossRef]
- Monte-Silva, K.; Kuo, M.-F.; Hessenthaler, S.; Fresnoza, S.; Liebetanz, D.; Paulus, W.; Nitsche, M.A. Induction of Late LTP-Like Plasticity in the Human Motor Cortex by Repeated Non-Invasive Brain Stimulation. Brain Stimul. 2013, 6, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Tucker, D.M.; Poulsen, C.; Luu, P. Critical Periods for the Neurodevelopmental Processes of Externalizing and Internalizing. Dev. Psychopathol. 2015, 27, 321–346. [Google Scholar] [CrossRef]
- Razza, L.B.; Palumbo, P.; Moffa, A.H.; Carvalho, A.F.; Solmi, M.; Loo, C.K.; Brunoni, A.R. A Systematic Review and Meta-analysis on the Effects of Transcranial Direct Current Stimulation in Depressive Episodes. Depress. Anxiety 2020, 37, 594–608. [Google Scholar] [CrossRef] [PubMed]
- Zewdie, E.; Ciechanski, P.; Kuo, H.C.; Giuffre, A.; Kahl, C.; King, R.; Cole, L.; Godfrey, H.; Seeger, T.; Swansburg, R.; et al. Safety and Tolerability of Transcranial Magnetic and Direct Current Stimulation in Children: Prospective Single Center Evidence from 3.5 Million Stimulations. Brain Stimul. 2020, 13, 565–575. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Amadera, J.; Berbel, B.; Volz, M.S.; Rizzerio, B.G.; Fregni, F. A Systematic Review on Reporting and Assessment of Adverse Effects Associated with Transcranial Direct Current Stimulation. Int. J. Neuropsychopharmacol. 2011, 14, 1133–1145. [Google Scholar] [CrossRef]
- Vöckel, J.; Spitznagel, N.; Markser, A.; Sigrist, C.; Koenig, J. A Paucity of Evidence in Youth: The Curious Case of Transcranial Direct Current Stimulation for Depression. Asian J. Psychiatr. 2024, 91, 103838. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Baibujiapu, G.; Rena, A. The Therapeutic Effect of Transcrnial Direct Current Stimulation Combined with Sandplay Therapy on Youth Depression. China Health Care Nutr. 2017, 15, 100. [Google Scholar]
- Zhang, Z.; Duan, J.; Wei, Y.; Zhao, P.; Wang, N.; Qin, S.; Zhu, R.; Zhang, J.; Wang, F. Study on Neuroimaging Features Related to the Efficacy of High-Definition Transcranial Direct Current Stimulation for Adolescents with Mood Disorder during Depressive Episode. J. Clin. Psychiatry 2023, 33, 351–355. [Google Scholar]
- Baliga, S.; Sreeraj, V.S.; Parlikar, R.; Rai, D.; Chhabra, H.; Kumar, V.; Venkatasubramanian, G. Role of Transcranial Direct Current Stimulation in Bipolar Depression: A Case Report. Asian J. Psychiatry 2020, 47, 101873. [Google Scholar] [CrossRef]
- Clayton, A.; Charlson, R.; Dobbs, B.; Howard, J.; Krupp, L.; Shaw, M.; Charvet, L. At-Home Transcranial Direct Current Stimulation Benefits Depression and Cognition in Multiple Sclerosis: Two Case Reports. Neurology 2018, 90 (Suppl. 15), 5.396. [Google Scholar] [CrossRef]
- Shankar, A.; Gupta, P.K.; Kar, S.K. Role of Transcranial Direct Current Stimulation (tDCS) in Moderate Depressive Episode After Failed Ketamine Therapy: A Case Study. Indian J. Psychiatry 2023, 65 (Suppl. 1), S144–S145. [Google Scholar]
- Sreeraj, V.S.; Bose, A.; Shanbhag, V.; Narayanaswamy, J.C.; Venkatasubramanian, G.; Benegal, V. Monotherapy With tDCS for Treatment of Depressive Episode During Pregnancy: A Case Report. Brain Stimul. 2016, 9, 457–458. [Google Scholar] [CrossRef]
