Orthopedic Manifestations of Syringomyelia: A Comprehensive Review
Abstract
1. Introduction
- A.
- Complete blockage, where the CSF pressure pulse wave is halted at the point of obstruction and transmitted into the spinal cord;
- B.
- Partial blockage, which allows some degree of CSF flow and is explained by two hemodynamic principles.
- Abnormal CSF flow dynamics associated with hindbrain malformations.
- Intramedullary or perimedullary congenital or acquired tissue damage.
- Syringomyelia resulting from intramedullary tumors (primarily ependymomas or hemangioblastomas) with secretory activity.
- Syringomyelia secondary to supratentorial hydrocephalus.
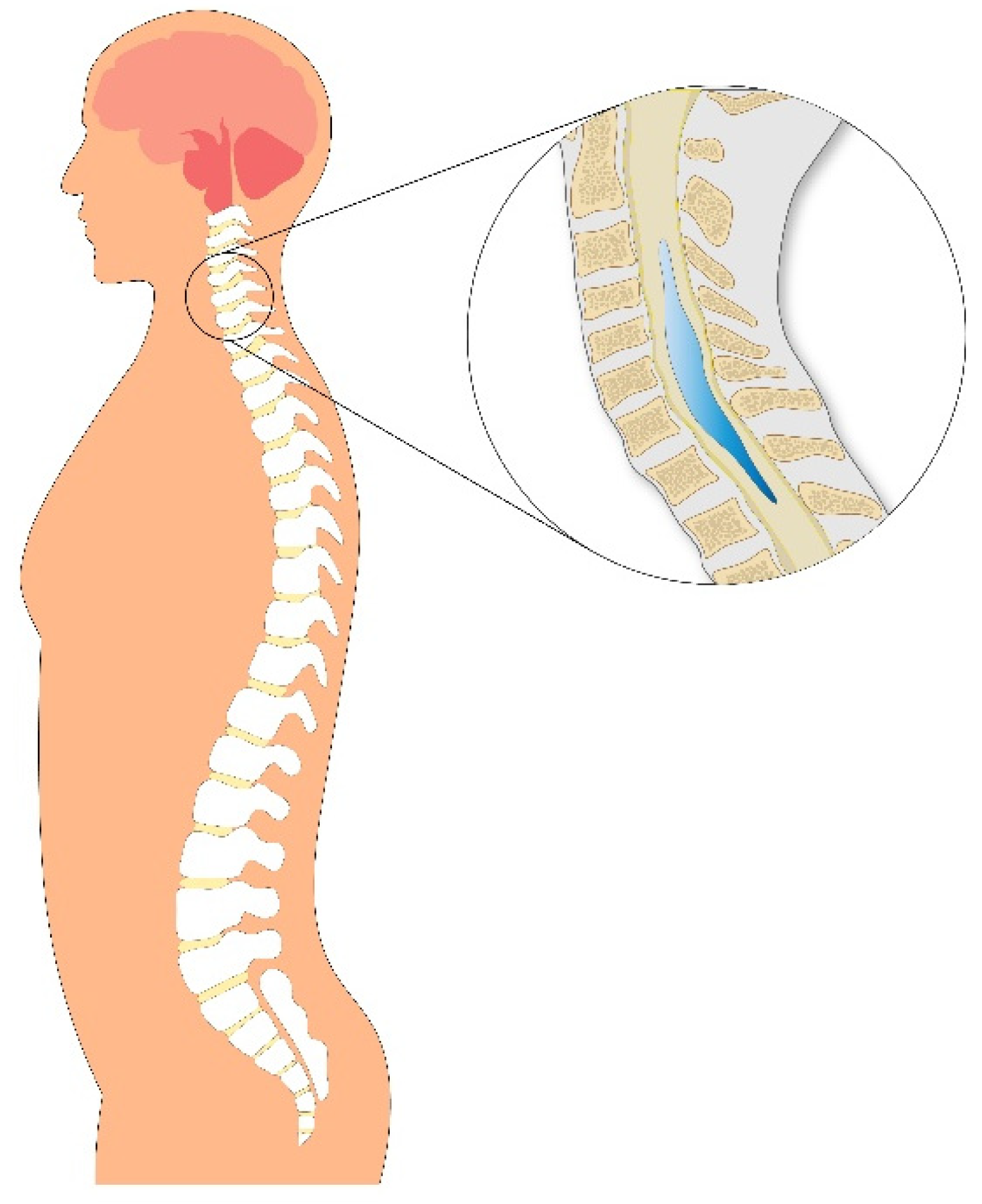
2. Subtypes and Morphological Variants of Syringomyelia
- Type I: Associated with hindbrain abnormalities such as Chiari malformation type I (CM-1), basilar invagination, or other lesions obstructing CSF flow at the foramen magnum, leading to central canal dilatation and syrinx formation.
- 4.
- Type II: Often considered idiopathic, this type also arises at the foramen magnum but without any identifiable obstruction.
- 5.
- Type III: Secondary to intraspinal disorders, such as tumors, traumatic myelopathy, arachnoiditis, pachymeningitis, or myelomalacia, which directly affect the spinal cord parenchyma.
- 6.
- Type IV: Referred to as pure hydromyelia, characterized by central canal dilatation without clear disruption to surrounding tissue.
- Hydromyelia, typically referring to a dilated ependymal-lined central canal.
- Syringomyelia, denoting glial-lined cavities that disrupt the cord parenchyma.
3. Prevalence
4. Etiology
4.1. Chiari-Related Syringomyelia
4.2. Primary Spinal Syringomyelia (PSS)
- Spinal trauma.
- Arachnoid cysts.
- Post-infectious scarring from meningitis or subarachnoid hemorrhage [11].
4.3. Tethered Cord Syndrome and Spina Bifida
4.4. Risk Factors for Syringomyelia
- Post-traumatic kyphosis exceeding 15°.
- Spinal canal stenosis greater than 25%.
5. Pathophysiology
5.1. Theoretical Perspectives on Syringomyelia Formation
5.2. Mechanisms of Syrinx Formation
- Water Hammer Theory suggests that partial obstruction at the fourth ventricle redirects systolic CSF pulses down a patent central canal, leading to syrinx formation [19].
