An Updated Perspective of the Clinical Features and Parathyroidectomy Impact in Primary Hyperparathyroidism Amid Multiple Endocrine Neoplasia Type 1 (MEN1): Focus on Bone Health
Abstract
1. Introduction
Objective
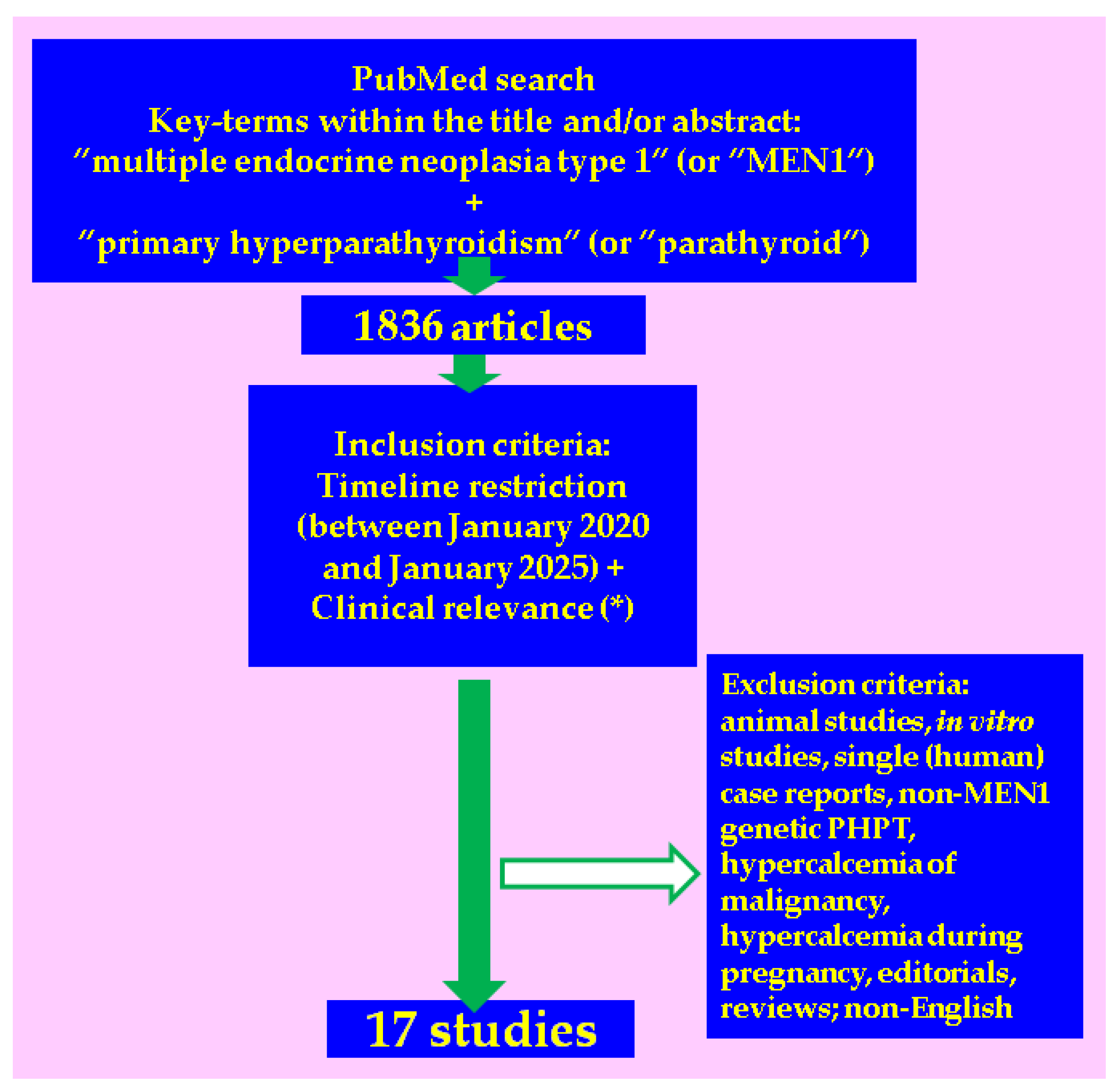
2. Methods
3. Results
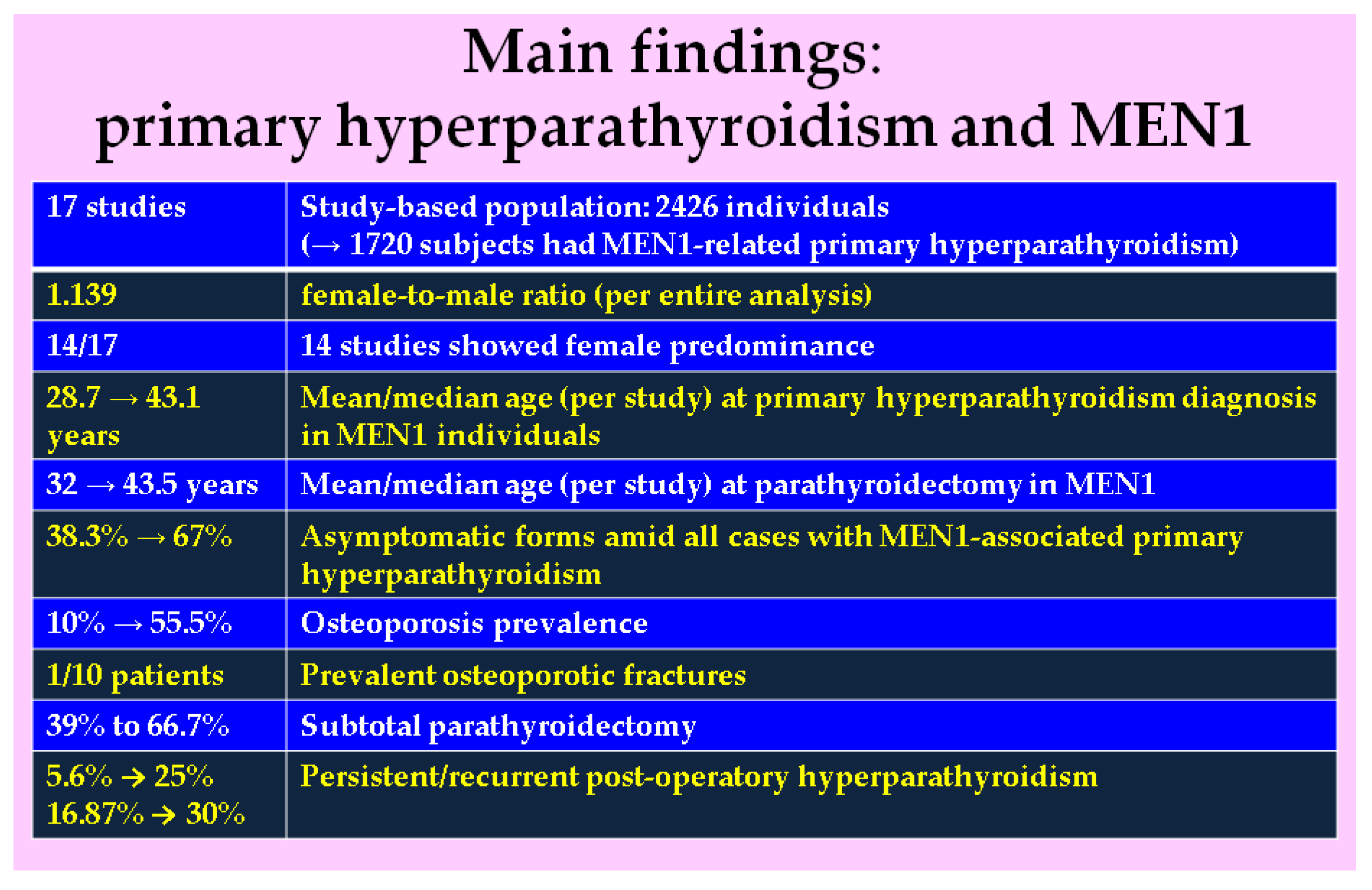
3.1. Studies-Based Analysis
3.2. Main Clinical Features and Mineral Metabolism Findings in MEN1-Related Primary Hyperparathyroidism
Sub-Analysis of the Bone Health Assessment
3.3. Parathyroidectomy Outcome in MEN1
3.3.1. Pre-Operatory Imaging Evaluation of the Parathyroid Masses in MEN1
3.3.2. Post-Parathyroidectomy Pathological Exam: Parathyroid Masses in MEN1
3.3.3. Management of the Primary Hyperparathyroidism and Its Impact on the Quality of Life in MEN1 Subjects
4. Discussion
4.1. MEN1: A Complex Lens to Look at Primary Hyperparathyroidism
- Age at MEN1 genetic testing (years): 30.3 ± 16.3 [26]
4.2. Contributing Factors for Bone Loss in MEN1: From Parathyroidectomy Timing and Benefits to the Impact of Non-Parathyroid Components
4.3. Current Limits and Further Research
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APN | atypical parathyroid neoplasm |
| BMD | bone mineral density |
| CI | confidence interval |
| DXA | Dual-Energy X-Ray Absorptiometry |
| F | females |
| F:M | female-to-male ratio |
| FCH-PET/CT | fluorine-18 positron emission tomography/computed tomography |
| GEP-NETs | gastro-entero-pancreatic neuroendocrine tumours |
| HR-pQCT | high-resolution peripheral quantitative computed tomography |
| IQR | interquartile range |
| MEN | multiple endocrine neoplasia |
| M | males |
| MPHPT | MEN1-related primary hyperparathyroidism |
| N | number of patients |
| n | number of studies |
| NET | neuroendocrine tumours |
| NA | not available |
| OR | odds ratio |
| PHPT | primary hyperparathyroidism |
| PTH | parathyroid hormone |
| PC | parathyroid carcinoma |
| PTx | parathyroidectomy |
| PitNETs | pituitary neuroendocrine tumours |
| STPTx | subtotal parathyroidectomy |
| <STPTx | less than subtotal PTx |
| SD | standard deviation |
| sestaMIBI | methoxyisobutylisonitrile labelled with technetium-99 m |
| SGE | single gland excisions |
| TPTx | total parathyroidectomy |
| TGF | tumour growth factor |
| TBS | trabecular bone score |
| vs. | versus |
References
- Greenberg, L.A. Multiple Endocrine Neoplasia Type 1, Type 2A, and Type 2B. Prim. Care 2024, 51, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Pereira Brabo, E.; Barbosa Moraes, A.; Dos Santos Marijuan Cabezas, B.S.; Vieira Neto, L. Multiple endocrine neoplasia type 1 in patients with gastroenteropancreatic neuroendocrine tumors: An opportunity for early diagnosis and appropriate management. Mol. Clin. Oncol. 2020, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Effraimidis, G.; Knigge, U.; Rossing, M.; Oturai, P.; Rasmussen, Å.K.; Feldt-Rasmussen, U. Multiple endocrine neoplasia type 1 (MEN-1) and neuroendocrine neoplasms (NENs). Semin. Cancer Biol. 2022, 79, 141–162. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.Y.; Hua, X.; Reidy-Lagunes, D.; Untch, B.R. MENIN loss as a tissue-specific driver of tumorigenesis. Mol. Cell. Endocrinol. 2018, 469, 98–106. [Google Scholar] [CrossRef]
- Manoharan, J.; Albers, M.B.; Rinke, A.; Adelmeyer, J.; Görlach, J.; Bartsch, D.K. Multiple Endocrine Neoplasia Type 1. Dtsch. Arztebl. Int. 2024, 121, 527–533. [Google Scholar] [CrossRef]
- Carsote, M.; Paduraru, D.N.; Nica, A.E.; Valea, A. Parathyroidectomy: Is vitamin D a player for a good outcome? J. Med. Life 2016, 4, 348–352. [Google Scholar]
- Balachandra, S.; Fazendin, J.; Chen, H. Complex Primary Hyperparathyroidism: Hereditary and Recurrent Disease. Surg. Clin. N. Am. 2024, 104, 811–823. [Google Scholar] [CrossRef]
- Minisola, S.; Arnold, A.; Belaya, Z.; Brandi, M.L.; Clarke, B.L.; Hannan, F.M.; Hofbauer, L.C.; Insogna, K.L.; Lacroix, A.; Liberman, U.; et al. Epidemiology, Pathophysiology, and Genetics of Primary Hyperparathyroidism. J. Bone Miner. Res. 2022, 37, 2315–2329. [Google Scholar] [CrossRef]
- Newey, P.J. Hereditary Primary Hyperparathyroidism. Endocrinol. Metab. Clin. N. Am. 2021, 50, 663–681. [Google Scholar] [CrossRef]
- Carsote, M.; Valea, A.; Dumitru, N.; Terzea, D.; Petrova, E.; Albu, S.; Buruiana, A.; Ghemigian, A. Metastases in daily endocrine practice. Arch. Balk. Med. Union. 2016, 51, 476–480. [Google Scholar]
- Romanet, P.; Coppin, L.; Molin, A.; Santucci, N.; Le Bras, M.; Odou, M.F. Chapter 5: The roles of genetics in primary hyperparathyroidism. Ann. Endocrinol. 2025, 86, 101694. [Google Scholar] [CrossRef] [PubMed]
- Al-Salameh, A.; Haissaguerre, M.; Tresallet, C.; Kuczma, P.; Marciniak, C.; Cardot-Bauters, C. Chapter 6: Syndromic primary hyperparathyroidism. Ann. Endocrinol. 2025, 86, 101695. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Igarashi, H.; Uehara, H.; Berna, M.J.; Jensen, R.T. Causes of death and prognostic factors in multiple endocrine neoplasia type 1: A prospective study: Comparison of 106 MEN1/Zollinger-Ellison syndrome patients with 1613 literature MEN1 patients with or without pancreatic endocrine tumors. Medicine 2013, 92, 135–181. [Google Scholar] [CrossRef] [PubMed]
- Bilezikian, J.P.; Khan, A.A.; Silverberg, S.J.; Fuleihan, G.E.; Marcocci, C.; Minisola, S.; Perrier, N.; Sitges-Serra, A.; Thakker, R.V.; Guyatt, G.; et al. Evaluation and Management of Primary Hyperparathyroidism: Summary Statement and Guidelines from the Fifth International Workshop. J. Bone Miner. Res. 2022, 37, 2293–2314. [Google Scholar] [CrossRef]
- Cormier, C.; Koumakis, E. Bone and primary hyperparathyroidism. Joint Bone Spine 2022, 89, 105129. [Google Scholar] [CrossRef]
- Tournis, S.; Makris, K.; Cavalier, E.; Trovas, G. Cardiovascular Risk in Patients with Primary Hyperparathyroidism. Curr. Pharm. Des. 2020, 26, 5628–5636. [Google Scholar] [CrossRef]
- Scheyer, N.; Frey, S.; Koumakis, E.; Guérin, C.; Desailloud, R.; Groussin, L.; Cariou, B.; Vergès, B.; Brunaud, L.; Mirallié, E.; et al. Chapter 3: Impact of primary hyperparathyroidism. Ann. Endocrinol. 2025, 86, 101692. [Google Scholar] [CrossRef]
- Iacobone, M.; Citton, M.; Viel, G.; Schiavone, D.; Torresan, F. Surgical approaches in hereditary endocrine tumors. Updates Surg. 2017, 69, 181–191. [Google Scholar] [CrossRef]
- Thakker, R.V.; Newey, P.J.; Walls, G.V.; Bilezikian, J.; Dralle, H.; Ebeling, P.R.; Melmed, S.; Sakurai, A.; Tonelli, F.; Brandi, M.L.; et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J. Clin. Endocrinol. Metab. 2012, 97, 2990–3011. [Google Scholar] [CrossRef]
- Frey, S.; Mosbah, H.; Donatini, G.; Brunaud, L.; Chabre, O.; Vezzosi, D. Chapter 9: Indications for the treatment of primary hyperparathyroidism. Ann. Endocrinol. 2025, 86, 101698. [Google Scholar] [CrossRef]
- Schubert, L.; Gaillard, M.; Melot, C.; Delbot, T.; Cottereau, A.S.; Koumakis, E.; Bonnet-Serrano, F.; Groussin, L. Management of primary hyperparathyroidism in MEN1: From initial subtotal surgery to complex treatment of the remaining gland. Ann. Endocrinol. 2025, 86, 101721. [Google Scholar] [CrossRef] [PubMed]
- Maraghelli, D.; Giusti, F.; Marini, F.; Brandi, M.L. Bone tissue and mineral metabolism in hereditary endocrine tumors: Clinical manifestations and genetic bases. Orphanet. J. Rare Dis. 2020, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, D.M., Jr.; Coutinho, F.L.; Toledo, R.A.; Gonçalves, T.D.; Montenegro, F.L.; Toledo, S.P. Biochemical, bone and renal patterns in hyperparathyroidism associated with multiple endocrine neoplasia type 1. Clinics 2012, 67 (Suppl. S1), 99–108. [Google Scholar] [CrossRef]
- Slouma, M.; Abbes, M.; Dhahri, R.; Litaiem, N.; Gueddiche, N.; Mansouri, N.; Msekni, I.; Gharsallah, I.; Metoui, L.; Louzir, B. Multiple endocrine neoplasia type 1 revealed by a hip pathologic fracture. Clin. Rheumatol. 2021, 40, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Eremkina, A.K.; Pylina, S.V.; Elfimova, A.R.; Gorbacheva, A.M.; Humbert, L.; López Picazo, M.; Hajrieva, A.V.; Solodovnikova, E.N.; Kovalevich, L.D.; Vetchinkina, E.A.; et al. Analysis of Bone Phenotype Differences in MEN1-Related and Sporadic Primary Hyperparathyroidism Using 3D-DXA. J. Clin. Med. 2024, 13, 6382. [Google Scholar] [CrossRef]