- Esmaeilpour, Z.; Shereen, A.D.; Ghobadi-Azbari, P.; Datta, A.; Woods, A.J.; Ironside, M.; O’Shea, J.; Kirk, U.; Bikson, M.; Ekhtiari, H. Methodology for tDCS Integration with fMRI. Hum. Brain Mapp. 2020, 41, 1950–1967. [Google Scholar] [CrossRef]
- Agboada, D.; Mosayebi Samani, M.; Jamil, A.; Kuo, M.-F.; Nitsche, M.A. Expanding the Parameter Space of Anodal Transcranial Direct Current Stimulation of the Primary Motor Cortex. Sci. Rep. 2019, 9, 18185. [Google Scholar] [CrossRef]
- Buchanan, D.M.; Bogdanowicz, T.; Khanna, N.; Lockman-Dufour, G.; Robaey, P.; D’Angiulli, A. Systematic Review on the Safety and Tolerability of Transcranial Direct Current Stimulation in Children and Adolescents. Brain Sci. 2021, 11, 212. [Google Scholar] [CrossRef] [PubMed]
- Maina, G.; Adami, M.; Ascione, G.; Bondi, E.; De Berardis, D.; Delmonte, D.; Maffezzoli, S.; Martinotti, G.; Nivoli, A.; Ottavianelli, E.; et al. Nationwide Consensus on the Clinical Management of Treatment-Resistant Depression in Italy: A Delphi Panel. Ann. Gen. Psychiatry 2023, 22, 48. [Google Scholar] [CrossRef]
- Zanão, T.A.; Moffa, A.H.; Shiozawa, P.; Lotufo, P.A.; Benseñor, I.M.; Brunoni, A.R. Impact of Two or Less Missing Treatment Sessions on tDCS Clinical Efficacy: Results from a Factorial, Randomized, Controlled Trial in Major Depression. Neuromodulation 2014, 17, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Kuo, M.-F.; Paulus, W.; Antal, A. Transcranial Direct Current Stimulation: Protocols and Physiological Mechanisms of Action. In Textbook of Neuromodulation; Springer: New York, NY, USA, 2015; pp. 101–111. ISBN 978-1-4939-1407-4. [Google Scholar]
- Charvet, L.E.; Shaw, M.T.; Bikson, M.; Woods, A.J.; Knotkova, H. Supervised Transcranial Direct Current Stimulation (tDCS) at Home: A Guide for Clinical Research and Practice. Brain Stimul. 2020, 13, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Borrione, L.; Cavendish, B.A.; Aparicio, L.V.M.; Luethi, M.S.; Goerigk, S.; Carneiro, A.M.; Valiengo, L.; Moura, D.O.; de Souza, J.P.; Baptista, M.; et al. Home-Use Transcranial Direct Current Stimulation for the Treatment of a Major Depressive Episode: A Randomized Clinical Trial. JAMA Psychiatry 2024, 81, 329–337. [Google Scholar] [CrossRef]
- Palm, U.; Reisinger, E.; Keeser, D.; Kuo, M.-F.; Pogarell, O.; Leicht, G.; Mulert, C.; Nitsche, M.A.; Padberg, F. Evaluation of Sham Transcranial Direct Current Stimulation for Randomized, Placebo-Controlled Clinical Trials. Brain Stimul. 2013, 6, 690–695. [Google Scholar] [CrossRef]
- Wallace, D.; Cooper, N.R.; Paulmann, S.; Fitzgerald, P.B.; Russo, R. Perceived Comfort and Blinding Efficacy in Randomised Sham-Controlled Transcranial Direct Current Stimulation (tDCS) Trials at 2 mA in Young and Older Healthy Adults. PLoS ONE 2016, 11, e0149703. [Google Scholar] [CrossRef]
- Hamilton, M. The Hamilton Rating Scale for Depression. In Assessment of Depression; Sartorius, N., Ban, T.A., Eds.; Springer: Berlin/Heidelberg, Germany, 1986; pp. 143–152. ISBN 978-3-642-70486-4. [Google Scholar]
- Snaith, R.P.; Hamilton, M.; Morley, S.; Humayan, A.; Hargreaves, D.; Trigwell, P. A Scale for the Assessment of Hedonic Tone the Snaith–Hamilton Pleasure Scale. Br. J. Psychiatry 1995, 167, 99–103. [Google Scholar] [CrossRef]
- Rizvi, S.J.; Quilty, L.C.; Sproule, B.A.; Cyriac, A.; Michael Bagby, R.; Kennedy, S.H. Development and Validation of the Dimensional Anhedonia Rating Scale (DARS) in a Community Sample and Individuals with Major Depression. Psychiatry Res. 2015, 229, 109–119. [Google Scholar] [CrossRef]