- Cranial–Spinal Pressure Dissociation Theory proposes that a caudal CSF block results in higher intracranial pressure relative to spinal intrathecal pressure, forcing CSF downward into the central canal, thereby producing communicating syringomyelia. However, this proposed communication is rarely seen in MRI or autopsy studies.
- Ball and Dayan Theory posits that intermittent increases in spinal CSF pressure, caused by cerebellar tonsil obstruction during activities like coughing or Valsalva maneuver, force CSF through extracellular spaces along the spinal cord surface, initiating syrinx formation [20].
- Piston Theory suggests that cerebellar tonsils act as a piston, creating pressure waves in the spinal subarachnoid space that drive CSF into the perivascular or subpial spaces, ultimately contributing to syrinx expansion [21].
- Perivascular Flow Disruption Theory proposes that CSF normally flows along perivascular spaces, but disruption of this flow, as seen in Chiari malformations, can lead to increased inflow or reduced outflow, resulting in syrinx development and enlargement [22].
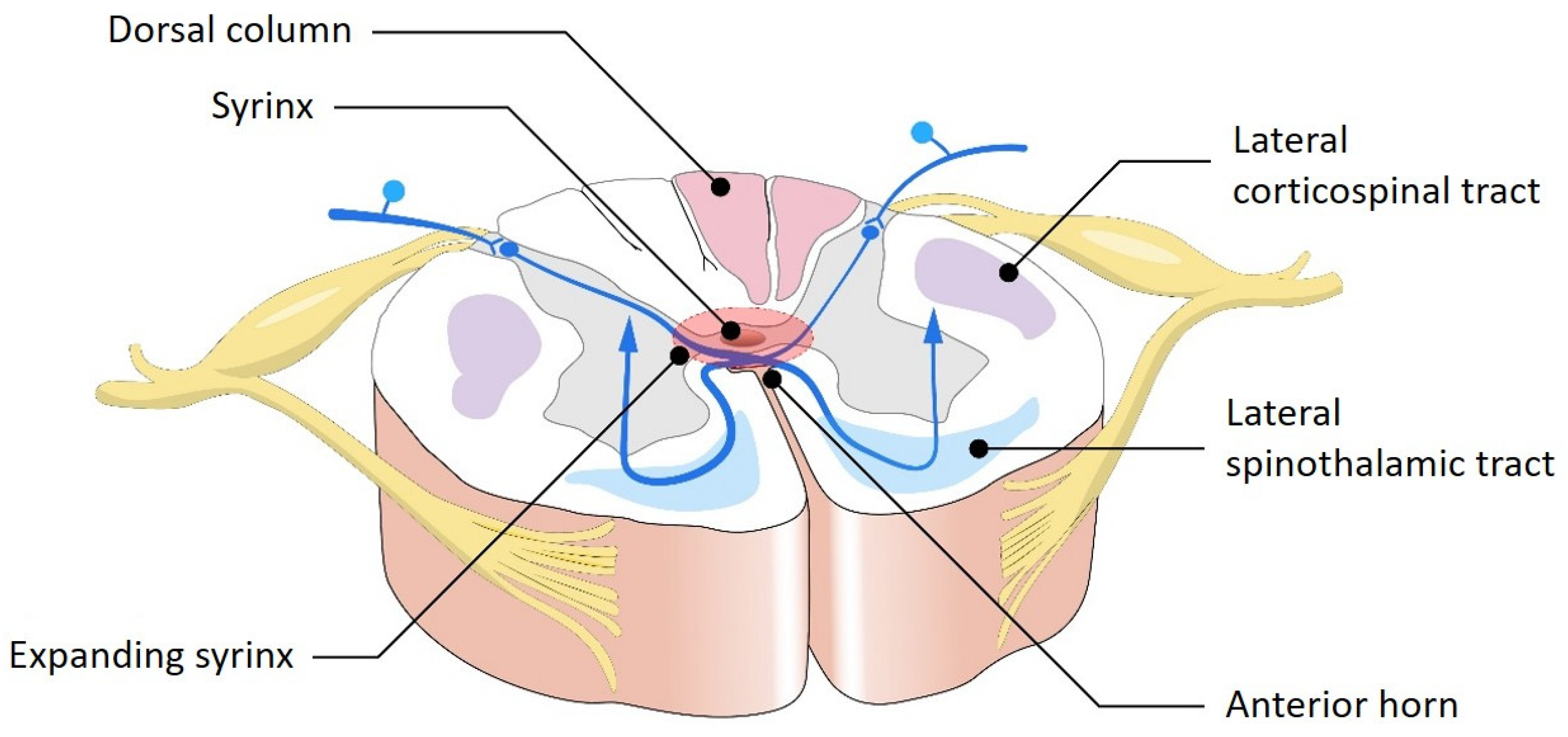
5.3. Impact on the Spinal Cord
6. Orthopedic Manifestations and Musculoskeletal Involvement
6.1. Scoliosis in Syringomyelia
6.2. Neurological Features with Musculoskeletal Implications
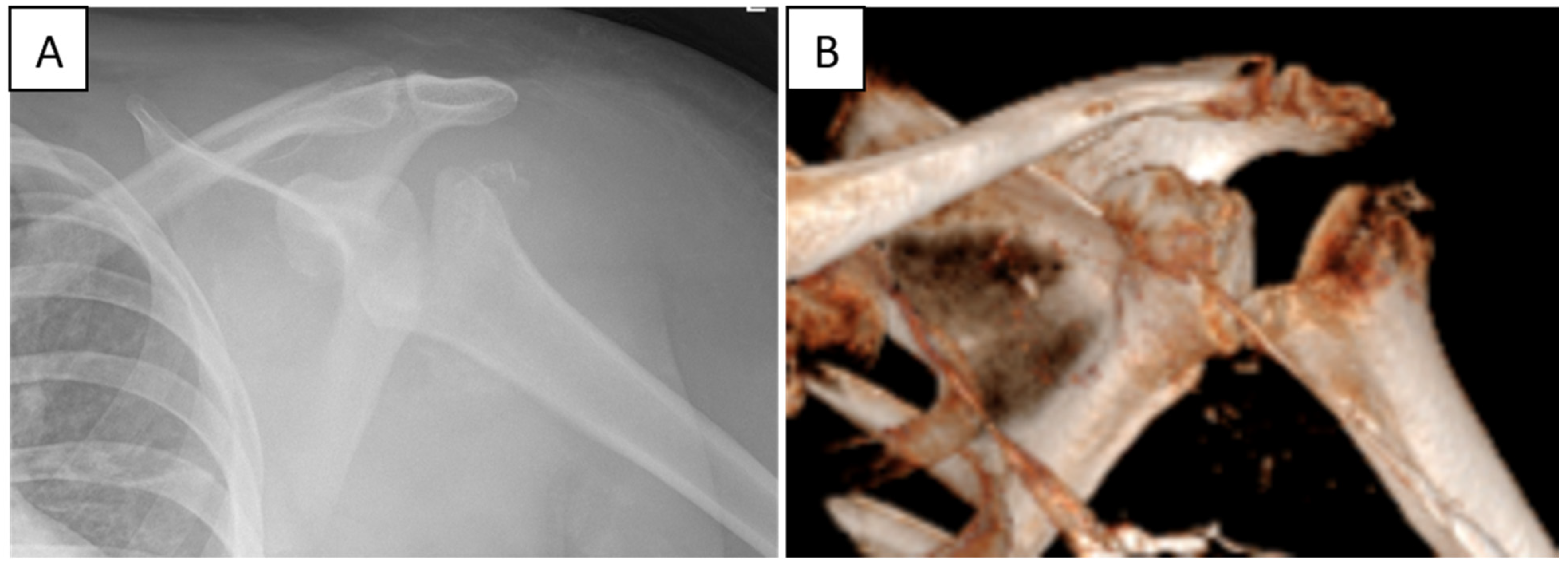