- Kuusela, E.; Kostiainen, I.; Ritvonen, E.; Ryhänen, E.M.; Schalin-Jäntti, C. Bone mineral density over ten years after primary parathyroidectomy in multiple endocrine neoplasia type 1. JBMR Plus 2024, 8, ziae129. [Google Scholar] [CrossRef]
- Santucci, N.; Ksiazek, E.; Pattou, F.; Baud, G.; Mirallié, E.; Frey, S.; Trésallet, C.; Sébag, F.; Guérin, C.; Mathonnet, M.; et al. Recurrence After Surgery for Primary Hyperparathyroidism in 517 Patients With Multiple Endocrine Neoplasia Type 1: An Association Francophone de Chirurgie Endocrinienne and Groupe d’étude des Tumeurs Endocrines study. Ann. Surg. 2024, 279, 340–345. [Google Scholar] [CrossRef]
- Shariq, O.A.; Abrantes, V.B.; Lu, L.Y.; Tebben, P.J.; Foster, T.M.; Dy, B.M.; Lyden, M.L.; Young, W.F.; McKenzie, T.J. Primary hyperparathyroidism in patients with multiple endocrine neoplasia type 1: Impact of genotype and surgical approach on long-term postoperative outcomes. Surgery 2024, 175, 8–16. [Google Scholar] [CrossRef]
- Figueiredo, A.A.; Saramago, A.; Cavaco, B.M.; Simões-Pereira, J.; Leite, V. Familial parathyroid tumours-comparison of clinical profiles between syndromes. J. Endocrinol. Investig. 2023, 46, 1799–1806. [Google Scholar] [CrossRef]
- Libánský, P.; Čarková, J.; Kushnir, I.; Matějková Běhanová, M.; Procyklová, K.; Šedý, J.; Vaculová, M.; Včelák, J.; Zikán, V.; Adámek, S. Recurrent Primary Hyperparathyroidism in Multiple Endocrine Neoplasia Type 1 Syndrome. Physiol. Res. 2023, 72 (Suppl. S4), S423–S427. [Google Scholar] [CrossRef]
- Song, A.; Chen, R.; Guan, W.; Yu, W.; Yang, Y.; Wang, J.; Nie, M.; Jiang, Y.; Li, M.; Xia, W.; et al. Trabecular Bone Score as a More Sensitive Tool to Evaluate Bone Involvement in MEN1-related Primary Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2023, 109, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Brescia, M.D.G.; Rodrigues, K.C.; d’Alessandro, A.F.; Alves Filho, W.; van der Plas, W.Y.; Kruijff, S.; Arap, S.S.; Toledo, S.P.A.; Montenegro, F.L.M.; Lourenço, D.M., Jr. Impact of parathyroidectomy on quality of life in multiple endocrine neoplasia type 1. Endocr. Connect. 2022, 11, e220021. [Google Scholar] [CrossRef] [PubMed]
- Landry, J.P.; Pieterman, C.R.C.; Clemente-Gutierrez, U.; Grubbs, E.G.; Fisher, S.B.; Graham, P.H.; Waguespack, S.G.; Perrier, N.D. Evaluation of risk factors, long-term outcomes, and immediate and delayed autotransplantation to minimize postsurgical hypoparathyroidism in multiple endocrine neoplasia type 1 (MEN1): A retrospective cohort study. Surgery 2022, 171, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Yavropoulou, M.P.; Vlachou, S.; Tsoli, M.; Fostira, F.; Kaltsas, G.; Kassi, E. Management and Long-Term Follow-Up of Hyperparathyroidism in Multiple Endocrine Neoplasia Type 1: Single Center Experience. J. Clin. Med. 2022, 11, 1967. [Google Scholar] [CrossRef]
- Marini, F.; Giusti, F.; Cioppi, F.; Maraghelli, D.; Cavalli, T.; Tonelli, F.; Brandi, M.L. Bone and Mineral Metabolism Phenotypes in MEN1-Related and Sporadic Primary Hyperparathyroidism, before and after Parathyroidectomy. Cells 2021, 10, 1895. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, S.; Liu, H.; Zhao, R.N.; Lai, X.J.; Lv, K.; Jiang, Y.X.; Li, J.C. Parathyroid disorder and concomitant thyroid cancer in patients with multiple endocrine neoplasia type 1: A retrospective cohort study. Medicine 2021, 100, e27098. [Google Scholar] [CrossRef]
- Choi, H.R.; Choi, S.H.; Choi, S.M.; Kim, J.K.; Lee, C.R.; Kang, S.W.; Lee, J.; Jeong, J.J.; Nam, K.H.; Chung, W.Y.; et al. Benefit of diverse surgical approach on short-term outcomes of MEN1-related hyperparathyroidism. Sci. Rep. 2020, 10, 10634. [Google Scholar] [CrossRef]
- Gauthé, M.; Dierick-Gallet, A.; Delbot, T.; Bricaire, L.; Bertherat, J.; North, M.O.; Cochand-Priollet, B.; Bouchard, P.; Talbot, J.N.; Groussin, L.; et al. 18F-fluorocholine PET/CT in MEN1 Patients with Primary Hyperparathyroidism. World J. Surg. 2020, 44, 3761–3769. [Google Scholar] [CrossRef]
- Manoharan, J.; Albers, M.B.; Bollmann, C.; Maurer, E.; Mintziras, I.; Wächter, S.; Bartsch, D.K. Single gland excision for MEN1-associated primary hyperparathyroidism. Clin. Endocrinol. 2020, 92, 63–70. [Google Scholar] [CrossRef]
- Song, A.; Yang, Y.; Liu, S.; Nie, M.; Jiang, Y.; Li, M.; Xia, W.; Wang, O.; Xing, X. Prevalence of Parathyroid Carcinoma and Atypical Parathyroid Neoplasms in 153 Patients With Multiple Endocrine Neoplasia Type 1: Case Series and Literature Review. Front. Endocrinol. 2020, 11, 557050. [Google Scholar] [CrossRef]
- Wang, W.; Nie, M.; Jiang, Y.; Li, M.; Meng, X.; Xing, X.; Wang, O.; Xia, W. Impaired geometry, volumetric density, and microstructure of cortical and trabecular bone assessed by HR-pQCT in both sporadic and MEN1-related primary hyperparathyroidism. Osteoporos. Int. 2020, 31, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Gokozan, H.N.; Scognamiglio, T. Advances and Updates in Parathyroid Pathology. Adv. Anat. Pathol. 2023, 30, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Erickson, L.A.; Mete, O.; Juhlin, C.C.; Perren, A.; Gill, A.J. Overview of the 2022 WHO Classification of Parathyroid Tumors. Endocr. Pathol. 2022, 33, 64–89. [Google Scholar] [CrossRef] [PubMed]
- Dušková, J. Parathyroid tumors in the 5th edition of the WHO Classification of Tumors of the Endocrine Organs. Cesk Patol. 2024, 60, 68–70. [Google Scholar]
- Brunetti, A.; Cosso, R.; Vescini, F.; Falchetti, A. Molecular Pathophysiology of Parathyroid Tumorigenesis-The Lesson from a Rare Disease: The “MEN1 Model”. Int. J. Mol. Sci. 2024, 25, 11586. [Google Scholar] [CrossRef]
- Simonds, W.F. Clinical and Molecular Genetics of Primary Hyperparathyroidism. Horm. Metab. Res. 2020, 52, 578–587. [Google Scholar] [CrossRef]
- Alberto, F. Genetics of parathyroids disorders: Overview. Best. Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 781–790. [Google Scholar] [CrossRef]
- Goudet, P.; Dalac, A.; Le Bras, M.; Cardot-Bauters, C.; Niccoli, P.; Lévy-Bohbot, N.; du Boullay, H.; Bertagna, X.; Ruszniewski, P.; Borson-Chazot, F.; et al. MEN1 disease occurring before 21 years old: A 160-patient cohort study from the Groupe d’étude des Tumeurs Endocrines. J. Clin. Endocrinol. Metab. 2015, 100, 1568–1577. [Google Scholar] [CrossRef]
- Kamilaris, C.D.C.; Stratakis, C.A. Multiple Endocrine Neoplasia Type 1 (MEN1): An Update and the Significance of Early Genetic and Clinical Diagnosis. Front. Endocrinol. 2019, 10, 339. [Google Scholar] [CrossRef]
- Valea, A.; Carsote, M.; Moldovan, C.; Georgescu, C. Chronic autoimmune thyroiditis and obesity. Arch. Balk. Med. Union. 2018, 53, 64–69. [Google Scholar]
- Răcătăianu, N.; Leach, N.; Bondor, C.I.; Mârza, S.; Moga, D.; Valea, A.; Ghervan, C. Thyroid disorders in obese patients. Does insulin resistance make a difference? Arch. Endocrinol. Metab. 2017, 61, 575–583. [Google Scholar] [CrossRef]
- Newey, P.J.; Newell-Price, J. MEN1 Surveillance Guidelines: Time to (Re)Think? J Endocr Soc. 2022, 6, bvac001. [Google Scholar] [CrossRef] [PubMed]
- Pieterman, C.R.C.; Valk, G.D. Update on the clinical management of multiple endocrine neoplasia type 1. Clin. Endocrinol. 2022, 97, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Al-Salameh, A.; Cadiot, G.; Calender, A.; Goudet, P.; Chanson, P. Clinical aspects of multiple endocrine neoplasia type 1. Nat. Rev. Endocrinol. 2021, 17, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Nastos, C.; Papaconstantinou, D.; Kofopoulos-Lymperis, E.; Peppa, M.; Pikoulis, A.; Lykoudis, P.; Palazzo, F.; Patapis, P.; Pikoulis, E. Optimal extent of initial parathyroid resection in patients with multiple endocrine neoplasia syndrome type 1: A meta-analysis. Surgery 2021, 169, 302–310. [Google Scholar] [CrossRef]
- Nobecourt, P.F.; Zagzag, J.; Asare, E.A.; Perrier, N.D. Intraoperative Decision-Making and Technical Aspects of Parathyroidectomy in Young Patients With MEN1 Related Hyperparathyroidism. Front. Endocrinol. 2018, 9, 618. [Google Scholar] [CrossRef]