- Young, R.C.; Biggs, J.T.; Ziegler, V.E.; Meyer, D.A. A Rating Scale for Mania: Reliability, Validity and Sensitivity. Br. J. Psychiatry 1978, 133, 429–435. [Google Scholar] [CrossRef]
- Grootenboer, E.M.V.; Giltay, E.J.; van der Lem, R.; van Veen, T.; van der Wee, N.J.A.; Zitman, F.G. Reliability and Validity of the Global Assessment of Functioning Scale in Clinical Outpatients with Depressive Disorders. J. Eval. Clin. Pract. 2012, 18, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.H.; Sewell, D.R.; Cooley, E.L.; Leavitt, N. Assessing Levels of Adaptive Functioning: The Role Functioning Scale. Community Ment. Health J. 1993, 29, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Brunoni, A.R.; Fregni, F. Clinical Trial Design in Non-Invasive Brain Stimulation Psychiatric Research. Int. J. Methods Psychiatr. Res. 2011, 20, e19–e30. [Google Scholar] [CrossRef]
- Khazanov, G.K.; Forbes, C.N.; Dunn, B.D.; Thase, M.E. Addressing Anhedonia to Increase Depression Treatment Engagement. Br. J. Clin. Psychol. 2022, 61, 255–280. [Google Scholar] [CrossRef]
- Rohden, A.I.; Benchaya, M.C.; Camargo, R.S.; de Campos Moreira, T.; Barros, H.M.; Ferigolo, M. Dropout Prevalence and Associated Factors in Randomized Clinical Trials of Adolescents Treated for Depression: Systematic Review and Meta-Analysis. Clin. Ther. 2017, 39, 971–992.e4. [Google Scholar] [CrossRef] [PubMed]
- Woodham, R.D.; Selvaraj, S.; Lajmi, N.; Hobday, H.; Sheehan, G.; Ghazi-Noori, A.-R.; Lagerberg, P.J.; Rizvi, M.; Kwon, S.S.; Orhii, P.; et al. Home-Based Transcranial Direct Current Stimulation Treatment for Major Depressive Disorder: A Fully Remote Phase 2 Randomized Sham-Controlled Trial. Nat. Med. 2024, 31, 87–95. [Google Scholar] [CrossRef]
- van ’t Wout-Frank, M.; Arulpragasam, A.R.; Faucher, C.; Aiken, E.; Shea, M.T.; Jones, R.N.; Greenberg, B.D.; Philip, N.S. Virtual Reality and Transcranial Direct Current Stimulation for Posttraumatic Stress Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2024, 81, 437–446. [Google Scholar] [CrossRef]
- Leffa, D.T.; Grevet, E.H.; Bau, C.H.D.; Schneider, M.; Ferrazza, C.P.; da Silva, R.F.; Miranda, M.S.; Picon, F.; Teche, S.P.; Sanches, P.; et al. Transcranial Direct Current Stimulation vs Sham for the Treatment of Inattention in Adults with Attention-Deficit/Hyperactivity Disorder: The TUNED Randomized Clinical Trial. JAMA Psychiatry 2022, 79, 847–856. [Google Scholar] [CrossRef]
- Karyotaki, E.; Kleiboer, A.; Smit, F.; Turner, D.T.; Pastor, A.M.; Andersson, G.; Berger, T.; Botella, C.; Breton, J.M.; Carlbring, P.; et al. Predictors of Treatment Dropout in Self-Guided Web-Based Interventions for Depression: An ‘Individual Patient Data’ Meta-Analysis. Psychol. Med. 2015, 45, 2717–2726. [Google Scholar] [CrossRef]
- Charvet, L.; George, A.; Charlson, E.; Lustberg, M.; Vogel-Eyny, A.; Eilam-Stock, T.; Cho, H.; Best, P.; Fernandez, L.; Datta, A.; et al. Home-Administered Transcranial Direct Current Stimulation Is a Feasible Intervention for Depression: An Observational Cohort Study. Front. Psychiatry 2023, 14, 1199773. [Google Scholar] [CrossRef]