6.3. Charcot Joints and Neurogenic Arthropathy
6.4. Illustrative Clinical Case
7. Diagnosis
7.1. Clinical Presentation
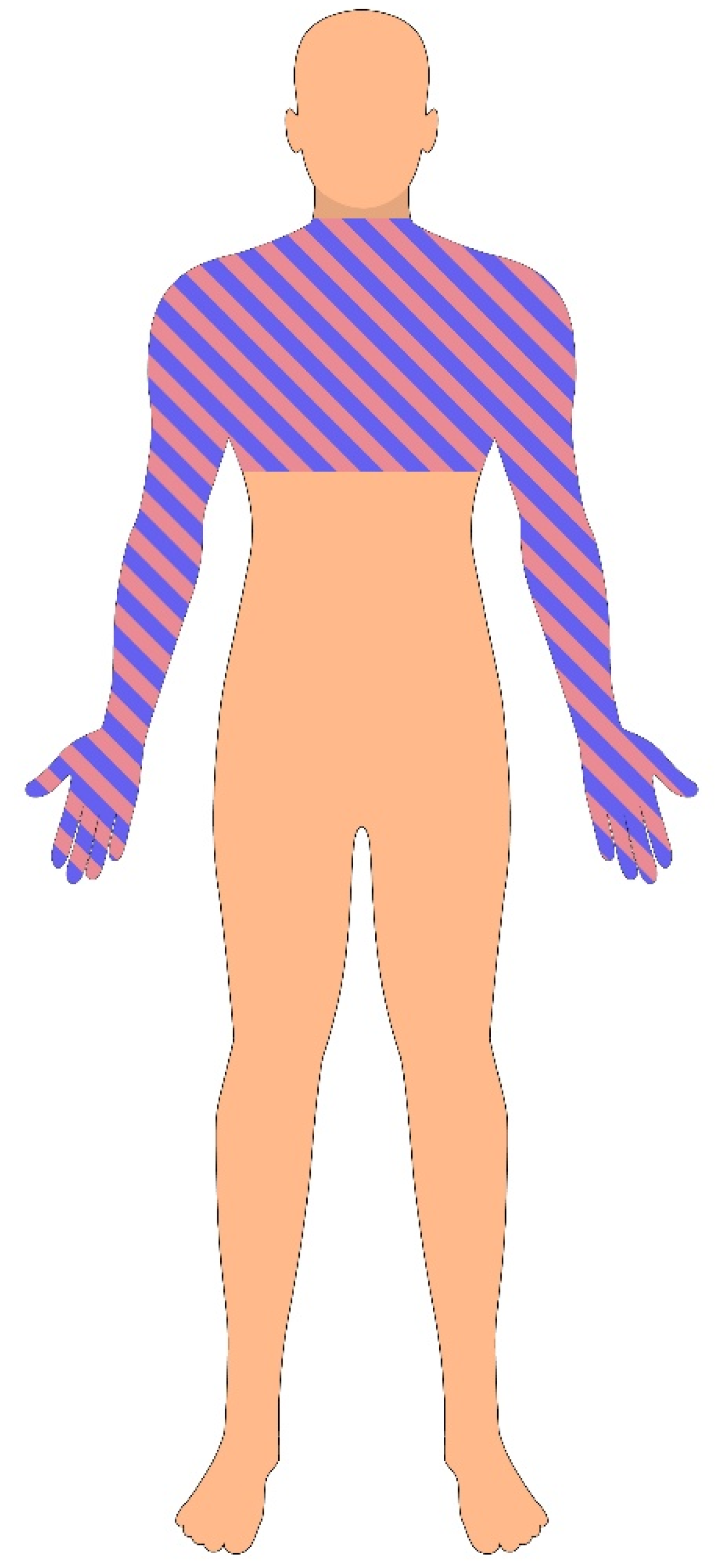
- Segmental sensory loss refers to localized sensory deficits corresponding to the dermatomal level of spinal cord involvement.
- Pyramidal signs indicate upper motor neuron dysfunction, often presenting as spasticity, hyperreflexia, and a positive Babinski sign, reflecting disruption of the corticospinal tract.
- Dissociated sensory loss is a hallmark of syringomyelia and describes the selective loss of pain and temperature sensation, with preservation of light touch, vibration, and proprioception. This pattern occurs because spinothalamic fibers, which carry pain and temperature sensations, cross within the central spinal cord and are affected early by syrinx expansion, whereas dorsal column pathways—carrying vibration and proprioception signals—are located more peripherally and remain intact.
- Radicular pain: Pain radiating along a dermatome due to nerve root irritation.
- Gait ataxia: Impaired coordination and unsteady walking due to sensory or motor deficits.
- Dysesthesias: Abnormal sensations often described as burning, tingling, or electric-shock-like pain.
- Spasms and spasticity: Involuntary muscle contractions or stiffness due to upper motor neuron involvement.