- Dumitru, N.; Carsote, M.; Cocolos, A.; Petrova, E.; Olaru, M.; Dumitrache, C.; Ghemigian, A. The Link Between Bone Osteocalcin and Energy Metabolism in a Group of Postmenopausal Women. Curr. Health Sci. J. 2019, 45, 47–51. [Google Scholar] [CrossRef]
- Agarwal, S.K. The future: Genetics advances in MEN1 therapeutic approaches and management strategies. Endocr. Relat. Cancer. 2017, 24, T119–T134. [Google Scholar] [CrossRef]
- Brandi, M.L.; Agarwal, S.K.; Perrier, N.D.; Lines, K.E.; Valk, G.D.; Thakker, R.V. Multiple Endocrine Neoplasia Type 1: Latest Insights. Endocr. Rev. 2021, 42, 133–170. [Google Scholar] [CrossRef]
- Thakker, R.V. Multiple endocrine neoplasia type 1 (MEN1) and type 4 (MEN4). Mol. Cell. Endocrinol. 2014, 386, 2–15. [Google Scholar] [CrossRef]
- Matkar, S.; Thiel, A.; Hua, X. Menin: A scaffold protein that controls gene expression and cell signaling. Trends Biochem. Sci. 2013, 38, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Hendy, G.N.; Kaji, H.; Canaff, L. Cellular functions of menin. Adv. Exp. Med. Biol. 2009, 668, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.; Agarwal, S.K. Epigenetic regulation in the tumorigenesis of MEN1-associated endocrine cell types. J. Mol. Endocrinol. 2018, 61, R13–R24. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, V.; Muscarella, L.A.; Verdelli, C.; Corbetta, S. Alterations of DNA methylation in parathyroid tumors. Mol. Cell. Endocrinol. 2018, 469, 60–69. [Google Scholar] [CrossRef]
- Davenport, C.; Agha, A. The role of menin in parathyroid tumorigenesis. Adv. Exp. Med. Biol. 2009, 668, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Alvelos, M.I.; Vinagre, J.; Fonseca, E.; Barbosa, E.; Teixeira-Gomes, J.; Sobrinho-Simões, M.; Soares, P. MEN1 intragenic deletions may represent the most prevalent somatic event in sporadic primary hyperparathyroidism. Eur. J. Endocrinol. 2012, 168, 119–128. [Google Scholar] [CrossRef]
- Mele, C.; Mencarelli, M.; Caputo, M.; Mai, S.; Pagano, L.; Aimaretti, G.; Scacchi, M.; Falchetti, A.; Marzullo, P. Phenotypes Associated With MEN1 Syndrome: A Focus on Genotype-Phenotype Correlations. Front. Endocrinol. 2020, 11, 591501. [Google Scholar] [CrossRef]
- Marini, F.; Brandi, M.L. Role of miR-24 in Multiple Endocrine Neoplasia Type 1: A Potential Target for Molecular Therapy. Int. J. Mol. Sci. 2021, 22, 7352. [Google Scholar] [CrossRef]
- Lines, K.E.; Javid, M.; Reed, A.A.C.; Walls, G.V.; Stevenson, M.; Simon, M.; Kooblall, K.G.; Piret, S.E.; Christie, P.T.; Newey, P.J.; et al. Genetic background influences tumour development in heterozygous Men1 knockout mice. Endocr. Connect. 2020, 9, 426–437. [Google Scholar] [CrossRef]
- Gao, Y.; Li, R.; Wu, L.; Yang, H.; Mao, J.; Zhao, W. Thymoma in multiple endocrine neoplasia type 1: A case report and systematic review. Endocrine. 2023, 82, 442–449. [Google Scholar] [CrossRef]
- Araujo-Castro, M.; Pascual-Corrales, E.; Molina-Cerrillo, J.; Moreno Mata, N.; Alonso-Gordoa, T. Bronchial Carcinoids: From Molecular Background to Treatment Approach. Cancers 2022, 14, 520. [Google Scholar] [CrossRef] [PubMed]
- Green, D.; Richards, K.; Doyle, B.; Thompson, C.; Hill, A.; O’Reilly, M.W.; Sherlock, M. Extra-adrenal adrenocortical cancer associated with multiple endocrine neoplasia type 1. Endocrinol. Diabetes Metab. Case Rep. 2024, 2024, 23–0068. [Google Scholar] [CrossRef] [PubMed]
- Marini, F.; Giusti, F.; Tonelli, F.; Brandi, M.L. Pancreatic Neuroendocrine Neoplasms in Multiple Endocrine Neoplasia Type 1. Int. J. Mol. Sci. 2021, 22, 4041. [Google Scholar] [CrossRef] [PubMed]
- Mitrica, M.; Vasiliu, O.; Plesa, A.; Sirbu, O.M. Multinodular and vacuolating neuronal tumor. Rom. J. Mil. Med. 2025, 128, 10–16. [Google Scholar] [CrossRef]
- Niederle, B.; Selberherr, A.; Bartsch, D.K.; Brandi, M.L.; Doherty, G.M.; Falconi, M.; Goudet, P.; Halfdanarson, T.R.; Ito, T.; Jensen, R.T.; et al. Multiple Endocrine Neoplasia Type 1 and the Pancreas: Diagnosis and Treatment of Functioning and Non-Functioning Pancreatic and Duodenal Neuroendocrine Neoplasia within the MEN1 Syndrome—An International Consensus Statement. Neuroendocrinology 2021, 111, 609–630. [Google Scholar] [CrossRef]
- Papadopoulou-Marketou, N.; Tsoli, M.; Chatzellis, E.; Alexandraki, K.I.; Kaltsas, G. Hereditary Syndromes Associated with Pancreatic and Lung Neuroendocrine Tumors. Cancers 2024, 16, 2075. [Google Scholar] [CrossRef]
- Valea, A.; Ghervan, C.; Morar, A.; Pop, D.D.; Carsote, M.; Albu, S.E.; Georgescu, C.E.; Chiorean, A. Hashimoto’s thyroiditis and breast cancer: Coincidence or correlation? Arch. Balk. Med. Union 2016, 51, 129–132. [Google Scholar]
- Geurts, J.L. Inherited syndromes involving pancreatic neuroendocrine tumors. J. Gastrointest. Oncol. 2020, 11, 559–566. [Google Scholar] [CrossRef]
- Kos-Kudła, B.; Castaño, J.P.; Denecke, T.; Grande, E.; Kjaer, A.; Koumarianou, A.; de Mestier, L.; Partelli, S.; Perren, A.; Stättner, S.; et al. European Neuroendocrine Tumour Society (ENETS) 2023 guidance paper for nonfunctioning pancreatic neuroendocrine tumours. J. Neuroendocrinol. 2023, 35, e13343. [Google Scholar] [CrossRef]
- Klein Haneveld, M.J.; van Treijen, M.J.C.; Pieterman, C.R.C.; Dekkers, O.M.; van de Ven, A.; de Herder, W.W.; Zandee, W.T.; Drent, M.L.; Bisschop, P.H.; Havekes, B.; et al. Initiating Pancreatic Neuroendocrine Tumor (pNET) Screening in Young MEN1 Patients: Results from the DutchMEN Study Group. J. Clin. Endocrinol. Metab. 2021, 106, 3515–3525. [Google Scholar] [CrossRef]
- Ramamoorthy, B.; Nilubol, N. Multiple Endocrine Neoplasia Type 1 Syndrome Pancreatic Neuroendocrine Tumor Genotype/Phenotype: Is There Any Advance on Predicting or Preventing? Surg. Oncol. Clin. N. Am. 2023, 32, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Răcătăianu, N.; Leach, N.V.; Bolboacă, S.D.; Soran, M.L.; Opriş, O.; Dronca, E.; Valea, A.; Ghervan, C. Interplay between metabolic and thyroid parameters in obese pubertal children. Does visceral adipose tissue make the first move? Acta Clin. Belg. 2021, 76, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Cohen, S.; Brown, D.A.; Himes, B.T.; Wheeler, L.P.; Ruff, M.W.; Major, B.T.; Singh Ospina, N.M.; Atkinson, J.L.D.; Meyer, F.B.; Bancos, I.; et al. Pituitary adenomas in the setting of multiple endocrine neoplasia type 1: A single-institution experience. J. Neurosurg. 2020, 134, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Le Bras, M.; Leclerc, H.; Rousseau, O.; Goudet, P.; Cuny, T.; Castinetti, F.; Bauters, C.; Chanson, P.; Tabarin, A.; Gaujoux, S.; et al. Pituitary adenoma in patients with multiple endocrine neoplasia type 1: A cohort study. Eur. J. Endocrinol. 2021, 185, 863–873. [Google Scholar] [CrossRef]
- Vasiliu, O. Therapeutic management of atypical antipsychotic-related metabolic dysfunctions using GLP-1 receptor agonists: A systematic review. Exp. Ther. Med. 2023, 26, 355. [Google Scholar] [CrossRef]
- Das, L.; Dutta, P. Association of primary hyperparathyroidism with pituitary adenoma and management issues. Best Pract. Res. Clin. Endocrinol. Metab. 2025, 39, 101978. [Google Scholar] [CrossRef]
- Nica, S.; Sionel, R.; Maciuca, R.; Csutak, O.; Ciobica, M.L.; Nica, M.I.; Chelu, I.; Radu, I.; Toma, M. Gender-Dependent Associations Between Digit Ratio and Genetic Polymorphisms, BMI, and Reproductive Factors. Rom. J. Mil. Med. 2025, 128, 78–86. [Google Scholar] [CrossRef]
- Halperin, R.; Tirosh, A. Progress report on multiple endocrine neoplasia type 1. Fam. Cancer 2025, 24, 15. [Google Scholar] [CrossRef]
- Pierotti, L.; Pardi, E.; Dinoi, E.; Piaggi, P.; Borsari, S.; Della Valentina, S.; Sardella, C.; Michelucci, A.; Caligo, M.A.; Bogazzi, F.; et al. Cutaneous lesions and other non-endocrine manifestations of Multiple Endocrine Neoplasia type 1 syndrome. Front. Endocrinol. 2023, 14, 1191040. [Google Scholar] [CrossRef]
- Stratakis, C.A. Hereditary syndromes predisposing to endocrine tumors and their skin manifestations. Rev. Endocr. Metab. Disord. 2016, 17, 381–388. [Google Scholar] [CrossRef]
- Waguespack, S.G. Beyond the “3 Ps”: A critical appraisal of the non-endocrine manifestations of multiple endocrine neoplasia type 1. Front. Endocrinol. 2022, 13, 1029041. [Google Scholar] [CrossRef]