- Carvalho Lima, V.L.; Collange Grecco, L.A.; Marques, V.C.; Fregni, F.; Brandão de Ávila, C.R. Transcranial Direct Current Stimulation Combined with Integrative Speech Therapy in a Child with Cerebral Palsy: A Case Report. J. Bodyw. Mov. Ther. 2016, 20, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Dechantsreiter, E.; Padberg, F.; Morash, A.; Kumpf, U.; Nguyen, A.; Menestrina, Z.; Windel, F.; Burkhardt, G.; Goerigk, S.; Morishita, T.; et al. Examining the Synergistic Effects of a Cognitive Control Video Game and a Home-Based, Self-Administered Non-Invasive Brain Stimulation on Alleviating Depression: The DiSCoVeR Trial Protocol. Eur. Arch. Psychiatry Clin. Neurosci. 2023, 273, 85–98. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Moffa, A.H.; Fregni, F.; Palm, U.; Padberg, F.; Blumberger, D.M.; Daskalakis, Z.J.; Bennabi, D.; Haffen, E.; Alonzo, A.; et al. Transcranial Direct Current Stimulation for Acute Major Depressive Episodes: Meta-Analysis of Individual Patient Data. Br. J. Psychiatry 2016, 208, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Meron, D.; Hedger, N.; Garner, M.; Baldwin, D.S. Transcranial Direct Current Stimulation (tDCS) in the Treatment of Depression: Systematic Review and Meta-Analysis of Efficacy and Tolerability. Neurosci. Biobehav. Rev. 2015, 57, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Mutz, J.; Edgcumbe, D.R.; Brunoni, A.R.; Fu, C.H. Efficacy and Acceptability of Non-Invasive Brain Stimulation for the Treatment of Adult Unipolar and Bipolar Depression: A Systematic Review and Meta-Analysis of Randomised Sham Controlled Trials. Neurosci. Biobehav. Rev. 2018, 92, 291–303. [Google Scholar] [CrossRef]
- Mutz, J.; Vipulananthan, V.; Carter, B.; Hurlemann, R.; Fu, C.H.Y.; Young, A.H. Comparative Efficacy and Acceptability of Non-Surgical Brain Stimulation for the Acute Treatment of Major Depressive Episodes in Adults: Systematic Review and Network Meta-Analysis. BMJ 2019, 364, l1079. [Google Scholar] [CrossRef]
- Moffa, A.H.; Martin, D.; Alonzo, A.; Bennabi, D.; Blumberger, D.M.; Benseñor, I.M.; Daskalakis, Z.; Fregni, F.; Haffen, E.; Lisanby, S.H.; et al. Efficacy and Acceptability of Transcranial Direct Current Stimulation (tDCS) for Major Depressive Disorder: An Individual Patient Data Meta-Analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 99, 109836. [Google Scholar] [CrossRef]
- Davey, C.G.; McGorry, P.D. Early Intervention for Depression in Young People: A Blind Spot in Mental Health Care. Lancet Psychiatry 2019, 6, 267–272. [Google Scholar] [CrossRef]
- Tang, S.; Lu, L.; Zhang, L.; Hu, X.; Bu, X.; Li, H.; Hu, X.; Gao, Y.; Zeng, Z.; Gong, Q.; et al. Abnormal Amygdala Resting-State Functional Connectivity in Adults and Adolescents with Major Depressive Disorder: A Comparative Meta-Analysis. EBioMedicine 2018, 36, 436–445. [Google Scholar] [CrossRef]
- Pizzagalli, D.A.; Roberts, A.C. Prefrontal Cortex and Depression. Neuropsychopharmacology 2022, 47, 225–246. [Google Scholar] [CrossRef]
- Miller, C.H.; Hamilton, J.P.; Sacchet, M.D.; Gotlib, I.H. Meta-Analysis of Functional Neuroimaging of Major Depressive Disorder in Youth. JAMA Psychiatry 2015, 72, 1045–1053. [Google Scholar] [CrossRef]
