Impact of the Enhanced Recovery After Surgery Protocol on the Perioperative Outcomes of Robot-Assisted Radical Cystectomy
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
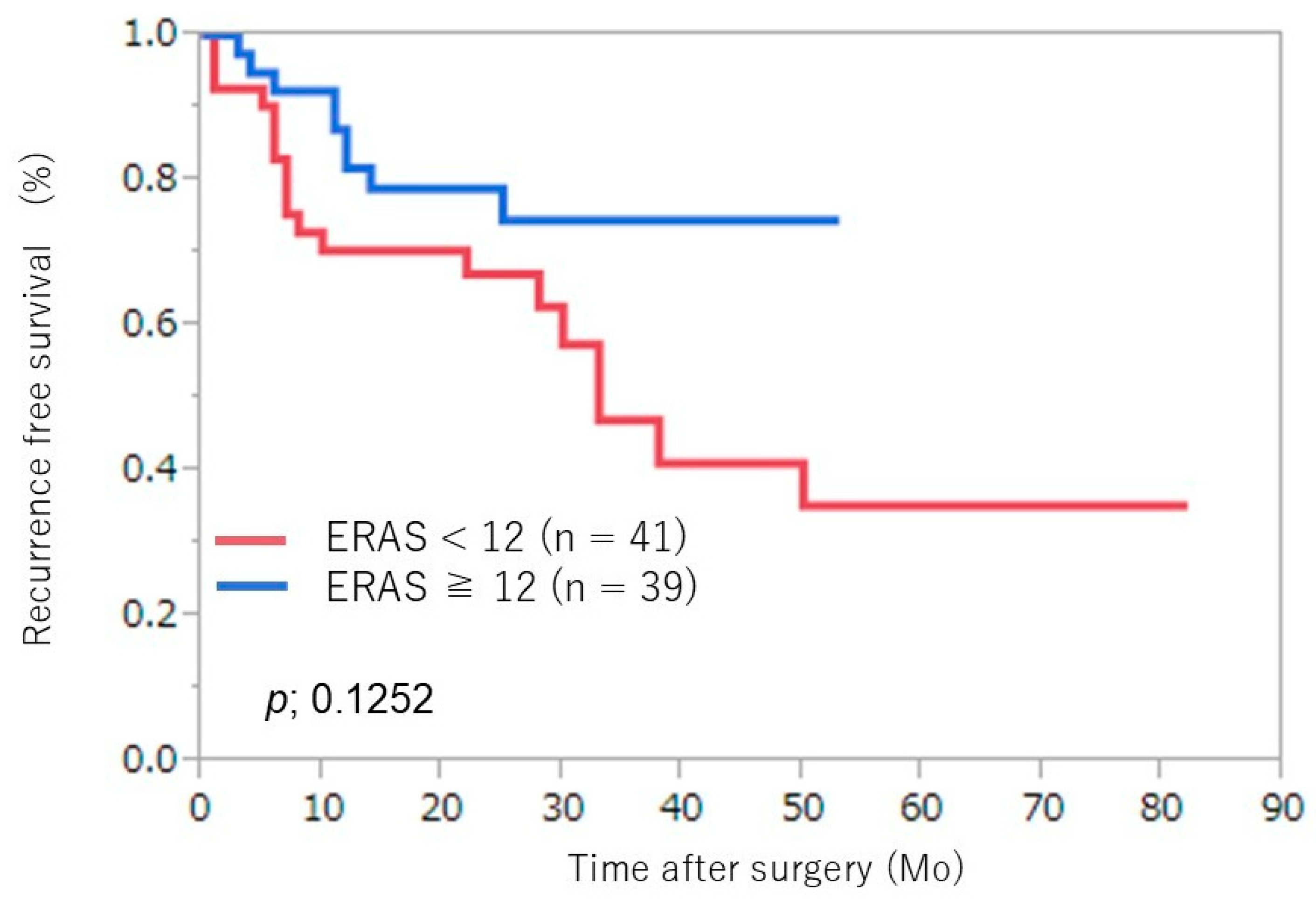
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ERAS | Enhanced recovery after surgery |
| RARC | Robot-assisted radical cystectomy |
| LOS | Length of stay |
| GDFT | Goal-directed fluid therapy |
| ROC | Receiver operating characteristic |
| ECUD | Extracorporeal urinary diversion |
| ICUD | Intracorporeal urinary diversion |
| IC | Ileal conduit |
| NB | Neobladder |
References
- Parekh, D.J.; Reis, I.M.; Castle, E.P.; Gonzalgo, M.L.; Woods, M.E.; Svatek, R.S.; Weizer, A.Z.; Konety, B.R.; Tollefson, M.; Krupski, T.L.; et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): An open-label, randomised, phase 3, non-inferiority trial. Lancet 2018, 391, 2525–2536. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.B.; Cumberbatch, M.G.K.; Kamat, A.M.; Jubber, I.; Kerr, P.S.; McGrath, J.S.; Djaladat, H.; Collins, J.W.; Packiam, V.T.; Steinberg, G.D.; et al. Reporting Radical Cystectomy Outcomes Following Implementation of Enhanced Recovery After Surgery Protocols: A Systematic Review and Individual Patient Data Meta-analysis. Eur. Urol. 2020, 78, 719–730. [Google Scholar] [CrossRef]
- Cerantola, Y.; Valerio, M.; Persson, B.; Jichlinski, P.; Ljungqvist, O.; Hubner, M.; Kassouf, W.; Muller, S.; Baldini, G.; Carli, F.; et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS(®)) society recommendations. Clin. Nutr. 2013, 32, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Ripollés-Melchor, J.; Abad-Motos, A.; Zorrilla-Vaca, A. Enhanced Recovery After Surgery (ERAS) in Surgical Oncology. Curr. Oncol. Rep. 2022, 24, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, J.B.; Kaye, A.D.; Tong, Y.; Belani, K.; Urman, R.D.; Hoffman, C.; Liu, H. Goal-directed fluid therapy in the perioperative setting. J. Anaesthesiol. Clin. Pharmacol. 2019, 35 (Suppl. S1), S29–S34. [Google Scholar]
- Hoshino, N.; Takada, T.; Hida, K.; Hasegawa, S.; Furukawa, T.A.; Sakai, Y. Daikenchuto for reducing postoperative ileus in patients undergoing elective abdominal surgery. Cochrane Database Syst. Rev. 2018, 4, Cd012271. [Google Scholar] [CrossRef]