- Jhawar, S.; Lakhotia, R.; Suzuki, M.; Welch, J.; Agarwal, S.K.; Sharretts, J.; Merino, M.; Ahlman, M.; Blau, J.E.; Simonds, W.F.; et al. Clinical presentation and management of primary ovarian neuroendocrine tumor in multiple endocrine neoplasia type 1. Endocrinol. Diabetes Metab. Case Rep. 2019, 2019, 19-0040. [Google Scholar] [CrossRef] [PubMed]
- Febrero, B.; Segura, P.; Ruiz-Manzanera, J.J.; Teruel, E.; Ros, I.; Ríos, A.; Hernández, A.M.; Rodríguez, J.M. Uncommon tumors in multiple endocrine neoplasia (MEN) type 1: Do they have a relationship with the prognosis of these patients? J. Endocrinol. Investig. 2021, 44, 1327–1330. [Google Scholar] [CrossRef] [PubMed]
- Popa, F.L.; Boicean, L.C.; Iliescu, M.G.; Stanciu, M. The importance of association between sex steroids deficiency, reduction of bone mineral density and falling risk in men with implications in medical rehabilitation. Balneo PRM Res. J. 2021, 12, 318–322. [Google Scholar] [CrossRef]
- Anghel, D.; Ciobica, L.M.; Negru, M.M.; Jurcut, C.; Otlocan, L.; Coca, A. Bone mineral density and vitamin D levels in patients with rheumatoid arthritis. Osteoporos. Int. 2017, 28 (Suppl. S1), S435–S436. [Google Scholar]
- Rusu, C.C.; Kacso, I.; Moldovan, D.; Potra, A.; Tirinescu, D.; Ticala, M.; Rotar, A.M.; Orasan, R.; Budurea, C.; Barar, A.; et al. Triiodothyronine and Protein Malnutrition Could Influence Pulse Wave Velocity in Pre-Dialysis Chronic Kidney Disease Patients. Diagnostics 2023, 13, 2462. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Silverberg, S.J.; Bandeira, F.; Cetani, F.; Chandran, M.; Cusano, N.E.; Ebeling, P.R.; Formenti, A.M.; Frost, M.; Gosnell, J.; et al. Management of Primary Hyperparathyroidism. J. Bone Miner. Res. 2022, 37, 2391–2403. [Google Scholar] [CrossRef]
- Ejlsmark-Svensson, H.; Rolighed, L.; Harsløf, T.; Rejnmark, L. Risk of fractures in primary hyperparathyroidism: A systematic review and meta-analysis. Osteoporos. Int. 2021, 32, 1053–1060. [Google Scholar] [CrossRef]
- Narayanan, N.; Palui, R.; Merugu, C.; Kar, S.S.; Kamalanathan, S.; Sahoo, J.; Selvarajan, S.; Naik, D. The Risk of Fractures in Primary Hyperparathyroidism: A Meta-Analysis. JBMR Plus 2021, 5, e10482. [Google Scholar] [CrossRef]
- Kanis, J.A.; Harvey, N.C.; Liu, E.; Vandenput, L.; Lorentzon, M.; McCloskey, E.V.; Bouillon, R.; Abrahamsen, B.; Rejnmark, L.; Johansson, H.; et al. Primary hyperparathyroidism and fracture probability. Osteoporos. Int. 2023, 34, 489–499. [Google Scholar] [CrossRef]
- Balachandra, S.; Wang, R.; Akhund, R.; Allahwasaya, A.; Lindeman, B.; Fazendin, J.; Gillis, A.; Chen, H. Patients with normocalcemic versus hypercalcemic hyperparathyroidism: What’s really the difference? Am. J. Surg. 2025, 244, 116272. [Google Scholar] [CrossRef] [PubMed]
- Yankova, I.; Lilova, L.; Petrova, D.; Dimitrova, I.; Stoynova, M.; Shinkov, A.; Kovatcheva, R. Biochemical characteristics and clinical manifestation of normocalcemic primary hyperparathyroidism. Endocrine 2024, 85, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Rusu, C.C.; Anton, F.; Valea, A.; Bondor, C.I. N-Terminal Pro-Brain Natriuretic Peptide Correlates with Ghrelin and Acyl-Ghrelin in Pre-Dialysis Chronic Kidney Disease. Int. J. Mol. Sci. 2024, 25, 5696. [Google Scholar] [CrossRef] [PubMed]
- Popa, F.L.; Diaconu, C.; Canciu, A.; Ciortea, V.M.; Iliescu, M.G.; Stanciu, M. Medical management and rehabilitation in posttraumatic common peroneal nerve palsy. Balneo PRM Res. J. 2022, 13, 496. [Google Scholar] [CrossRef]
- Kužma, M.; Jackuliak, P.; Killinger, Z.; Payer, J. Parathyroid Hormone-Related Changes of Bone Structure. Physiol Res. 2021, 70 (Suppl. S1), S3–S11. [Google Scholar] [CrossRef]
- Hua, Y.; Li, Y.; Zhou, J.; Fan, L.; Huang, F.; Wu, Z.; Xue, H.; Yang, B.; Chen, P.; Rui, Y.; et al. Mortality following fragility hip fracture in China: A record linkage study. Arch. Osteoporos. 2023, 18, 105. [Google Scholar] [CrossRef]
- Schaffler-Schaden, D.; Schweighofer-Zwink, G.; Hehenwarter, L.; van der Zee-Neuen, A.; Flamm, M.; Beheshti, M.; Pirich, C. Bone Mineral Density and First Line Imaging with [(18)F]fluorocholine PET/CT in Normocalcemic and Hypercalcemic Primary Hyperparathyroidism: Results from a Single Center. Diagnostics 2024, 14, 2466. [Google Scholar] [CrossRef]
- Eller-Vainicher, C.; Chiodini, I.; Battista, C.; Viti, R.; Mascia, M.L.; Massironi, S.; Peracchi, M.; D’Agruma, L.; Minisola, S.; Corbetta, S.; et al. Sporadic and MEN1-related primary hyperparathyroidism: Differences in clinical expression and severity. J. Bone Miner. Res. 2009, 24, 1404–1410. [Google Scholar] [CrossRef]
- Kong, J.; Wang, O.; Nie, M.; Shi, J.; Hu, Y.; Jiang, Y.; Li, M.; Xia, W.; Meng, X.; Xing, X. Clinical and Genetic Analysis of Multiple Endocrine Neoplasia Type 1-Related Primary Hyperparathyroidism in Chinese. PLoS ONE 2016, 11, e0166634. [Google Scholar] [CrossRef]
- Silva, F.F.; Lima, M.L.; Pedreira, C.C.; Matos, M.A. Influence of disease activity and gonadal status on bone mineral density and turnover in acromegaly. J. Bone Miner. Metab. 2024, 43, 123–132. [Google Scholar] [CrossRef]
- Bioletto, F.; Berton, A.M.; Barale, M.; Aversa, L.S.; Sauro, L.; Presti, M.; Mocellini, F.; Sagone, N.; Ghigo, E.; Procopio, M.; et al. Skeletal fragility in pituitary disease: How can we predict fracture risk? Pituitary 2024, 27, 789–801. [Google Scholar] [CrossRef]
- Gorbacheva, A.; Eremkina, A.; Goliusova, D.; Krupinova, J.; Mokrysheva, N. The role of menin in bone pathology. Endocr. Connect. 2022, 11, e210494. [Google Scholar] [CrossRef] [PubMed]
- Uygur, M.M.; Frara, S.; di Filippo, L.; Giustina, A. New tools for bone health assessment in secreting pituitary adenomas. Trends Endocrinol. Metab. 2023, 34, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Frara, S.; Melin Uygur, M.; di Filippo, L.; Doga, M.; Losa, M.; Santoro, S.; Mortini, P.; Giustina, A. High Prevalence of Vertebral Fractures Associated With Preoperative GH Levels in Patients With Recent Diagnosis of Acromegaly. J. Clin. Endocrinol. Metab. 2022, 107, e2843–e2850. [Google Scholar] [CrossRef] [PubMed]
- di Filippo, L.; Bilezikian, J.P.; Canalis, E.; Terenzi, U.; Giustina, A. New insights into the vitamin D/PTH axis in endocrine-driven metabolic bone diseases. Endocrine 2024, 85, 1007–1019. [Google Scholar] [CrossRef]
- Yun, S.J.; Sang, H.; Park, S.Y.; Chin, S.O. Effect of Hyperprolactinemia on Bone Metabolism: Focusing on Osteopenia/Osteoporosis. Int. J. Mol. Sci. 2024, 25, 1474. [Google Scholar] [CrossRef]
- di Filippo, L.; Doga, M.; Resmini, E.; Giustina, A. Hyperprolactinemia and bone. Pituitary 2020, 23, 314–321. [Google Scholar] [CrossRef]
- Coss, D.; Yang, L.; Kuo, C.B.; Xu, X.; Luben, R.A.; Walker, A.M. Effects of prolactin on osteoblast alkaline phosphatase and bone formation in the developing rat. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E1216–E1225. [Google Scholar] [CrossRef]
- Seriwatanachai, D.; Thongchote, K.; Charoenphandhu, N.; Pandaranandaka, J.; Tudpor, K.; Teerapornpuntakit, J.; Suthiphongchai, T.; Krishnamra, N. Prolactin directly enhances bone turnover by raising osteoblast-expressed receptor activator of nuclear factor kappaB ligand/osteoprotegerin ratio. Bone 2008, 42, 535–546. [Google Scholar] [CrossRef]
- Fleseriu, M.; Auchus, R.; Bancos, I.; Ben-Shlomo, A.; Bertherat, J.; Biermasz, N.R.; Boguszewski, C.L.; Bronstein, M.D.; Buchfelder, M.; Carmichael, J.D.; et al. Consensus on diagnosis and management of Cushing’s disease: A guideline update. Lancet Diabetes Endocrinol. 2021, 9, 847–875. [Google Scholar] [CrossRef]
- Sun, H.; Wu, C.; Hu, B.; Xie, G. High prevalence of vertebral fractures associated with preoperative cortisol levels in patients with recent diagnosis of Cushing disease. Ann. Med. 2023, 55, 2282183. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kyrou, I.; Kaltsas, G.; Grossman, A.B.; Randeva, H.S.; Weickert, M.O. Mechanisms of Central Hypogonadism. Int. J. Mol. Sci. 2021, 22, 8217. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.V.O.; Boguszewski, C.L. Fertility issues in aggressive pituitary tumors. Rev. Endocr. Metab. Disord. 2020, 21, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, S.; Bruyère, O.; Bergmann, P.; Cavalier, E.; Gielen, E.; Goemaere, S.; Kaufman, J.M.; Lapauw, B.; Laurent, M.R.; De Schepper, J.; et al. How to manage osteoporosis before the age of 50. Maturitas 2020, 138, 14–25. [Google Scholar] [CrossRef]