- Autonomic dysreflexia: A potentially life-threatening condition characterized by sudden hypertension, bradycardia, and profuse sweating, often triggered by noxious stimuli below the level of spinal cord injury.
- Neuropathic pain: Chronic pain caused by direct injury to the nervous system, often disproportionate to physical findings [6].
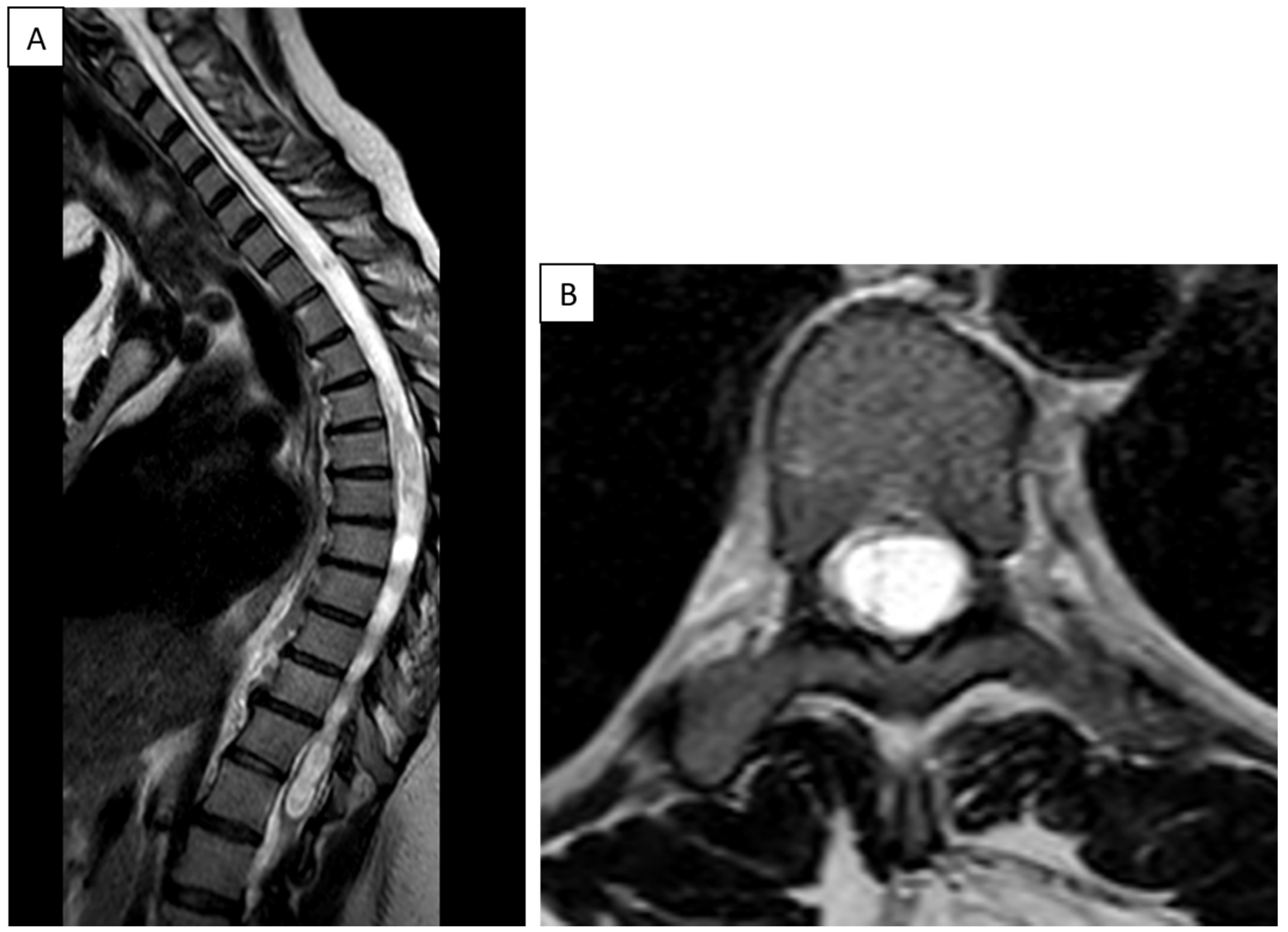
7.2. Imaging Studies
- Persistent spinal cord lesions;
- A history of spinal surgery or trauma;
- Gradually progressive neurological symptoms;
- Abrupt symptom exacerbation without a clear alternative diagnosis.
7.3. MRI and Cine MRI
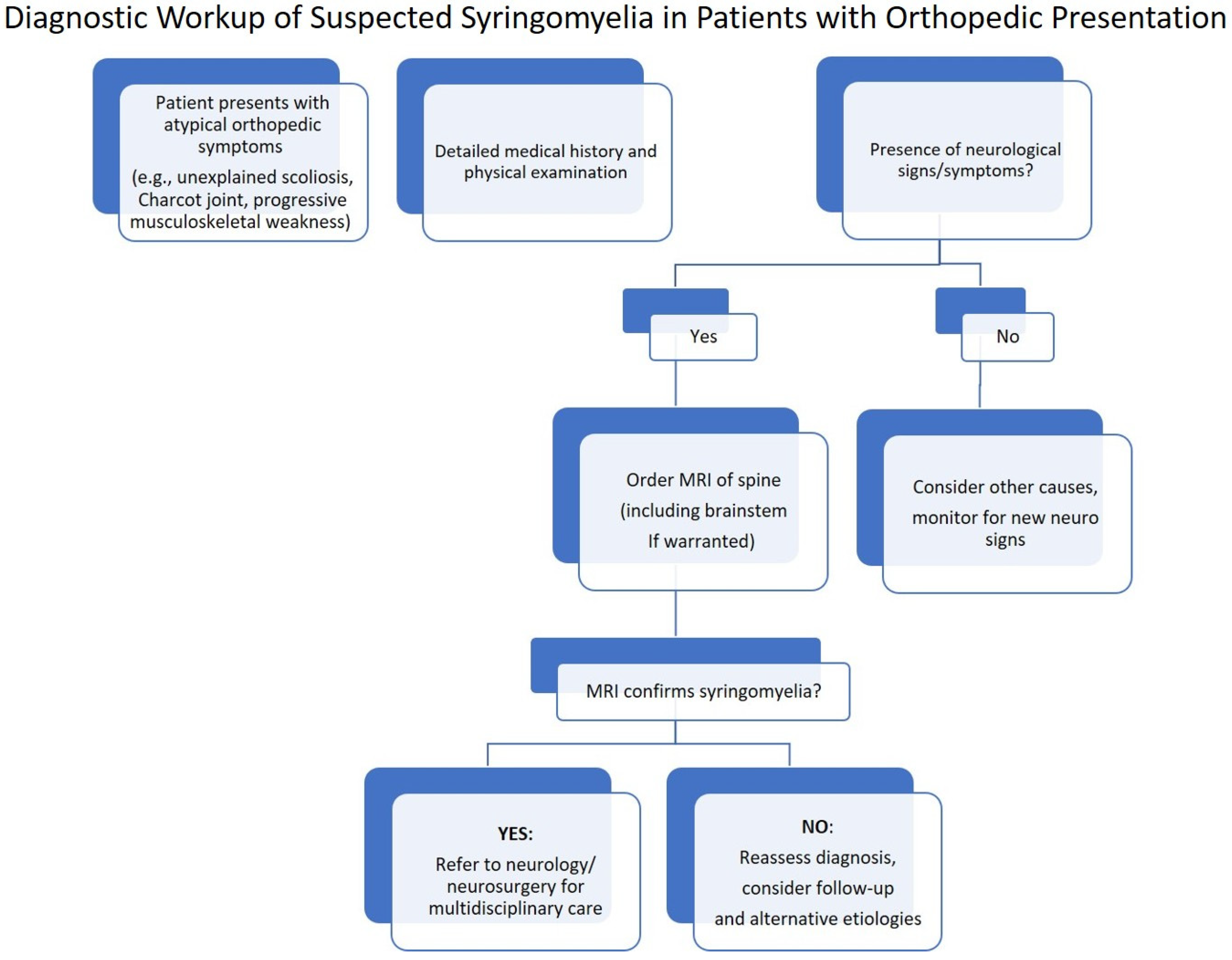
7.4. Diagnostic Workup: Stepwise Flowchart
8. Management
8.1. Conservative Management
- Medical Management
- A.
- Anticonvulsant Agents: Gabapentin and pregabalin have been shown to improve neuropathic pain, including hyperalgesia and allodynia, through modulation of voltage-gated calcium channels [34,35,36,37,38]. Pregabalin has also demonstrated efficacy in reducing phantom scratching and other sensory disturbances [35].
- B.
- C.
- D.
- Carbonic Anhydrase Inhibitors (e.g., Acetazolamide): These agents may reduce CSF flow and have been proposed as adjuncts in conservative management [41].
- E.
- F.
- G.
- 2.
- Management of Spasticity
- A.
- B.
- C.
- Botulinum toxin injections—effective in focal spasticity, often used in combination with physiotherapy [49].
- D.
- 3.
- Physical Therapy and Rehabilitation
- Stretching and strengthening exercises.
- Weight-bearing and postural training.
- Orthotic support (e.g., ankle–foot orthoses).
- Serial casting.
- Cryotherapy, thermotherapy, and electrical stimulation.
8.2. Surgical Management
- A.
- Suboccipital Decompression (Posterior Fossa Decompression): In patients with Chiari malformation type I (CM-I), the most commonly performed procedure is posterior fossa decompression (PFD) or craniocervical decompression, which aims to reestablish normal CSF circulation by removing bone at the foramen magnum and often includes dural opening and duraplasty [56,57,58,59]. There is ongoing debate regarding the extent of decompression, particularly the need to open the dura. Some surgeons advocate dural opening with a patch graft, citing its importance in identifying and releasing arachnoid scarring or other obstructions, which are present in up to 55% of cases [57]. Others argue that dural opening may increase the risk of complications. Intraoperative ultrasonography has been proposed to optimize decompression and tailor surgery to individual CSF flow dynamics [17].
- B.
- Shunt Placement: In cases where decompression does not result in syrinx regression, or in post-traumatic syringomyelia, shunt placement may be considered. Various shunt types include the following:
- Syringosubarachnoid shunt.
- Syringoperitoneal shunt.
- Syringopleural shunt.
- C.
- Spinal Cord Surgery and Direct Decompression: In select cases, direct surgical decompression of spinal cord cavities may be required. Techniques include restoration of subarachnoid pathways or syrinx drainage via myelotomy. However, these procedures pose significant risks, including dorsal column injury, especially in patients who retain some neurological function [60]. Moreover, septated syrinx cavities may limit the effectiveness of shunting, and unless the underlying cause of fluid accumulation is addressed, new cavities may continue to form despite successful decompression [60].
- D.
- Etiology-Directed Surgery: In cases where the syrinx is caused by an identifiable compressive lesion, such as a tumor or scar tissue, removal of the obstruction can restore CSF flow and often lead to syrinx resolution [61]. Tumor resection, when feasible, remains the primary approach, although radiation therapy may be considered in certain scenarios.
- E.
- Drainage Procedures: In patients with idiopathic or progressively enlarging syrinx cavities, drainage via stent or shunt placement may be considered. A stent allows for internal diversion of syrinx fluid, while a shunt system, typically composed of a catheter and valve mechanism, diverts fluid to pleural or peritoneal spaces [62]. These procedures may stabilize or improve symptoms, although long-term success varies, and some patients may experience recurrence requiring reoperation [63].
9. Treatment Considerations in Charcot Shoulder
Back to Our Patient
10. Summary and Conclusions
11. Key Messages
- Syringomyelia is a complex spinal cord disorder characterized by fluid-filled cavities within the spinal cord, often resulting from disrupted cerebrospinal fluid (CSF) dynamics due to Chiari malformation, trauma, or intradural lesions.
- Orthopedic manifestations are frequent but under-recognized and may represent the earliest clinical signs. These include neurogenic arthropathy (Charcot joints)—most notably involving the shoulder—as well as pes cavus, scoliosis, gait disturbances, and unexplained limb weakness.
- Neuropathic shoulder arthropathy, though rare, can lead to progressive joint destruction due to impaired proprioception and pain perception. Early identification is crucial to prevent irreversible structural damage.
- Clinical symptoms of syringomyelia vary widely, often mimicking other neurological or orthopedic conditions. Early signs may include dissociated sensory loss, spasticity, paresthesia, or segmental motor weakness.