- Tyson, M.D.; Chang, S.S. Enhanced Recovery Pathways Versus Standard Care After Cystectomy: A Meta-analysis of the Effect on Perioperative Outcomes. Eur. Urol. 2016, 70, 995–1003. [Google Scholar] [CrossRef]
- Raynor, M.C.; Lavien, G.; Nielsen, M.; Wallen, E.M.; Pruthi, R.S. Elimination of preoperative mechanical bowel preparation in patients undergoing cystectomy and urinary diversion. Urol. Oncol. 2013, 31, 32–35. [Google Scholar] [CrossRef]
- Bergqvist, D.; Agnelli, G.; Cohen, A.T.; Eldor, A.; Nilsson, P.E.; Le Moigne-Amrani, A.; Dietrich-Neto, F. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N. Engl. J. Med. 2002, 346, 975–980. [Google Scholar] [CrossRef]
- Kassie, G.M.; Nguyen, T.A.; Kalisch Ellett, L.M.; Pratt, N.L.; Roughead, E.E. Preoperative medication use and postoperative delirium: A systematic review. BMC Geriatr. 2017, 17, 298. [Google Scholar] [CrossRef]
- Zganjar, A.; Glavin, K.; Mann, K.; Dahlgren, A.; Thompson, J.; Wulff-Burchfield, E.; Winright, S.; Heim, A.; Wyre, H.; Lee, E.; et al. Intensive preoperative ostomy education for the radical cystectomy patient. Urol. Oncol. 2022, 40, 481–486. [Google Scholar] [CrossRef]
- Karl, A.; Rittler, P.; Buchner, A.; Fradet, V.; Speer, R.; Walther, S.; Stief, G.C. Prospective assessment of malnutrition in urologic patients. Urology 2009, 73, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Hollenbeck, B.K.; Miller, D.C.; Taub, D.; Dunn, R.L.; Khuri, S.F.; Henderson, W.G.; Montie, J.E.; Underwood, W., 3rd; Wei, J.T. Identifying risk factors for potentially avoidable complications following radical cystectomy. J. Urol. 2005, 174 Pt 1, 1231–1237; discussion 7. [Google Scholar] [CrossRef]
- Azhar, R.A.; Bochner, B.; Catto, J.; Goh, A.C.; Kelly, J.; Patel, H.D.; Pruthi, R.S.; Thalmann, G.N.; Desai, M. Enhanced Recovery after Urological Surgery: A Contemporary Systematic Review of Outcomes, Key Elements, and Research Needs. Eur. Urol. 2016, 70, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Nygren, J.; Thacker, J.; Carli, F.; Fearon, K.C.; Norderval, S.; Lobo, D.N.; Ljungqvist, O.; Soop, M.; Ramirez, J.; Enhanced Recovery After Surgery Society. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin. Nutr. 2012, 31, 801–816. [Google Scholar] [CrossRef]