- Cheng, C.H.; Chen, L.R.; Chen, K.H. Osteoporosis Due to Hormone Imbalance: An Overview of the Effects of Estrogen Deficiency and Glucocorticoid Overuse on Bone Turnover. Int. J. Mol. Sci. 2022, 23, 1376. [Google Scholar] [CrossRef]
- Golds, G.; Houdek, D.; Arnason, T. Male Hypogonadism and Osteoporosis: The Effects, Clinical Consequences, and Treatment of Testosterone Deficiency in Bone Health. Int. J. Endocrinol. 2017, 2017, 4602129. [Google Scholar] [CrossRef]
- Bandeira, L.; Silva, B.C.; Bilezikian, J.P. Male osteoporosis. Arch. Endocrinol. Metab. 2022, 66, 739–747. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kim, D.J. Case Report: Severe osteoporosis misunderstood by bone metastasis after total gastrectomy and multiple metastasectomy. Front Oncol. 2023, 13, 1216705. [Google Scholar] [CrossRef]
- Altieri, B.; Di Dato, C.; Modica, R.; Bottiglieri, F.; Di Sarno, A.; Pittaway, J.F.H.; Martini, C.; Faggiano, A.; Colao, A. Bone Metabolism and Vitamin D Implication in Gastroenteropancreatic Neuroendocrine Tumors. Nutrients 2020, 12, 1021. [Google Scholar] [CrossRef]
- Kaji, H. Menin and bone metabolism. J. Bone Miner. Metab. 2012, 30, 381–387. [Google Scholar] [CrossRef]
- Kanazawa, I.; Canaff, L.; Abi Rafeh, J.; Angrula, A.; Li, J.; Riddle, R.C.; Boraschi-Diaz, I.; Komarova, S.V.; Clemens, T.L.; Murshed, M.; et al. Osteoblast menin regulates bone mass in vivo. J. Biol. Chem. 2015, 290, 3910–3924. [Google Scholar] [CrossRef]
- Liu, P.; Lee, S.; Knoll, J.; Rauch, A.; Ostermay, S.; Luther, J.; Malkusch, N.; Lerner, U.H.; Zaiss, M.M.; Neven, M.; et al. Loss of menin in osteoblast lineage affects osteocyte-osteoclast crosstalk causing osteoporosis. Cell Death Differ. 2017, 24, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Abi-Rafeh, J.; Asgari, M.; Troka, I.; Canaff, L.; Moussa, A.; Pasini, D.; Goltzman, D. Genetic Deletion of Menin in Mouse Mesenchymal Stem Cells: An Experimental and Computational Analysis. JBMR Plus 2022, 6, e10622. [Google Scholar] [CrossRef] [PubMed]
- Troka, I.; Griffanti, G.; Canaff, L.; Hendy, G.N.; Goltzman, D.; Nazhat, S.N. Effect of Menin Deletion in Early Osteoblast Lineage on the Mineralization of an In Vitro 3D Osteoid-like Dense Collagen Gel Matrix. Biomimetics 2022, 7, 101. [Google Scholar] [CrossRef]
- van Leeuwaarde, R.S.; Pieterman, C.R.C.; Bleiker, E.M.A.; Dekkers, O.M.; van der Horst-Schrivers, A.N.; Hermus, A.R.; de Herder, W.W.; Drent, M.L.; Bisschop, P.H.; Havekes, B.; et al. High Fear of Disease Occurrence Is Associated With Low Quality of Life in Patients With Multiple Endocrine Neoplasia Type 1: Results From the Dutch MEN1 Study Group. J. Clin. Endocrinol. Metab. 2018, 103, 2354–2361. [Google Scholar] [CrossRef]
- Cipriani, C.; Cianferotti, L. Quality of Life in Primary Hyperparathyroidism. Endocrinol. Metab. Clin. N. Am. 2022, 51, 837–852. [Google Scholar] [CrossRef]
- Yavari, M.; Feizi, A.; Haghighatdoost, F.; Ghaffari, A.; Rezvanian, H. The influence of parathyroidectomy on cardiometabolic risk factors in patients with primary hyperparathyroidism: A systematic review and meta-analysis. Endocrine 2021, 72, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.D.; Silverberg, S.J. Quality of Life in Primary Hyperparathyroidism Revisited: Keep Calm and Carry on? J. Bone Miner. Res. 2021, 36, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.W.; Thøgersen, K.F.; Jensen, L.T.; Krakauer, M.; Kristensen, B.; Bennedbæk, F.N.; Zerahn, B. Changes in quality of life 6 months after parathyroidectomy for primary hyperparathyroidism. Endocr. Connect. 2022, 11, e210630. [Google Scholar] [CrossRef]
- Marini, F.; Giusti, F.; Tonelli, F.; Brandi, M.L. Management impact: Effects on quality of life and prognosis in MEN1. Endocr. Relat. Cancer 2017, 24, T227–T242. [Google Scholar] [CrossRef]
- Bjornsdottir, S.; Ing, S.; Mitchell, D.M.; Sikjaer, T.; Underbjerg, L.; Hassan-Smith, Z.; Sfeir, J.; Gittoes, N.J.; Clarke, L.B.L. Epidemiology and Financial Burden of Adult Chronic Hypoparathyroidism. J. Bone Miner. Res. 2022, 37, 2602–2614. [Google Scholar] [CrossRef] [PubMed]
- Cetani, F.; Dinoi, E.; Pierotti, L.; Pardi, E. Familial states of primary hyperparathyroidism: An update. J. Endocrinol. Investig. 2024, 47, 2157–2176. [Google Scholar] [CrossRef] [PubMed]
- Blau, J.E.; Simonds, W.F. Familial Hyperparathyroidism. Front. Endocrinol. 2021, 12, 623667. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; Benevento, E.; De Cicco, F.; Grossrubatscher, E.M.; Hasballa, I.; Tarsitano, M.G.; Centello, R.; Isidori, A.M.; Colao, A.; Pellegata, N.S.; et al. Multiple endocrine neoplasia type 4 (MEN4): A thorough update on the latest and least known men syndrome. Endocrine 2023, 82, 480–490. [Google Scholar] [CrossRef]
- Halperin, R.; Arnon, L.; Nasirov, S.; Friedensohn, L.; Gershinsky, M.; Telerman, A.; Friedman, E.; Bernstein-Molho, R.; Tirosh, A. Germline CDKN1B variant type and site are associated with phenotype in MEN4. Endocr. Relat. Cancer 2022, 30, e220174. [Google Scholar] [CrossRef]
- Mathiesen, J.S.; Effraimidis, G.; Rossing, M.; Rasmussen, Å.K.; Hoejberg, L.; Bastholt, L.; Godballe, C.; Oturai, P.; Feldt-Rasmussen, U. Multiple endocrine neoplasia type 2: A review. Semin. Cancer Biol. 2022, 79, 163–179. [Google Scholar] [CrossRef]
- Machens, A.; Dralle, H. Multiple endocrine neoplasia type 2: Towards a risk-based approach integrating molecular and biomarker results. Curr. Opin. Oncol. 2024, 36, 1–12. [Google Scholar] [CrossRef]
- Larsen, L.V.; Mirebeau-Prunier, D.; Imai, T.; Alvarez-Escola, C.; Hasse-Lazar, K.; Censi, S.; Castroneves, L.A.; Sakurai, A.; Kihara, M.; Horiuchi, K.; et al. Primary hyperparathyroidism as first manifestation in multiple endocrine neoplasia type 2A: An international multicenter study. Endocr. Connect. 2020, 9, 489–497. [Google Scholar] [CrossRef]
| First Author/Year/Reference | Study Design | Study Population |
|---|---|---|
| Eremkina/2024 [25] | Retrospective study | N = 59 with MHPT vs. sporadic PHPT; F:M = 6.375:1 (86.44% females) N1 = 22 with MPHPT; F:M = 18:4 (81.81% females); age [median (IQR)] = 36 (28, 39) y N2 (from N1) = 11 with MPHPT with dynamic follow-up N3 = 37 with sporadic PHPT; F:M = 8.25:1 (89.18% females); age [median (IQR)] = 34 (30, 38) y N4 (from N3) = 14 with sporadic PHPT with dynamic follow-up |
| Kuusela/2024 [26] | Observational study | N = 70 with MPHPT vs. controls N1 = 35 with MPHPT; F:M = 18:17 (51% females); age (mean ± SD) = 42.8 ± 15.7 y Age at genetic testing (mean ± SD) = 30.3 ± 16.3 y Age at MPHPT diagnosis (mean ± SD) = 28.7 ± 13.6 y N2 = 35 age- and sex-matched controls; F:M = 18:17 (51% females); age (mean ± SD) = 43.2 ± 9.71 y |
| Santucci/2024 [27] | Retrospective cohort study | N = 517 surgery candidates (who underwent PTx for MPHPT); F:M = 287:230 (55.5% females); Age at diagnosis [median (IQR)] = 36.2 (25, 48) y N1 = 178 who underwent <STPTx; F:M = 101:77 (57% females); age at diagnosis [median (IQR)] = 36.4 (25, 49) Y N2 = 339 who underwent STPTx; F:M = 186:153 (55% females); age at diagnosis [median (IQR)] = 36.1 (25, 46) Y |
| Shariq/2024 [28] | Retrospective study | N = 209 with MEN1 N1 = 194 with MPHPT; F:M = 109:85 (56.18% females); age [median (IQR)] = 30 (22, 38) y N2 = 73 with MPHPT and truncating variant in exon 2, 9 or 10; F:M = 39:34 (53% females); age [median (IQR)] = 50 (39, 62) N3 = 121 with MPHPT and other pathogenic variants; F:M = 70:51 (58% females); age [median (IQR)] = 55 (40,64) |
| Figueiredo/2023 [29] | Retrospective analysis | N = 48 with familial PHPT; F:M = 24:24 (50% females); age (mean ± SD) = 40 ± 15.5 yN1 = 17 (35.4%) with MPHPT; F:M = 8:9 (47.1% females)Age at PHPT diagnosis (mean ± SD) = 43.1 ± 14.2 yAge at first manifestation (mean ± SD) = 37.7 ± 17.6 y |
| Libánský/2023 [30] | Retrospective study | N = 101 surgery candidates (who underwent PTx for PHPT vs. MPHPT) N1 = 78 with PHPT and reoperation; F:M = 60:18 (76.92% females); age (mean ± SD) = 58.37 ± 1.56 y N2 = 27 with MPHPT; F:M = 17:10 (62.96% females); age (mean ± SD) = 38.7 ± 2.46 y |