- Diagnosis requires a high index of suspicion, especially in orthopedic settings, where patients may present with joint pain or dysfunction without a history of trauma or infection.
- Conservative treatment—including analgesics, anticonvulsants, physical therapy, and joint protection—remains the first-line strategy for both neurologic and orthopedic manifestations.
- Surgical intervention, including posterior fossa decompression, syrinx shunting, or lesion removal, is indicated in progressive cases and should be tailored to the underlying etiology.
- Long-term follow-up is essential, not only to assess neurological stability and syrinx progression, but also to monitor orthopedic complications, especially in joints affected by neurogenic arthropathy.
Funding
Conflicts of Interest
References
- Leclerc, A.; Matveeff, L.; Emery, E. Syringomyelia and hydromyelia: Current understanding and neurosurgical management. Rev. Neurol. 2021, 177, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.K.; Slimack, N.P.; Ganju, A. Idiopathic syringomyelia: Retrospective case series, comprehensive review, and update on management. Neurosurg. Focus 2011, 31, E15. [Google Scholar] [CrossRef]
- Sabellano, Z.; Batino, L.; Navarro, J.J. Atypical presentation of syringomyelia mimicking as motor neuron disease: A case report. Clin. Imag. Med. Case Rep. 2023, 4, 2685. [Google Scholar] [CrossRef]
- Heiss, J.D.; Snyder, K.; Peterson, M.M.; Patronas, N.J.; Butman, J.A.; Smith, R.K.; DeVroom, H.L.; Sansur, C.A.; Eskioglu, E.; Kammerer, W.A.; et al. Pathophysiology of primary spinal syringomyelia. J. Neurosurg. Spine 2012, 17, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Blegvad, C.; Grotenhuis, J.; Juhler, M.J. Syringomyelia: A practical, clinical concept for classification. Acta Neurochir. 2014, 156, 2127–2138. [Google Scholar] [CrossRef]
- Ciaramitaro, P.; Garbossa, D.; Peretta, P.; Piatelli, G.; Massimi, L.; Valentini, L.; Migliaretti, G.; Baldovino, S.; Roccatello, D.; Kodra, Y.; et al. Syringomyelia and Chiari Syndrome Registry: Advances in epidemiology, clinical phenotypes and natural history based on a North Western Italy cohort. J. Neurosurg. Sci. 2020, 56, 48–58. [Google Scholar]
- Flint, G.J. Syringomyelia: Diagnosis and management. Pr. Neurol. 2021, 21, 403–411. [Google Scholar] [CrossRef]
- Kahn, E.N.; Muraszko, K.M.; Maher, C.O. Prevalence of Chiari I malformation and syringomyelia. Neurosurg. Clin. N. Am. 2015, 26, 501–507. [Google Scholar] [CrossRef]
- Arnautovic, A.; Splavski, B.; Boop, F.A.; Arnautovic, K.I. Pediatric and adult Chiari malformation Type I surgical series 1965–2013: A review of demographics, operative treatment, and outcomes. J. Neurosurg. Pediatr. 2015, 15, 161–177. [Google Scholar] [CrossRef]
- Milhorat, T.H.; Capocelli, A.L.; Anzil, A.P.; Kotzen, R.M.; Milhorat, R.H. Pathological basis of spinal cord cavitation in syringomyelia: Analysis of 105 autopsy cases. J. Neurosurg. 1995, 82, 802–812. [Google Scholar] [CrossRef]
- Holly, L.T.; Batzdorf, U. Chiari malformation and syringomyelia: JNSPG 75th anniversary invited review article. J. Neurosurg. Spine 2019, 31, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Alperin, N.; Loftus, J.R.; Bagci, A.M.; Lee, S.H.; Oliu, C.J.; Shah, A.H.; Green, B.A. Magnetic resonance imaging–based measures predictive of short-term surgical outcome in patients with Chiari malformation Type I: A pilot study. J. Neurosurg. Pediatr. 2017, 26, 28–38. [Google Scholar] [CrossRef]
- Lapsiwala, S.B.; Iskandar, B.J. The tethered cord syndrome in adults with spina bifida occulta. Neurol. Res. 2004, 26, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Mosleh, M.M.; Sohn, M.-J. Optimizing Therapeutic Strategies for Syringomyelia Associated with Tethered Cord Syndrome: A Comprehensive Review. Children 2024, 11, 961. [Google Scholar] [CrossRef]
- Ko, H.Y.; Kim, W.; Kim, S.Y.; Shin, M.J.; Cha, Y.S.; Chang, J.H.; Shin, Y.B. Factors associated with early onset post-traumatic syringomyelia. Spinal Cord. 2012, 50, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Sakas, D.E.; Korfias, S.I.; Wayte, S.C.; Beale, D.J.; Papapetrou, K.P.; Stranjalis, G.S.; Whittaker, K.W.; Whitwell, H.L. Chiari malformation: CSF flow dynamics in the craniocervical junction and syrinx. Acta Neurochir. 2005, 147, 1223–1233. [Google Scholar] [CrossRef]
- Rusbridge, C.; Greitz, D.; Iskandar, B.J. Syringomyelia: Current concepts in pathogenesis, diagnosis, and treatment. J. Vet. Intern. Med. 2006, 20, 469–479. [Google Scholar] [CrossRef]
- Giner, J.; López, C.P.; Hernández, B.; de la Riva, Á.G.; Isla, A.; Roda, J. Update on the pathophysiology and management of syringomyelia unrelated to Chiari malformation. Neurocirugia 2019, 34, 318–325. [Google Scholar] [CrossRef]
- Gardner, J.W.; Angel, J. The mechanism of syringomyelia and its surgical correction. Neurosurgery 1959, 6, 131–140. [Google Scholar] [CrossRef]
- Ball, M.; Dayan, A. Pathogenesis of syringomyelia. Lancet 1972, 300, 799–801. [Google Scholar] [CrossRef]