- Antal, A.; Luber, B.; Brem, A.-K.; Bikson, M.; Brunoni, A.R.; Cohen Kadosh, R.; Dubljević, V.; Fecteau, S.; Ferreri, F.; Flöel, A.; et al. Non-Invasive Brain Stimulation and Neuroenhancement. Clin. Neurophysiol. Pract. 2022, 7, 146–165. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.M.; Chan, M.M.; Shea, C.K.; Mo, F.Y.; Yiu, K.W.; Chung, R.C.; Cheung, M.-C.; Chan, A.S. Effects of Prefrontal Transcranial Direct Current Stimulation on Social Functioning in Autism Spectrum Disorder: A Randomized Clinical Trial. Autism Int. J. Res. Pract. 2023, 27, 2465–2482. [Google Scholar] [CrossRef] [PubMed]
- Hunold, A.; Haueisen, J.; Freitag, C.M.; Siniatchkin, M.; Moliadze, V. Cortical Current Density Magnitudes during Transcranial Direct Current Stimulation Correlate with Skull Thickness in Children, Adolescent and Young Adults. Prog. Brain Res. 2021, 264, 41–56. [Google Scholar] [CrossRef]
- Kessler, S.K.; Minhas, P.; Woods, A.J.; Rosen, A.; Gorman, C.; Bikson, M. Dosage Considerations for Transcranial Direct Current Stimulation in Children: A Computational Modeling Study. PLoS ONE 2013, 8, e76112. [Google Scholar] [CrossRef] [PubMed]
- Antonenko, D.; Nierhaus, T.; Meinzer, M.; Prehn, K.; Thielscher, A.; Ittermann, B.; Flöel, A. Age-Dependent Effects of Brain Stimulation on Network Centrality. NeuroImage 2018, 176, 71–82. [Google Scholar] [CrossRef]
- Schneider, H.D.; Hopp, J.P. The Use of the Bilingual Aphasia Test for Assessment and Transcranial Direct Current Stimulation to Modulate Language Acquisition in Minimally Verbal Children with Autism. Clin. Linguist. Phon. 2011, 25, 640–654. [Google Scholar] [CrossRef]
- Amatachaya, A.; Auvichayapat, N.; Patjanasoontorn, N.; Suphakunpinyo, C.; Ngernyam, N.; Aree-uea, B.; Keeratitanont, K.; Auvichayapat, P. Effect of Anodal Transcranial Direct Current Stimulation on Autism: A Randomized Double-Blind Crossover Trial. Behav. Neurol. 2014, 2014, 173073–173077. [Google Scholar] [CrossRef]
- D’Urso, G.; Bruzzese, D.; Ferrucci, R.; Priori, A.; Pascotto, A.; Galderisi, S.; Altamura, A.C.; Bravaccio, C. Transcranial Direct Current Stimulation for Hyperactivity and Noncompliance in Autistic Disorder. World J. Biol. Psychiatry 2015, 16, 361–366. [Google Scholar] [CrossRef]
- Zemestani, M.; Hoseinpanahi, O.; Salehinejad, M.A.; Nitsche, M.A. The Impact of Prefrontal Transcranial Direct Current Stimulation (tDCS) on Theory of Mind, Emotion Regulation and Emotional-behavioral Functions in Children with Autism Disorder: A Randomized, Sham-controlled, and Parallel-group Study. Autism Res. 2022, 15, 1985–2003. [Google Scholar] [CrossRef]
- Soff, C.; Sotnikova, A.; Christiansen, H.; Becker, K.; Siniatchkin, M. Transcranial Direct Current Stimulation Improves Clinical Symptoms in Adolescents with Attention Deficit Hyperactivity Disorder. J. Neural Transm. 2017, 124, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Schertz, M.; Karni-Visel, Y.; Genizi, J.; Manishevitch, H.; Lam, M.; Akawi, A.; Dudai, M.; Fenton, A.A.; Bikson, M. Transcranial Direct Current Stimulation (tDCS) in Children with ADHD: A Randomized, Sham-Controlled Pilot Study. J. Psychiatr. Res. 2022, 155, 302–312. [Google Scholar] [CrossRef]
- Jensen, F.E.; Rakhade, S.N. Epileptogenesis in the Immature Brain: Emerging Mechanisms. Nat. Rev. Neurol. 2009, 5, 380–391. [Google Scholar] [CrossRef]
- Sudbrack-Oliveira, P.; Barbosa, M.Z.; Thome-Souza, S.; Razza, L.B.; Gallucci-Neto, J.; da Costa Lane Valiengo, L.; Brunoni, A.R. Transcranial Direct Current Stimulation (tDCS) in the Management of Epilepsy: A Systematic Review. Seizure 2021, 86, 85–95. [Google Scholar] [CrossRef]