| Song/2023 [31] | Retrospective observational study | N = 480 with MPHPT vs. sporadic PHPT N1 = 120 with MPHPT; F:M = 70:50 (58.33% females); age [median (IQR)] = 43.5 (31.5, 52) y N2 = 360 with sporadic PHPT; F:M = 255:105 (70.83% females); age [median (IQR)] = 52 (40.5, 61) y N3 (from N1) = 86 with MPHPT with bone data; F:M = 39:47 (45.34% females); age at onset [median (IQR)] = 44 (31.5, 55) y N4 (from N2) = 86 age and sex matched with sporadic PHPT and bone data; F:M = 32:54 (37% females); age at onset [median (IQR)] = 48.5 (38, 57) y |
| Bresci/2022 [32] | Prospective study | N = 30 surgery candidates (who underwent PTx for MPHPT); F:M = 16:14 (53.33% females) Age at PTx [median (IQR)] = 38 (22, 44) y |
| Landry/2022 [33] | Retrospective study | N = 206 surgery candidates (who underwent PTx for MPHPT); F:M = 106:100 (51% females) Age at first PTx (mean ± SD) = 32 ± 12.7 y |
| Yavropoulou/2022 [34] | Retrospective cohort study | N = 68 with MPHPT; F:M = 29:39 (42.6% females) Age at MEN diagnosis (mean ± SD) = 39 ± 13.06 y Age at MPHPT diagnosis (mean ± SD = 35.2 ± 14 y |
| Marini/2021 [35] | Retrospective study | N = 180 with MPHPT vs. sporadic PHPT N1 = 133 with MPHPT; F:M = 87:46 (65.4% females); age at MPHPT diagnosis (mean ± SD) = 34.1 ± 13.5 y N2 = 47 with sporadic PHPT; F:M = 44:3 (93.6% females) |
| Wang/2021 [36] | Retrospective cohort study | N = 45 surgery candidates (who underwent PTx for MPHPT vs. sporadic PHPT + thyroidectomy for thyroid nodules; F:M = 12:33 (26.7% females) N1 = 15 with MPHPT; F:M = 4:11 (26.7% females); age at thyroidectomy (mean ± SD) = 52.87 ± 9.92 y N2 = 30 with sporadic PHPT (age and sex matched with N1); F:M = 8:22 (26.7% females); age at thyroidectomy (mean ± SD) = 53.43 ± 9.2 y |
| Choi/2020 [37] | Retrospective study | N = 33 surgery candidates (who underwent PTx for MPHPT); age (mean ± SD) = 43.4 ± 14.1 y N1 = 12 with MPHPT who underwent <STPTx; age (mean ± SD) = 37.4 ± 8.9 y N2 = 4 with MPHPT who underwent STPTx; age (mean ± SD) = 42 ± 10.8 y N3 = 17 with MPHPT who underwent TPTx; age (mean ± SD) = 48 ± 16.49 y |
| Gauthé/2020 [38] | Retrospective study | N = 22 with MPHPT; F:M = 6:16 (37.5% females) Age at MEN1 diagnosis [median (IQR)] = 35 (18, 76) y Age at MPHPT diagnosis [median (IQR)] = 34 (21, 69) y |
| Manoharan/2020 [39] | Retrospective study | N = 89 surgery candidates (who underwent PTx for MPHPT); F:M = 44:45 (49.43% females); age [median (range)] = 35 (18–70) y N1 = 28 with MPHPT who underwent SGE; age [median (range)] = 40 (range 18–67) N2 = 23 with MPHPT who underwent STPTx; age [median (range)] = 36 (range 18–68) N3 = 38 with MPHPT who underwent TPTx; age [median (range)] = 32 (range 18–70) |
| Song/2020 [40] | Retrospective study | N = 153 with MPHPT N1 = 150 with MPHPT without PC/APN; F:M = 87:63 (58% females); age at disease onset (mean ± SD) = 43 ± 15.5 y N2 = 3 with MPHPT and PC/APN; F:M = 2:1; age at disease onset >49 y |
| Wang/2020 [41] | Case control study | N = 116 with PHPT (MPHPT sub-group) vs. controls (sporadic PHPT sub-group) N1 = 58 with PHPT; F:M = 41:17 (70.68% females); age (mean ± SD) = 49.9 ± 15.1 y N2 = 58 age and sex matched controls; F:M = 41:17 (70.68% females); age (mean ± SD) = 50.6 ± 15.7 y N3 (from N1) = 11 with MPHPT; F:M = 8:3 (72.72% females); age (mean ± SD) = 38.64 ± 15.25 y N4 (from N2) = 47 with sporadic PHPT; F:M = 33:14 (70.21% females); age (mean ± SD) = 52.57 ± 13.99 y N3 vs. N4 age p = 0.005 |
| Reference | Assessment of the Calcium Metabolism | Clinical Features |
|---|---|---|
| [25] | N1 vs. N3 PTH: 131.6 (95.92, 198.3) vs. 117.3 (102.3, 169.5) pg/mL, p = 0.931 Albumin corrected Ca: 2.69 (2.62, 2.80) vs. 2.69 (2.63, 2.77) mmol/L, p = 0.911 24-h urine calcium: 8.22 (6.42, 10.29) vs. 8.61 (6.60, 10.90) mmol/L, p = 0.651 | Nephrolithiasis: 54.5% (12/22) vs. 62.2% (23/37), p = 0.594 |
| [26] | N1: PTH: 96.6 ± 68.9 ng/L N1 vs. N2: Ionized Ca: 1.31 ± 0.12 vs. 1.24 ± 0.03 mmol/L, p = 0.001 24-h urinary calcium: 6.7 ± 4.9 vs. 2.3 ± 1.2, p < 0.001 | NA |
| [28] | N2 vs. N3: PTH: 72 (59, 110) vs. 83 (60, 114) pg/mL Total Ca: 11 (10.6, 11.3) vs. 11 (10.4, 11.3) mg/dL | Bone mineral density loss: 45% (87/194) Nephrolithiasis: 60% (115/194) N2 vs. N3: Median (IQR) age at PHPT onset: 27 (21, 34) vs. 31 (22, 41) y, p = 0.007 Bone mineral density: p > 0.05 Nephrolithiasis: p > 0.05 |
| [29] | PTH: 169.9 (210.5) pg/mL Total Ca: 11.7 ± 1.2 mg/dL | N1: Diagnosis of PHPT: Screening: 35.3% (6/17) Clinical manifestations: 41.2% (7/17) Routine blood analysis: 23.5% (4/17) Nephrolithiasis: 47.1% (8/17) Osteoporosis/osteopenia: 17.6% (3/17) Chronic kidney disease: 11.8% (2/17) |
| [30] | PTH: 18.12 ± 3.74 pmol/L Total Ca: 2.88 ± 2.46 mmol/L | NA |
| [31] | N1 vs. N2 PTH: 317.2 (130.1, 353.0) vs. 514.9 (135.0, 520.2) pg/mL, p = 0.08 Total Ca: 2.84 ± 0.24 vs. 2.90 ± 0.34 mmol/L, p = 0.18 iCa: 1.40 ± 0.14 vs. 1.45 ± 0.26 mmol/L, p = 0.29 24-h urinary Ca: 7.7 (5.5, 10.2) vs. 7.8 (4.7, 10.9) mmol/day, p = 0.90 | N1 vs. N2 Skeletal symptoms: 10.8% vs. 24.4%, p = 0.002 Pathologic fracture: 7.5% vs. 8.9%, p = 0.78 Subperiosteal absorption: 1.7% vs. 17.2%, p < 0.001 Osteitis fibrosa cystica: 3.3% vs. 10.0%, p = 0.04 Osteomalacia: 1.7% vs. 5.6%, p = 0.13 Gastrointestinal symptoms: 25.8% vs. 27.2%, p = 0.86 Urinary involvement: 54.2% vs. 35.6%, p < 0.001 Hypercalcaemic crisis: 0.8% vs. 10.6%, p = 0.002 Asymptomatic: 38.3% vs. 39.2%, p = 0.96 |
| [32] | Preoperative: Ca: 10.8 (10.4, 11.1) mg/dL 9.4 and PTH: 104 (76, 137) pg/mL Symptomatic vs. asymptomatic: PTH: 111 (78, 171) vs. 101 (58, 116) pg/mL, p = 0.13 Total Ca: 10.7 (10.3, 11.1) vs. 10.9 (10.4, 11.2) mg/dL, p = 0.44 | Symptomatic: 63.3% (19/30) Asymptomatic: 36.7% (11/30) |
| [34] | NA | PHPT as first manifestation: 64.7% (44/68) Comorbidities: 72% (49/68) Type 2 diabetes mellitus: 35% (24/68) Hypertension: 29% (20/68) Thyroid pathology: 20.5% (14/68) Death: 11.7% (8/68) Osteoporosis: 17.6% (11/68) Osteopenia: 5.88% (4/68) Nephrolithiasis: 64.7% (22/68) Nephrocalcinosis: 1.47 (1/68) |
| [35] | N1 vs. N2: PTH: 17.2 ± 17.2 vs. 19.0 ± 18.0 pmol/L, p > 0.05 Ionized Ca: 5.67 ± 0.43 vs. 5.55 ± 0.35 mg/dL, p > 0.05 Total Ca: 10.5 ± 1.5 vs. 10.3 ± 0.9 mg/dL, p > 0.05 24-h urinary calcium: 328.7 ± 155.4 vs. 269.5 ± 123.7 mg/24 h, p > 0.05 | Nephrolithiasis: 47.4% (63/133) |
| [36] | N1 vs. N2: PTH: 470.67 ± 490.74 vs. 217.77 ± 165.60 pg/mL, p = 0.001 | NA |
| [37] | N1 vs. N2 vs. N3: PTH: 108.8 ± 37.7 vs. 138.1 ± 52.2 vs. 190.9 ± 90.6, p = 0.017 | NA |
| [38] | PTH: 54.9 (33.9, 114.1) ng/L Albumin corrected Ca: 2.70 (2.51, 3.01) mmol/L Ionized Ca: 1.41 (1.33, 1.60) mmol/L | Osteopenia/osteoporosis: 27.27% (6/22) Nephrolithiasis/nephrocalcinosis: 27.27% (6/22) |
| [39] | PTH median (range): 106.5 (51–2040) pg/mL Total Ca median (range): 2.85 (2.30–3.70) mmol/L | Symptoms: 33% (29/89) Nephrolithiasis: 72% (21/89) Ulcer: 10% (3/89) Bone pain: 17% (5/89) N1 vs. N2 vs. N3: Symptoms: 50% (14/28) vs. 30.1% (9/23) vs. 100% 31.5% (12/38), p > 0.05 Nephrolithiasis: 25% (7/28) vs. 21.7% (5/23) vs. 23.7% (9/38), p > 0.05 Ulcer: 3.5% (1/28) vs. 8.7% (2/23) vs. 0% (0/38), p > 0.05 Bone pain: 7.1% (2/28) vs. 8.7% (2/23) vs. 2.6% (1/38), p > 0.05 |
| [40] | N1: PTH: 185.5 (108.3, 297.0) pg/mL Total Ca: 2.78 (2.61, 2.88) mmol/L 24-h urinary calcium: 7.68 (5.09, 10.28) mmol/day | N1: Gastrointestinal involvement: 21.3% (32/150) Bone involvement: 49.3% (74/150) Bone pain: 19.3% (29/150) Pathological fracture: 9.3% (14/150) Subperiosteal absorption: 8% (12/150) Osteitis fibrosa cystica: 3.3% (5/150) Osteoporosis: 28.6% (43/150) Urinary tract involvement: 46.7% (70/150) N2: Nephrolithiasis: 100% (3/3) Bone pain and osteoporosis 66.6% (2/3) Gastrointestinal symptoms: 33.3% (1/3) |
| [41] | N3 vs. N4: Total Ca: 2.75 ± 0.13 vs. 2.81 ± 0.31 mmol/L, p = 0.526 iCa: 1.42 ± 0.06 vs. 1.41 ± 0.18 mmol/L, p = 0.779 Serum PTH: 141.5 (78.7, 245.7) vs. 185.2 (39.9, 1891.5) pg/mL, p = 0.207 | N3 vs. N4 Asymptomatic: 54.5% (6/11) vs. 38.3% (18/47), p = 0.325 |
| Reference | Prevalence of Osteoporosis/Osteopenia | Prevalence of Low Energy Fractures |