- Oldfield, E.H.; Muraszko, K.; Shawker, T.H.; Patronas, N.J. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils: Implications for diagnosis and treatment. J. Neurosurg. 1994, 80, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, M.A.; Jones, N.R.; Yang, L.; Brown, C.J. Mechanisms underlying the formation and enlargement of noncommunicating syringomyelia: Experimental studies. Neurosurg. Focus 2000, 8, E2. [Google Scholar] [CrossRef]
- Van Der Veken, J.; Harding, M.; Hatami, S.; Agzarian, M.; Vrodos, N. Syringomyelia intermittens: Highlighting the complex pathophysiology of syringomyelia. Illustrative case. J. Neurosurg. Case Lessons 2021, 2, CASE21341. [Google Scholar] [CrossRef]
- Klekamp, J. The pathophysiology of syringomyelia—Historical overview and current concept. Acta Neurochir. 2002, 144, 649–664. [Google Scholar] [CrossRef] [PubMed]
- Parida, M.K.; Pattanaik, S.S.; Panda, A.K.; Das, B.K.; Tripathy, S.R. Charcot arthropathy of elbow due to syringomyelia: A case series and systematic review of literature. Clin. Rheumatol. 2023, 42, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.P.; Kanhangad, M.P.; Saifuddin, S.; Kurup, J.K.N. Pattern of Syringomyelia in Presumed Idiopathic and Congenital Scoliosis. Asian Spine J. 2021, 15, 791–798. [Google Scholar] [CrossRef]
- Mikhaylovskiy, M.; Stupak, V.; Belozerov, V.; Fomichev, N.; Lutsik, A.; Afanasyev, L.; Bondarenko, A.; Krutko, A.; Dolzhenko, D.; Zaidman, A.; et al. Progressive Scoliosis and Syringomyelia—Questions of Surgical Approach. Folia Medica 2018, 60, 261–269. [Google Scholar] [CrossRef]
- Goel, A. Scoliosis and Syringomyelia. Neurol. India 2022, 70, 2192–2193. [Google Scholar] [CrossRef]
- Williams, B. Orthopaedic features in the presentation of syringomyelia. J. Bone Jt. Surgery. Br. 1979, 61-B, 314–323. [Google Scholar] [CrossRef]
- Klekamp, J. How should syringomyelia be defined and diagnosed? World Neurosurg. 2018, 111, e729–e745. [Google Scholar] [CrossRef]
- Ralot, T.; Jatav, V.S.; Karimji, S.; Sharma, V. Syringomyelia mimicking as bibrachial variant of motor neuron disease. J. Assoc. Physicians India 2024, 72, 93–94. [Google Scholar] [PubMed]
- Bonfield, C.M.; Levi, A.D.; Arnold, P.M.; Okonkwo, D.O. Surgical management of post-traumatic syringomyelia. Spine 2010, 35, S245–S258. [Google Scholar] [CrossRef]
- Mariani, C.; Cislaghi, M.G.; Barbieri, S.; Filizzolo, F.; Palama, F.; Farina, E.; D’Aliberti, G.; Scarlato, G. The natural history and results of surgery in 50 cases of syringomyelia. J. Neurol. 1991, 238, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Plessas, I.N.; Volk, H.A.; Rusbridge, C.; Vanhaesebrouck, A.E.; Jeffery, N.D. Comparison of gabapentin versus topiramate on clinically affected dogs with Chiari-like malformation and syringomyelia. Vet. Rec. 2015, 177, 288. [Google Scholar] [CrossRef]
- Thoefner, M.S.; Skovgaard, L.T.; McEvoy, F.J.; Berendt, M.; Bjerrum, O.J. Pregabalin alleviates clinical signs of syringomyelia-related central neuropathic pain in Cavalier King Charles Spaniel dogs: A randomized controlled trial. Veter-Anaesth. Analg. 2020, 47, 238–248. [Google Scholar] [CrossRef]
- Coderre, T.J.; Kumar, N.; Lefebvre, C.D.; Yu, J.S.C. Evidence that gabapentin reduces neuropathic pain by inhibiting the spinal release of glutamate. J. Neurochem. 2005, 94, 1131–1139. [Google Scholar] [CrossRef]
- Gilron, I.; Flatters, S.J. Gabapentin and Pregabalin for the Treatment of Neuropathic Pain: A Review of Laboratory and Clinical Evidence. Pain Res. Manag. 2006, 11, 16A–29A. [Google Scholar] [CrossRef]
- Hamandi, K.; Sander, J.W. Pregabalin: A new antiepileptic drug for refractory epilepsy. Seizure 2006, 15, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Girod, M.; Allerton, F.; Gommeren, K.; Tutunaru, A.; de Marchin, J.; Van Soens, I.; Ramery, E.; Peeters, D. Evaluation of the effect of oral omeprazole on canine cerebrospinal fluid production: A pilot study. Vet. J. 2016, 209, 119–124. [Google Scholar] [CrossRef]
- Rusbridge, C. New considerations about Chiari-like malformation, syringomyelia and their management. Practice 2020, 42, 252–267. [Google Scholar] [CrossRef]
- Carrion, E.; Hertzog, J.H.; Medlock, M.D.; Hauser, G.J.; Dalton, H.J. Use of acetazolamide to decrease cerebrospinal fluid production in chronically ventilated patients with ventriculopleural shunts. Arch. Dis. Child. 2001, 84, 68–71. [Google Scholar] [CrossRef]
- Takahashi, M.; Kawaguchi, M.; Shimada, K.; Nakashima, T.; Furuya, H. Systemic meloxicam reduces tactile allodynia development after L5 single spinal nerve injury in rats. Reg. Anesth. Pain Med. 2005, 30, 351–355. [Google Scholar] [CrossRef]
- Rusbridge, C.; Jeffery, N.D. Pathophysiology and treatment of neuropathic pain associated with syringomyelia. Vet. J. 2008, 175, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Moulin, D.E.; Palma, D.; Watling, C.; Schulz, V. Methadone in the Management of Intractable Neuropathic Noncancer Pain. Can. J. Neurol. Sci. 2005, 32, 340–343. [Google Scholar] [CrossRef]
- Yang, K.; Ellenbogen, Y.; Bhatt, C.; Aljoghaiman, M.; Zagzoog, N.; Reddy, K. Syringopleural shunt insertion using a minimally invasive approach: Technical note, case series, and review of the literature. J. Minim. Invasive Spine Surg. Tech. 2021, 6, 115–121. [Google Scholar] [CrossRef]