- Auvichayapat, N.; Rotenberg, A.; Gersner, R.; Ngodklang, S.; Tiamkao, S.; Tassaneeyakul, W.; Auvichayapat, P. Transcranial Direct Current Stimulation for Treatment of Refractory Childhood Focal Epilepsy. Brain Stimul. 2013, 6, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Brunoni, A.R.; Moffa, A.H.; Sampaio-Júnior, B.; Gálvez, V.; Loo, C.K. Treatment-Emergent Mania/Hypomania during Antidepressant Treatment with Transcranial Direct Current Stimulation (tDCS): A Systematic Review and Meta-Analysis. Brain Stimul. 2017, 10, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, V.; Alonzo, A.; Martin, D.; Mitchell, P.B.; Sachdev, P.; Loo, C.K. Hypomania Induction in a Patient with Bipolar II Disorder by Transcranial Direct Current Stimulation (tDCS). J. ECT 2011, 27, 256–258. [Google Scholar] [CrossRef]
- Salehinejad, M.A.; Nikolin, S.; Vicario, C.M.; Nitsche, M.A.; Loo, C.K.; Brunoni, A.R. Safety and Tolerability. In Transcranial Direct Current Stimulation in Neuropsychiatric Disorders: Clinical Principles and Management; Brunoni, A.R., Nitsche, M.A., Loo, C.K., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 667–676. ISBN 978-3-030-76136-3. [Google Scholar]
- D’Urso, G.; Toscano, E.; Sanges, V.; Sauvaget, A.; Sheffer, C.E.; Riccio, M.P.; Ferrucci, R.; Iasevoli, F.; Priori, A.; Bravaccio, C.; et al. Cerebellar Transcranial Direct Current Stimulation in Children with Autism Spectrum Disorder: A Pilot Study on Efficacy, Feasibility, Safety, and Unexpected Outcomes in Tic Disorder and Epilepsy. J. Clin. Med. 2021, 11, 143. [Google Scholar] [CrossRef]
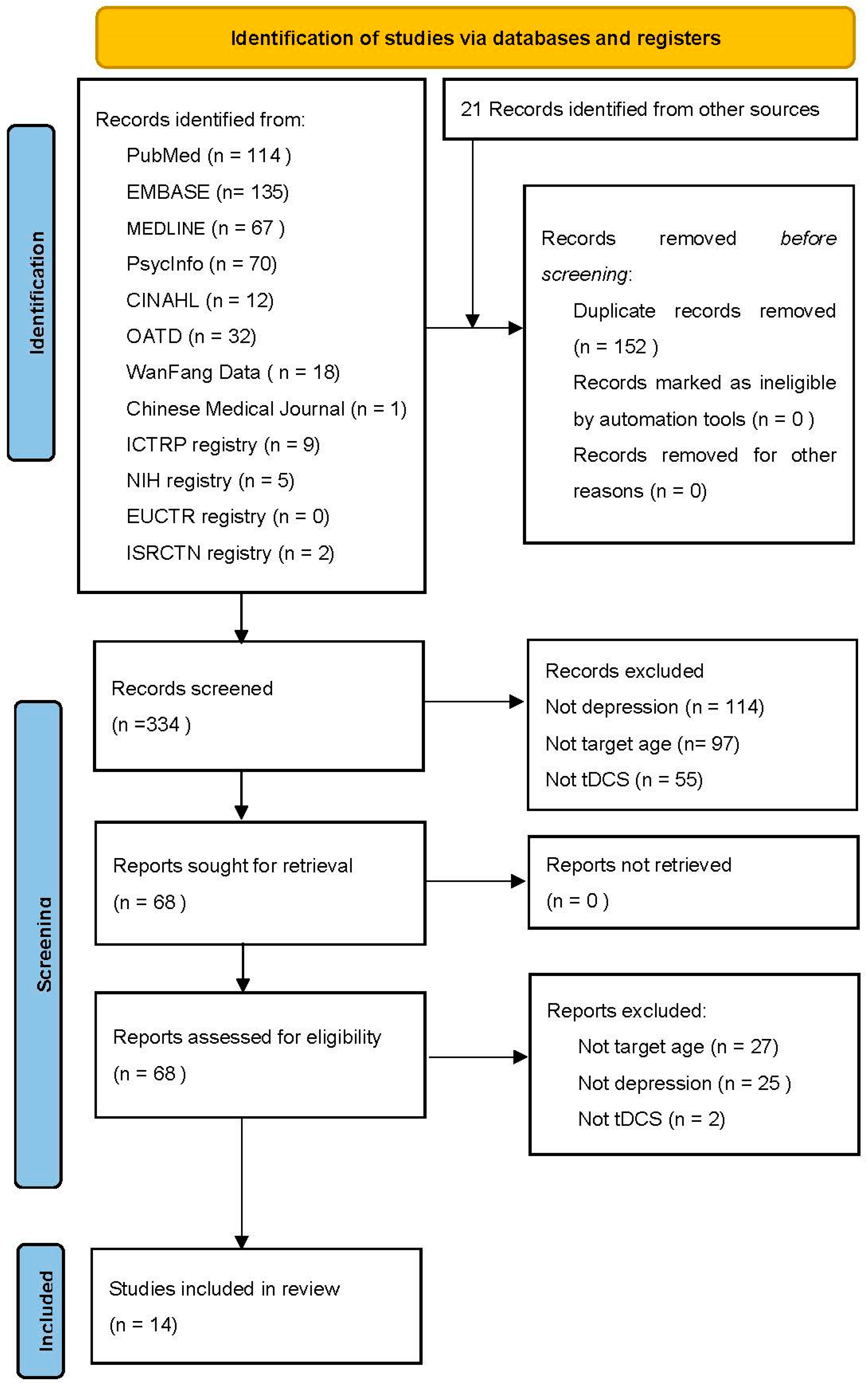


| ||||||||
| Name of First Author/Publication Year | Design | N | Diagnosis | Age Range (N) | Control | Anode/Cathode | Protocol | POM |
| Baibujiapu et al., 2017 [24] | Sham-Controlled Study (a) | Active = 32 Sham = 33 | Major Depressive Disorder | 10–17 | Sham-tDCS + Sandplay Therapy | Primary somatosensory cortex/contralateral shoulder | 15 sessions; 1.2 mA; 20 min | HDRS at (pre: 28.7 ± 4.3; post: 20.5 ± 3.6) (p < 0.001 between groups) (b) |
| Zhang et al., 2023 [25] | Open-label Study | 28 | Major Depressive Episode | 14–17 | - | HD-tDCS Central anode: L-DLPFC | 20 sessions; 2 mA; 20 min | HDRS-17 (pre: 24.14 ± 5.79; post: 13.14 ± 5.49; t = −11.383, p < 0.001) (b) |
| ||||||||
| Name of First Author/Publication Year | Design | N | Diagnosis | Age (N) | Control | Anode/Cathode | Protocol | POM |
| Baliga et al., 2020 [26] | Case Report | 1 | Bipolar Depression | 21 | - | L-DLPFC/R-DLPFC | 10 sessions; 2 mA; 30 min | HDRS (reduction from 20 to 9) (30%) |
| Clayton et al., 2018 [27] | Case Report | 1 | Major Depression with Multiple Sclerosis | 19 | - | L-DLPFC | 2 sessions; 1.5 mA; 20 min (c) | BDI (reduction from 12 to 0) |
| Shankar et al., 2023 [28] | Case Report | 1 | Treatment-resistant depression | 16 | - | Not reported | 20 sessions; n-r; after ketamine infusion | HDRS-17 (reduction from 15 to 8) |
| Sreeraj, et al., 2016 [29]. | Case Report | 1 | Depression with pregnancy | 23 | - | L-DLPFC/R-DLPFC | 10 sessions; 2 mA; 30 min | HDRS (reduction from 18 to 6), HAMA |
| ||||||||
| Trial ID (Status) | Design | N | Diagnosis | Age range (N) | Control | Anode/Cathode | Protocol | POM |