|---|---|---|
| [25] | N1 vs. N3: Z-score < −2.0 SD or low-energy fractures: 59.1% (13/22) vs. 27% (10/37), p = 0.026 | N1 vs. N3: 9.1% (1/22) vs. 5.4% (2/37), p = 0.624 |
| [28] | N2 vs. N3: Osteoporosis: 15% (11/73) vs. 7% (9/121) and osteopenia: 34% (25/73) vs. 35% (42/121) | NA |
| [31] | N3 vs. N4: 14% vs. 8.2%, p = 0.33 BMD below expected for age: 46.5% vs. 39.5%, p = 0.44 | N1 vs. N2: Pathological fractures 7.5% (9/120) vs. 8.9% (32/360), p = 0.78 N3 vs. N4: Pathological fractures 8.1% (7.86) vs. 5.8% (5/86), p = 0.76 |
| [34] | Osteoporosis: 17.6% (11/68) and osteopenia: 5.88% (4/68) | Fragility fracture: 0% |
| [35] | N1:Osteoporosis: 40.9% (27/66) and osteopenia: 43.9% (29/66) N2: Osteoporosis: 66.0% (31/47) and osteopenia: 27.6% (13/47) | NA |
| [38] | Osteopenia/osteoporosis: 27.27% (6/22) | NA |
| [40] | N1: Osteoporosis: 28.6% (43/150) N2: Bone pain and osteoporosis 66.6% (2/3) | N1: Pathological fracture: 9.3% (14/150) |
| [41] | N3 vs. N4: 54.5% (6/11) vs. 34.0% (16/47), p = 0.302 | NA |
| Reference | Lumbar Spine BMD/T-Score Mean ± SD or Median (IQR) | Total Hip BMD/T-Score Mean ± SD or Median (IQR) | Femoral Neck BMD/T-Score Mean ± SD or Median (IQR) |
|---|---|---|---|
| [25] | N1 vs. N3: BMD = 1.02 (0.93, 1.11) vs. 1.15 (1.07, 1.22), p = 0.002 g/cm2 Z-score = −1.50 (−1.90, −1.00) vs. −0.50 (−1.20, −0.10), p = 0.012 | N1 vs. N3: BMD = 0.89 (0.72, 0.92) vs. 0.97 (0.89, 1.10) g/cm2, p = 0.002 Z-score = −1.00 (−1.80, −0.40) vs. −0.40 (−0.9, 0.40), p = 0.018 | N1 vs. N3: BMD = 0.81 (0.67, 0.94) vs. 0.94 (0.88, 1.04) g/cm2, p = 0.001 Z-score = −1.60 (−1.90, −0.80) vs. −0.40 (−1.0, 0.00), p = 0.004 |
| [31] | N3 vs. N4 BMD = 0.91 ± 0.18 vs. 1.01 ± 0.17, p < 0.001 g/cm2 T-score = −1.69 ± 1.48 vs. −0.94 ± 1.40, p < 0.001 Z-score = −1.40 ± 1.39 vs. −0.50 ± 1.21, p < 0.001 | N3 vs. N4 BMD = 0.75 ± 0.30 vs. 0.81 ± 0.23, p = 0.17 g/cm2 T-score = −1.45 ± 1.00 vs. −0.97 ± 1.38, p = 0.01 Z-score = −1.31 ± 0.97 vs. −0.58 ± 1.04, p < 0.001 | N3 vs. N4 BMD = 0.73 ± 0.35 vs. 0.79 ± 0.18, p = 0.14 g/cm2 T-score = −1.53 ± 1.02 vs. −0.99 ± 1.09, p = 0.002 Z-score = −1.15 ± 1.05 vs. −0.43 ± 1.01, p < 0.001 |
| [35] | N1 vs. N2 BMD = 0.884 ± 0.154 vs. 0.855 ± 0.133 g/cm2, p > 0.05 T-score = −1.7 ± 1.4 vs. −2.1 ± 1.2, p > 0.05 | N1 vs. N2 BMD = 0.843 ± 0.177 vs. 0.816 ± 0.141 g/cm2, p > 0.05 T-score = −1.3 ± 1.0 vs. −1.5 ± 0.9, p > 0.05 | N1 vs. N2 BMD = 0.704 ± 0.120 vs. 0.702 ± 0.150 g/cm2, p > 0.05 T-score = −1.7 ± 0.9 vs. −1.9 ± 1.2, p > 0.05 |
| [41] | N3 vs. N4 T-score = −2.0 (−3.0, 1.7) vs. −1.2 (−5.2,0.8), p = 0.498 Z-score = −1.8 (−2.5, 1.9) vs. −0.3 (−2.7, 2.3), p = 0.042 | N3 vs. N4 T-score = −1.6 (−2.9, 1.3) vs. −1.1 (−3.3,0.6), p = 0.052 Z-score = −1.6 (−2.8, 1.6) vs. −0.8 (−3.2, 0.9), p = 0.042 | N3 vs. N4 T-score = −1.8 (−3.1, 0.7) vs. −1.4 (−3.5, 0.7), p = 0.218 Z-score = −1.7 (−2.5, 1.6) vs. −0.8 (−3.0, 1.3), p = 0.054 |
| Reference | Lumbar BMD | Total Hip BMD | Femoral Neck BMD |
|---|---|---|---|
| [25] | +8.5%, p = 0.008 | +2.1%, p = 0.005 | +4.3%, p = 0.007 |
| [26] | N1 vs. N2 BMD = 0.986 ± 0.123 vs. 1.172 ± 0.139 g/cm2, p < 0.001 T-score = −0.79 ± 1.14 vs. −0.15 ± 1.19, p = 0.03 Z-score = −0.29 ± 1.14 vs. −0.10 ± 1.18, p = 0.49 | N1 vs. N2 BMD = 0.931 ± 0.130 vs. 1.022 ± 0.128 g/cm2, p = 0.004 T-score = −0.44 ± 0.98 vs. −0.19 ± 1.01, p = 0.309 Z-score = −0.10 ± 0.80 vs. −0.04 ± 0.95, p = 0.778 | N1 vs. N2 BMD = 0.782 ± 0.119 vs. 0.967 ± 0.129 g/cm2, p < 0.001 T-score = −0.99 ± 0.89 vs. −0.45 ± 1.03 p = 0.012 Z-score = −0.37 ± 0.67 vs. −0.19 ± 0.98 p = 0.356 |
| [35] | N1BMD = 0.818 ± 0.157 vs. 0.879 ± 0.164 g/cm2, p > 0.05 T-score = −2.3 ± 1.3 vs. −1.7 ± 1.4, p > 0.05 | N1 BMD = 0.801 ± 0.161 vs. 0.841 ± 0.170 g/cm2, p > 0.05 T-score = −1.6 ± 0.9 vs. −1.2 ± 1.0, p > 0.05 | N1 BMD = 0.673 ± 0.114 vs. 0.697 ± 0.128 g/cm2, p > 0.05 T-score = −1.9 ± 0.9 vs. −1.6 ± 1.0, p > 0.05 |
| Reference | Trabecular Bone Score Median (IQR) | 3D DXA Analysis Median (IQR) |
|---|---|---|
| [25] | N1 vs. N3: 1.39 (1.32–1.45) vs. 1.49 (1.40–1.51), p = 0.136 | N1 vs. N3: Cortical sBMD TH = 131.15 (106.96–150.63) vs. 151.95 (141.89–163.72) g/cm2, p = 0.001 Cortical sBMD FN = 102.06 (92.54–118.58) vs. 130.10 (119.68–138.09) g/cm2, p < 0.001 Trabecular vBMD TH = 142.22 (105.29–181.17) vs. 168.81 (150.22–212.23) g/cm3, p = 0.029 Trabecular vBMD FN = 181.93 (154.69–235.27) vs. 237.74 (212.92–265.67) g/cm3, p = 0.008 Cortical vBMD TH = 724.79 (652.67–779.78) vs. 800.74 (751.19–857.710) g/cm3, p = 0.007 Cortical vBMD FN = 713.81 (671.471–768.502) vs. 797.82 (758.03–858.38) g/cm3, p = 0.002 Cortical Thickness TH = 1.77 (1.65–1.83) vs. 1.910 (1.86–2.01) mm, p < 0.001 Cortical Thickness FN = 1.48 (1.40–1.59) vs. 1.65 (1.49–1.80) mm, p = 0.002 N2 before vs. after PTx: Cortical sBMD TH = 135.70 (100.65–153.83) vs. 147.71 (106.21–168.08) g/cm2, p = 0.001 Cortical sBMD FN = 112.20 (95.04–123.62) vs. 121.33 (101.16–132.55) g/cm2, p = 0.001 Trabecular vBMD TH = 157.17 (113.95–177.26) vs. 172.62 (120.75–226.64) g/cm3, p = 0.019 Trabecular vBMD FN = 204.18 (170.26–226.75) vs. 207.64 (170.02–286.12) g/cm3, p = 0.019 Cortical vBMD TH = 745.44 (597.69–776.26) vs. 761.41 (614.26–835.44) g/cm3, p = 0.005 Cortical vBMD FN = 738.11 (636.25–781.54) vs. 767.69 (667.84–816.35) g/cm3, p = 0.019 Cortical Thickness TH = 1.79 (1.68–1.96) vs. 1.878 (1.73–2.01) mm, p = 0.005 Cortical Thickness FN = 1.61 (1.50–1.71) vs. 1.65 (1.58–1.71) mm, p = 0.007 |
| [31] | N3 vs. N4 1.230 < TBS < 1.310: 20.9% vs. 26.7%, p = 0.47 TBS ≤ 1.230: 53.4% vs. 26.7%, p < 0.001 Serum ionized calcium and TBS in N3: B = 0.275, SE = 0.132, p = 0.04 |
| Reference | Age at PTx | Surgical Approach | Post-Surgery Outcome |
|---|---|---|---|
| [26] | 33.3 ± 13.7 y | <STPTx: 46.9% (15/32) STPTx: 15.6% (5/32) TPTx: 37.5% (12/32) | Recurrent PHPT: <STPTx: 86.7% (13/15) STPTx: 0% TPTx: 66.7% (8/12) Persistent PHPT: 62.9% (22/35) |
| [27] | N: 37.7 (27, 49) y N1: 37.0 (26, 50) y N2: 37.9 (28, 48) y | <STPTx: 34.43% (178/517) STPTx: 65.57% (339/517) | N vs. N1 vs. N2: Recurrent PHPT: 53.2% vs. 68.5% vs. 45%, p < 0.001 Persistent PHPT: 8.3% vs. 18% vs. 3.2%, p < 0.001 Hypoparathyroidism at 6 mo: 16% vs. 3.4% vs. 22.7%, p < 0.001 Hypoparathyroidism at 12 mo: 13.5% vs. 2.3% vs. 19.5%, p < 0.001 Risk of recurrence OR (95% CI): Exon 10 pathogenic variant: 2.19 (1.31–3.69), p = 0.003 <STPTx: 2.61 (2.03–3.31), p < 0.001 Sex: p = 0.490 Age at surgery: p = 0.612 Exon 2 pathogenic variant: p = 0.767 Exon 9 pathogenic variant: p = 0.111 |
| [28] | <STPTx vs. STPTx vs. TPTx: 30 (22, 38) vs. 31 (24, 38) vs. 32 (22, 37) | <STPTx: 40% (67/167) STPTx: 57% (95/167) TPTx: 3% (7/167) Diagnosis of MEN1 before surgery: 36% vs. 82% vs. 80%, p < 0.0001 | <STPTx vs. STPTx vs. TPTx Persistent PHPT: 25% vs. 3% vs. 0% Recurrent PHPT: 64% vs. 58% vs. 60% Persistent/recurrent PHPT: 84% vs. 61% vs. 60%, p = 0.0003 Second surgery: 69% 25% 20% Third surgery: 24% vs. 8% vs. 20% Fourth surgery: 1% vs. 2% vs. 0% Prolonged hypoparathyroidism: 9% vs. 7% vs. 40%, p > 0.05 Permanent laryngeal nerve palsy: 0% |
| [29] | PTx in 76.5% (13/17) | <STPTx: 38.5% (5/13) STPTx: 23.1% (3/13) STPTx and thymectomy: 15.4% (2/13) TPTx: 15.4% (2/13) <STPTx and hemithyroidectomy: 7.7% (1/13) | Persistent PHPT: 25% (3/17) Recurrent PHPT: 16.7% (2/13) Hypoparathyroidism: 41.7% (5/13) |
| [30] | NA | NA | N2: Reoperation: 25.9% (7/27) Recurrent PHPT: 71.4% (5/7) Persistent PHPT: 28.6% (2/7) Transient hypoparathyroidism: 66.7% (18/27) Permanent hypoparathyroidism: 14.8% (4/27) Transitory laryngeal nerve palsy: 11.1% (3/27) Permanent laryngeal nerve palsy: 3.7% (1/27) |
| [31] | NA | NA | N1: PTx in 80% (96/120) Persistent PHPT: 13.5% (13/96) Recurrent PHPT: 28.9% (24/83) Reoperation: 17.7% (17/96) |
| [32] | Median (Q1, Q3) age at PTx = 38 (22, 44) y | STPTx: 66.7% (20/30) TPTx: 33.3% (10/30) | Hypoparathyroidism: 23.33% (7/30) |