- Ushewokunze, S.O.S.; Gan, Y.C.; Phillips, K.; Thacker, K.; Flint, G. Surgical treatment of post-traumatic syringomyelia. Spinal Cord. 2010, 48, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Cabahug, P.; Pickard, C.; Edmiston, T.; Lieberman, J.A. A Primary Care Provider’s Guide to Spasticity Management in Spinal Cord Injury. Top. Spinal Cord. Inj. Rehabil. 2020, 26, 157–165. [Google Scholar] [CrossRef]
- Rekand, T.; Hagen, E.M.; Grønning, M. Spasticity following spinal cord injury. Tidsskr. Nor. Laegeforen 2012, 132, 1742–1746. [Google Scholar]
- Elbasiouny, S.M.; Moroz, D.; Bakr, M.M.; Mushahwar, V.K. Management of spasticity after spinal cord injury: Current techniques and future directions. Neurorehabil Neural Repair. 2010, 24, 23–33. [Google Scholar] [CrossRef]
- Khan, F.; Amatya, B.; Bensmail, D.; Yelnik, A. Non-pharmacological interventions for spasticity in adults: An overview of systematic reviews. Ann. Phys. Rehabil. Med. 2019, 62, 265–273. [Google Scholar] [CrossRef]
- McMahon, M. Physical Therapy Management and Treatment of a Patient with Post-Traumatic Syringomyelia. Ph.D. Thesis, University of Iowa, Iowa City, IA, USA, 2017. [Google Scholar]
- Ghobrial, G.M.; Dalyai, R.T.; Maltenfort, M.G.; Prasad, S.K.; Harrop, J.S.; Sharan, A.D. Arachnolysis or cerebrospinal fluid diversion for adult-onset syringomyelia? A systematic review of the literature. World Neurosurg. 2015, 83, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Killeen, T.; Rosner, J.; Jutzeler, C.R.; Hupp, M.; Heilbronner, R.; Curt, A. Spontaneous resolution of an extensive posttraumatic syrinx. Neurology 2016, 87, 1299–1301. [Google Scholar] [CrossRef] [PubMed]
- Albright, L.; Pollack, I.F.; Adelson, D.; Abbott, R.; Adamson, D.C.; Alden, T.D.; Yang, M. Principles and Practice of Pediatric Neurosurgery; Thieme: Stuttgart, Germany, 2008. [Google Scholar]
- Schijman, E.; Steinbok, P. International survey on the management of Chiari I malformation and syringomyelia. Child’s Nerv. Syst. 2004, 20, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Milhorat, T.H.; Bolognese, P.A. Tailored operative technique for Chiari type I malformation using intraoperative color Doppler ultrasonography. Neurosurgery 2003, 53, 899–906. [Google Scholar] [CrossRef]
- Zhang, F.; Norvell, D.C.; Hermsmeyer, J.T.; Schuster, J.M. Persistent/recurrent syringomyelia after Chiari decompression—Natural history and management strategies: A systematic review. Evid.-Based Spine-Care J. 2013, 04, 116–125. [Google Scholar] [CrossRef]
- Wetjen, N.M.; Heiss, J.D.; Oldfield, E.H. Time course of syringomyelia resolution following decompression of Chiari malformation Type I. J. Neurosurg. Pediatr. 2008, 1, 118–123. [Google Scholar] [CrossRef]
- Srikantha, U.; Hari, A.; Lokanath, Y.K.; Varma, R.G. Syringo-subarachnoid shunt placement: A minimally invasive technique using fixed tubular retractors—Three case reports and literature review. Int. J. Spine Surg. 2020, 14, 133–139. [Google Scholar] [CrossRef]
- Zuev, A.A.; Kostenko, G.V. Treatment of syringomyelia associated with Chiari 1 malformation. Zhurnal Nevrol. I Psikhiatrii im. S.S. Korsakova 2017, 117, 102–106. [Google Scholar] [CrossRef]
- Zheng, Y.-C.; Liu, Y.-T.; Wei, K.-C.; Huang, Y.-C.; Chen, P.-Y.; Hsu, Y.-H.; Lin, C.-L. Outcome predictors and clinical presentation of syringomyelia. Asian J. Surg. 2023, 46, 705–711. [Google Scholar] [CrossRef]
- Yuan, C.; Guan, J.; Du, Y.; Fang, Z.; Wang, X.; Yao, Q.; Zhang, C.; Liu, Z.; Wang, K.; Duan, W.; et al. Neurological deterioration after posterior fossa decompression for adult syringomyelia: Proposal for a summarized treatment algorithm. Front. Surg. 2022, 9, 968906. [Google Scholar] [CrossRef]
- Snoddy, M.C.; Lee, D.H.; Kuhn, J.E. Charcot shoulder and elbow: A review of the literature and update on treatment. J. Shoulder Elb. Surg. 2017, 26, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, B.F.; Kızıldağ, B. Neuropathic arthropathy of the shoulder secondary to operated syringomyelia: A case-based review. Rheumatol. Int. 2023, 43, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak, A.; Lubiatowski, P.; Kordasiewicz, B.; Brzóska, R.; Laprus, H. Shoulder arthropathy secondary to syringomyelia: Case series of 10 patients. Eur. J. Orthop. Surg. Traumatol. 2022, 32, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadila, M.; Sarrabia, G.; Shapira, S.; Yaacobi, E.; Baruch, Y.; Engel, I.; Ohana, N. Orthopedic Manifestations of Syringomyelia: A Comprehensive Review. J. Clin. Med. 2025, 14, 3145. https://doi.org/10.3390/jcm14093145
Fadila M, Sarrabia G, Shapira S, Yaacobi E, Baruch Y, Engel I, Ohana N. Orthopedic Manifestations of Syringomyelia: A Comprehensive Review. Journal of Clinical Medicine. 2025; 14(9):3145. https://doi.org/10.3390/jcm14093145
Chicago/Turabian StyleFadila, Mohamad, Geva Sarrabia, Shay Shapira, Eyal Yaacobi, Yuval Baruch, Itzhak Engel, and Nissim Ohana. 2025. "Orthopedic Manifestations of Syringomyelia: A Comprehensive Review" Journal of Clinical Medicine 14, no. 9: 3145. https://doi.org/10.3390/jcm14093145
APA StyleFadila, M., Sarrabia, G., Shapira, S., Yaacobi, E., Baruch, Y., Engel, I., & Ohana, N. (2025). Orthopedic Manifestations of Syringomyelia: A Comprehensive Review. Journal of Clinical Medicine, 14(9), 3145. https://doi.org/10.3390/jcm14093145