| ChiCTR2000039503 (ongoing) | Case–Control Observation Study | 50 | Major Depressive Disorder | 10–22 | - | Not reported | Not reported | ASIQ, BSI, BRMS, CGI, CTQ, HAMA, HDRS, MPQ-SF, PSQI, SHAPS, SDSS |
| ChiCTR2400079464 (ongoing) | Double-blind RCT | 40 | Major Depressive Disorder | 13–17 | Sham tDCS | Not reported | Not reported | HDRS-17 |
| DRKS00027066 (ongoing) | Double-blind RCT | 100 | Major Depressive episode | 13–17 | Sham tDCS | L-DLPFC/R-DLPFC | 10 sessions; 2 mA | BDI-II |
| NCT03368469 (withdrawn) (d) | Open-label, Single-arm Study | 20 (e) | Epilepsy and Depressive Disorder | 10–21 | Sham tDCS | lDPFC/R-SOA | 10 sessions; 2 mA; 20 min | CDRS-R |
| NCT03897699 (completed) | Quadruple-blind RCT | 68 (Actual: 36) | Major Depressive Disorder (girls only) | 17–24 (mean age 20.2) | Sham tDCS + Mindful Breathing Training | L-DLPFC/R-DLPFC | 10 sessions; 2 mA; 20 min | Change in DLPFC Connectivity; Amygdala/DMN Secondary outcome measure: MARDS: Active group 6.47 (4.69); Control Group 5.95 (4.04) (f) Adverse Effects: |
| NCT04780152 (ongoing) | Double-blind RCT | 172 | Major Depressive Disorder | 10–17 | Sham tDCS + fluoxetine | L-DLPFC/R-DLPFC | 10 sessions; 2 mA; 30 min | CDI |
| NCT05498441 (ongoing) | Double-blind RCT | 120 | Major Depressive Episode | 13–18 | Routine HD-tDCS | Central anode: personalised HD-tDCS with 4 × 1 ring montage on L- DLPFC | 20 sessions; 20 min | HDRS-17, RBANS, MRI and DTI imaging |
| NCT06061653 (ongoing) | Double-blind RCT | 60 | Major Depressive Disorder | 12–18 | IPT + HD-tDCS | Not reported | Not reported | HDRS-24; CRDS-R |
| Sex/Age | Education (Years) | Time Since Depression (Weeks) | HDRS Severity (Baseline) | Device Assigned | Treatment Completion | Self-Administration Compliance | Adverse Effects | Blinding Guess | |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Male/16 | 8 | 104 | Mild (14) | Sooma (Home-Based) | 5 sessions completed without issues | Completed 5/5 home sessions, no issues | None | Not correct |
| 2. | Male/20 | 13 | 156 | Severe (21) | Soterix (Hospital-Based) | 5 sessions completed (1 session rescheduled) | - | Tingling (Mild) | Correct |
| 3. | Female/20 | 13 | 312 | Mild (14) | Sooma (Home-Based) | 5 sessions completed, with prompting | Completed 5/5 home sessions, 1 missed, prompted the same day for makeup session | None | Not correct |
| 4. | Female/17 | 10 | - | Severe (20) | Sooma (Home-Based) | 5 sessions completed without issues | Completed 5/5 home sessions, no issues | Tingling (Mild) | Correct |
| 5. | Male/16 | 11 | 260 | Moderate (18) | Soterix (Hospital-Based) | 5 sessions completed (2 sessions rescheduled) | - | None | Not correct |
| 6. | Male/21 | 15 | 312 | Moderate (15) | Sooma (Home-Based) | 5 sessions completed (minor delays) | Completed 5/5 home sessions, minor delays | None | Correct |
| 7. | Male/20 | 13 | 52 | Moderate (15) | Soterix (Hospital-Based) | 5 sessions | - | None | Not correct |
| 8. | Female/24 | 15 | 312 | Moderate (15) | Soterix (Hospital-Based) | 5 sessions (rescheduling required) | - | Tingling, Headache (Mild) | Correct |
| Score/Group | T0 Mean | (S.D.) | T1 Mean | (S.D.) | Mean Difference | (S.D.) | t | p | d |
|---|---|---|---|---|---|---|---|---|---|
| HDRS: p = 1 | |||||||||
| Active tDCS | 18.50 | (2.65) | 13.75 | (2.06) | −4.75 | (0.96) | −0.72 | 0.48 | −0.32 |