| [33] | Mean ± SD = 32 ± 12.7 y Age at most recent PTx: 42 ± 12 y | <STPTx: 42% (85/206) STPTx: 47% (95/204) TPTx and autotransplantation: 12% (24/206) | Prolonged hypoparathyroidism: 23% (47/206) Recovered hypoparathyroidism: 40% (19/47) At last follow-up: Aparathyroid: 1% (2/206) Hypoparathyroid: 13% (26/206) Euparathyroid: 54% (112/206) Hyperparathyroid: 31% (64/206) OR (95% CI) of prolonged hypoparathyroidism: Age at last operation: 1 (0.98, 1.03), p = 1 Female: 1.18 (0.61, 2.27), p = 0.6 4 or more glands resected: 6.02 (2.96, 12.24), p < 0.001 PTx before 2010: 2.07 (1.02, 4.23), p = 0.045 Immediate postoperative PTH < 15 ng/mL: 13.1 (3.61, 47.47), p < 0.001 OR (95% CI) of hypoparathyroidism recovery: Age at last operation: 0.96 (0.91, 1.01), p = 0.13 Female: 1.69 (0.47, 6.15), p = 0.42 4 or more glands resected: 0.19 (0.05, 0.72), p = 0.02 Reoperation: 1.02 (0.29, 3.6), p = 0.98 |
| [34] | NA | PTx in 83.8% (57/68) <STPTx: 38.5% (22/57) STPTx: 61.5% (35/57) | Long-term remission: 56% (32/57) Persistent PHPT: 12.2% (7/57) Recurrent PHPT: 31.5% (18/57) Reoperation: 61% (11/18) Permanent hypoparathyroidism: 19.2% (11/57) Laryngeal nerve palsy: 0% Long-term remission and STPTx: OR (95% CI) = 1.7 (1.2–3.7, p < 0.001) Cinacalcet use: 33.8% (23/68) |
| [35] | N1 36.6 ± 14.3 y | N1 Did not undergo PTx: 21.1% (28/133) NCPHPT: 64.3% (18/28) PTx: 78.9% (105/133) <STPTx: 23.8% (25/105) STPTx: 39% (41/105) TPTx: 37.1% (39/105) | N1 Recurrent PHPT: 20% (21/105) Persistent PHPT: 11.4% (12/105) Permanent hypoparathyroidism: 12.4% (13/105) Reoperation: 14.3% (15/105) |
| [37] | 43.4 ± 14.1 y | <STPTx: 36.35% (12/33) STPTx: 12.12% (4/33) TPTx: 51.51% (17/33) | N1 vs. N2 vs. N3: Persistent PHPT: 0% vs. 0% vs. 0% Recurrent PHPT: 25% (3/12) vs. 50% (2/4) vs. 5.9% (1/17), p = 0.076 Transient hypoparathyroidism: 0% vs. 0% vs. 23.5% (4/17), p = 0.154 Permanent hypoparathyroidism: 0% vs. 0% vs. 35.3% (6/17), p = 0.031 Parathyroid venous sampling vs. non-parathyroid venous sampling: Persistent PHPT: 0% vs. 0% Recurrent PHPT: 0% vs. 10% (1/10), p = 1.00 Transient hypoparathyroidism: 22.2% (2/9) vs. 10% (1/10), p = 0.582 Permanent hypoparathyroidism: 0% vs. 50% (5/10), p = 0.033 TPTx: 44.4% (4/9) vs. 100% (10/10), p = 0.011 |
| [38] | NA | PTx in 68.18% (15/22) <STPTx: 20% (3/15) | Persistent PHPT: 6.7% (1/15) Recurrent PHPT: 0% Transient hypocalcaemia: 6.7% (1/15) Laryngeal nerve palsy: 0% |
| [39] | NA | <STPTx: 31.5% (28/89) STPTx: 25.8% (23/89) TPTx: 42.7% (38/89) | Persistent PHPT: 5.6% Recurrent PHPT: 36% Transient hypoparathyroidism: 49% Permanent hypoparathyroidism: 18% Permanent laryngeal nerve palsy: 0% Severe postoperative hypocalcaemia: 0% N1 vs. N2 vs. N3: Persistent PHPT: 14.2% vs. 0% vs. 2.6%, p = 0.052 Recurrent PHPT: 21.3% vs. 10.1% vs. 4.4%, N1 vs. N2 p = 0.03, N1 vs. N3 p = 0.001 Recurrence free survival: 101 (range 3301) vs. 139 (range 28–278) vs. 204 (range 75–396) months, N1 vs. N2 p = 0.018, N1 vs. N3 p = 0.049, N2 vs. N3 p = 0.35 Transient hypoparathyroidism: 0% vs. 26% vs. 100% Permanent hypoparathyroidism: 0% vs. 17% vs. 32%, N1 vs. N3 p = 0.01, N2 vs. N3 p = 0.06 |
| [40] | NA | N: PTx: 73.2% (112/153) | NA |
| Reference | Preoperative Detection Rate and Key Findings in Pre-Surgery Imaging Scans |
|---|---|
| [36] | 91.2% (51/56); N1 vs. N2: 87% vs. 93.9%, p = 0.33 US features in N1 vs. N2: Round lesions: 80% vs. 25.8%, p < 0.001 Irregular shape: 94% vs. 48.4%, p = 0.301 Vague boundary: 95% vs. 0%, p = 0.13 Heterogeneous: 96% vs. 45.2% p = 0.218 Abundant blood flow: 95% vs. 93.5%, p = 0.662 |
| [38] | US: 91% (20/22) SestaMIBI scintigraphy and SPECT/CT: 96% (21/22) FCH-PET/CT: 96% (21/22) SUVmax adenoma vs. hyperplasia: 4.0 (range 1.8–13.4) vs. 3.9 (range 1.8–13.4), p = 0.14 Sensitivity: US: 60% SestaMIBI and SPECT/CT: 66% US and sestaMIBI: 76% FCH-PET/CT: 76% US and FCH-PET/CT: 84% US and sestaMIBI and FCH-PET/CT: 90% Specificity: US: 91% SestaMIBI and SPECT/CT: 87% US and sestaMIBI: 84% FCH-PET/CT: 92% US and FCH-PET/CT: 87% US and sestaMIBI and FCH-PET/CT: 81% Positive predictive value: US: 91% SestaMIBI and SPECT/CT: 83% US and sestaMIBI: 83% FCH-PET/CT: 91% US and FCH-PET/CT: 87% US and sestaMIBI and FCH-PET/CT: 83% Negative predictive value: US: 60% SestaMIBI and SPECT/CT: 71% US and sestaMIBI: 78% FCH-PET/CT: 79% US and FCH-PET/CT: 84% US and sestaMIBI and FCH-PET/CT: 88% Accuracy: US: 70% SestaMIBI and SPECT/CT: 76% US and sestaMIBI: 80% FCH-PET/CT: 84% US and FCH-PET/CT: 85% US and sestaMIBI and FCH-PET/CT: 85% |
| Reference | Main Histological Findings |
|---|---|
| [26] | Hyperplasia: 59.4% (19/32) Adenoma: 9.4% (3/32) Carcinoma: 0% |
| [31] | N1 vs. N2 Multi-glandular involvement: 52.1% vs. 10%, p < 0.001 Carcinoma: 1% vs. 10% |
| [36] | N1 vs. N2: Multi-glandular involvement: 40% vs. 10%, p = 0.003 Mean parathyroid lesion numbers: 1.6 ± 0.91 vs. 1.1 ± 0.55, p = 0.002 Size: 1.68 ± 0.78 vs. 1.88 ± 0.73 cm, p = 0.349 Hyperplasia: 46.7% vs. 16.7%, p = 0.039 |
| [38] | Adenomas: 26% Hyperplasia: 69% Thymus carcinoid tumours: 5% |
| [40] | Hyperplasia: 40.2% (45/112) Adenomas: 57.1% (64/112) Atypical parathyroid neoplasm: 1.8% (2/112) Parathyroid carcinoma: 0.9% (1/112) |
| Reference | Physical Component Summary | Mental Component Summary |
|---|---|---|
| [32] | Preoperative vs. 6 mo vs. 12 mo PCS: 76 (44–91) vs. 72 (51–92) vs. 80 (46–92), p = 0.71 PF: 88 (59–100) vs. 90 (64–100) vs. 85 (64–96), p = 0.57 RP: 100 (0–100) vs. 84 (0–100) vs. 100 (0–100), p = 0.22 BP: 88 (41–100) vs. 61 (41–100) vs. 72 (28–100), p = 0.23 GH: 62 (40–77) vs. 62 (44–82) vs. 60 (39–77), p = 0.55 | Preoperative vs. 6 mo vs. 12 mo MCS: 66 (36–84) vs. 75 (33–87) vs. 76 (45–89), p = 0.23 VT: 60 (30–81) vs. 62 (40–80) vs. 65 (39–75), p = 0.51 SF: 69 (38–100) vs. 88 (57–100) vs. 82 (50–100), p = 0.04 RE: 100 (0–100) vs. 84 (0–100) vs. 100 (0–100), p = 0.22 MH: 66 (43–80) vs. 70 (44–88) vs. 72 (44–84), p = 0.23 |
| Symptomatic vs. asymptomatic PCS 61.2 (39.5–83.0) vs. 92.5 (83.5–94.2), p = 0.0051 PF 80.0 (40.0–90.0) vs. 100.0 (90.0–100.0), p = 0.0093 RP 50.0 (0.0–100.0) vs. 100.0 (100.0–100.0), p = 0.08 BP 62.0 (30.0–100.0) vs. 94.0 (62.0–100.0), p = 0.14 GH 47.0 (37.0–67.0) vs. 77.0 (62.0–82.0), p = 0.0062 | Symptomatic vs. asymptomatic MCS 56.0 (28.5–75.5) vs. 82.0 (46.2–92.2), p = 0.04 VT 45.0 (25.0–70.0) vs. 80.0 (55.0–90.0), p = 0.01 SF 50.0 (25.0–100.0) vs. 88.0 (38.0–100.0), p = 0.21 RE 100.0 (0.0–100.0) vs. 100.0 (33.0–100.0), p = 0.30 MH 56.0 (28.0–72.0) vs. 76.0 (64.0–84.0), p = 0.02 | |
| Pain score and PCS: r = −0.60, CI = (−0.78, −0.26), p = 0.0009 | MCS and remnant parathyroid volume at 6 mo: r = 0.3807, p = 0.038 | |
| Total parathyroid volume and RP: r = −0.44, CI = (−0.70, −0.09), p = 0.01) | Postoperative MCS: 80.25 (54.88–92.63) vs. 32.25 (16.38–83.75), p = 0.0365) | |
| PCS and remanent parathyroid volume at 12 mo: r = 0.3625, p = 0.049 | ||
| 1–2 comorbidities vs. 3–4 comorbidities: Preoperative PCS: 88.0 (65.38–93.63) vs. 39.50 (30–75–63–38), p = 0.0015 Postoperative PCS: 84.5 (63.38–84.50) vs. 28.75 (20.25–74.13), p = 0.0031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheorghe, A.-M.; Stanciu, M.; Lebada, I.C.; Nistor, C.; Carsote, M. An Updated Perspective of the Clinical Features and Parathyroidectomy Impact in Primary Hyperparathyroidism Amid Multiple Endocrine Neoplasia Type 1 (MEN1): Focus on Bone Health. J. Clin. Med. 2025, 14, 3113. https://doi.org/10.3390/jcm14093113
Gheorghe A-M, Stanciu M, Lebada IC, Nistor C, Carsote M. An Updated Perspective of the Clinical Features and Parathyroidectomy Impact in Primary Hyperparathyroidism Amid Multiple Endocrine Neoplasia Type 1 (MEN1): Focus on Bone Health. Journal of Clinical Medicine. 2025; 14(9):3113. https://doi.org/10.3390/jcm14093113
Chicago/Turabian StyleGheorghe, Ana-Maria, Mihaela Stanciu, Ioana Codruta Lebada, Claudiu Nistor, and Mara Carsote. 2025. "An Updated Perspective of the Clinical Features and Parathyroidectomy Impact in Primary Hyperparathyroidism Amid Multiple Endocrine Neoplasia Type 1 (MEN1): Focus on Bone Health" Journal of Clinical Medicine 14, no. 9: 3113. https://doi.org/10.3390/jcm14093113
APA StyleGheorghe, A.-M., Stanciu, M., Lebada, I. C., Nistor, C., & Carsote, M. (2025). An Updated Perspective of the Clinical Features and Parathyroidectomy Impact in Primary Hyperparathyroidism Amid Multiple Endocrine Neoplasia Type 1 (MEN1): Focus on Bone Health. Journal of Clinical Medicine, 14(9), 3113. https://doi.org/10.3390/jcm14093113