| Sham tDCS | 13.75 | (1.50) | 10.00 | (2.45) | −3.75 | (3.78) | |||
| SHAPS: p = 1 | |||||||||
| Active tDCS | 34.25 | (4.50) | 37.00 | (4.83) | 2.75 | (4.20) | 1.23 | 0.236 | 0.55 |
| Sham tDCS | 45.50 | (3.32) | 46.00 | (3.56) | 0.50 | (0.57) | |||
| C-DARS: p = 1 | |||||||||
| Active tDCS | 24.00 | (8.17) | 27.00 | (7.07) | 3.00 | (7.96) | 1.03 | 0.32 | 0.46 |
| Sham tDCS | 55.50 | (10.60) | 53.00 | (7.39) | −2.50 | (7.77) | |||
| YMRS: N.A. | |||||||||
| Active tDCS | 3.00 | (0.40) | 3.00 | (0.40) | - | - | N.A. | ||
| Sham tDCS | 2.80 | (0.54) | 2.80 | (0.54) | - | - | |||
| SOFAS: p = 0.429 | |||||||||
| Active tDCS | 6.00 | (0.82) | 6.75 | (1.50) | 0.75 | (0.96) | 0.65 | 0.52 | 0.29 |
| Sham tDCS | 8.50 | (0.71) | 9.50 | (0.01) | 1 | (0.56) | |||
| VAS—Comfort: p = 1 | |||||||||
| Active tDCS | 7.25 | (0.96) | 6.75 | (1.71) | −0.50 | (1.29) | −1.42 | 0.26 | −0.78 |
| Sham tDCS | 9.00 | (0.00) | 10.00 | (0.00) | 1 | (0.00) | |||
| Active Group (n = 4) | Sham Group (n = 4) | |||
| Adverse Effects | Frequency | % | Frequency | % |
| Tingling | 2 | 0.10% | 0 | |
| Skin redness | 0 | 0 | ||
| Burning sensation | 0 | 0 | ||
| Headache | 3 | 0.15% | 0 | |
| Fatigue | 0 | 0 | ||
| Itching | 0 | 0 | ||
| Scalp pain | 0 | 0 | ||
| Neck pain | 0 | 0 | ||
| Sleepiness | 0 | 0 | ||
| Trouble concentrating | 0 | 0 | ||
| Acute mood change | 0 | 0 | ||
| Compliance | 100% | 100% | ||
| Hospital-Based (n = 4) | Home-Based (n = 4) | |||
| Adverse Effects | Frequency | % | Frequency | % |
| Tingling | 1 | 0.05% | 1 | 0.05% |
| Skin redness | 0 | 0 | ||
| Burning sensation | 0 | 0 | ||
| Headache | 2 | 0.10% | 1 | 0.05% |
| Fatigue | 0 | 0 | ||
| Itching | 0 | 0 | ||
| Scalp pain | 0 | 0 | ||
| Neck pain | 0 | 0 | ||
| Sleepiness | 0 | 0 | ||
| Trouble concentrating | 0 | 0 | ||
| Acute mood change | 0 | 0 | ||
| Compliance | 100% | 100% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, H.K.Y.; Yuen, S.Y.; Tsui, I.W.T.; Yeung, W.F.; Ruan, J.Y.; Wong, C.S.M.; Jin, J.X.H.; Lee, C.T.; Chung, K.F. Transcranial Direct Current Stimulation (tDCS) in the Treatment of Youth Depression: Integrating Literature Review Insights in a Pilot Clinical Trial. J. Clin. Med. 2025, 14, 3152. https://doi.org/10.3390/jcm14093152
Lo HKY, Yuen SY, Tsui IWT, Yeung WF, Ruan JY, Wong CSM, Jin JXH, Lee CT, Chung KF. Transcranial Direct Current Stimulation (tDCS) in the Treatment of Youth Depression: Integrating Literature Review Insights in a Pilot Clinical Trial. Journal of Clinical Medicine. 2025; 14(9):3152. https://doi.org/10.3390/jcm14093152
Chicago/Turabian StyleLo, Heidi Ka Ying, Suet Ying Yuen, Iris Wai Tung Tsui, Wing Fai Yeung, Jia Yin Ruan, Corine Sau Man Wong, Joyce Xu Hao Jin, Chit Tat Lee, and Ka Fai Chung. 2025. "Transcranial Direct Current Stimulation (tDCS) in the Treatment of Youth Depression: Integrating Literature Review Insights in a Pilot Clinical Trial" Journal of Clinical Medicine 14, no. 9: 3152. https://doi.org/10.3390/jcm14093152
APA StyleLo, H. K. Y., Yuen, S. Y., Tsui, I. W. T., Yeung, W. F., Ruan, J. Y., Wong, C. S. M., Jin, J. X. H., Lee, C. T., & Chung, K. F. (2025). Transcranial Direct Current Stimulation (tDCS) in the Treatment of Youth Depression: Integrating Literature Review Insights in a Pilot Clinical Trial. Journal of Clinical Medicine, 14(9), 3152. https://doi.org/10.3390/jcm14093152