- Sarin, A.; Chen, L.L.; Wick, E.C. Enhanced recovery after surgery-Preoperative fasting and glucose loading-A review. J. Surg. Oncol. 2017, 116, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Svanfeldt, M.; Thorell, A.; Hausel, J.; Soop, M.; Nygren, J.; Ljungqvist, O. Effect of “preoperative” oral carbohydrate treatment on insulin action--a randomised cross-over unblinded study in healthy subjects. Clin. Nutr. 2005, 24, 815–821. [Google Scholar] [CrossRef]
- Taniguchi, H.; Sasaki, T.; Fujita, H.; Takamori, M.; Kawasaki, R.; Momiyama, Y.; Takano, O.; Shibata, T.; Goto, T. Preoperative fluid and electrolyte management with oral rehydration therapy. J. Anesth. 2009, 23, 222–229. [Google Scholar] [CrossRef]
- Yatabe, T.; Tamura, T.; Kitagawa, H.; Namikawa, T.; Yamashita, K.; Hanazaki, K.; Yokoyama, M. Preoperative oral rehydration therapy with 2.5% carbohydrate beverage alleviates insulin action in volunteers. J. Artif. Organs 2013, 16, 483–488. [Google Scholar] [CrossRef]
- Choi, J.W.; Kim, D.K.; Kim, J.K.; Lee, E.J.; Kim, J.Y. A retrospective analysis on the relationship between intraoperative hypothermia and postoperative ileus after laparoscopic colorectal surgery. PLoS ONE 2018, 13, e0190711. [Google Scholar] [CrossRef]
- Collins, J.W.; Patel, H.; Adding, C.; Annerstedt, M.; Dasgupta, P.; Khan, S.M.; Artibani, W.; Gaston, R.; Piechaud, T.; Catto, J.W.; et al. Enhanced Recovery After Robot-assisted Radical Cystectomy: EAU Robotic Urology Section Scientific Working Group Consensus View. Eur. Urol. 2016, 70, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Kawai, M.; Uesaka, K.; Kodera, Y.; Nagano, H.; Murakami, Y.; Morita, S.; Sakamoto, J.; Yamaue, H.; JAPAN-PD Investigators. Effect of Daikenchuto (TJ-100) on postoperative bowel motility and on prevention of paralytic ileus after pancreaticoduodenectomy: A multicenter, randomized, placebo-controlled phase II trial (the JAPAN-PD study). Jpn. J. Clin. Oncol. 2013, 43, 436–438. [Google Scholar] [CrossRef] [PubMed]
- Llorente, C.; Guijarro, A.; Hernández, V.; Fernández-Conejo, G.; Passas, J.; Aguilar, L.; Tejido, A.; Hernández, C.; Moralejo, M.; Subirá, D.; et al. Outcomes of an enhanced recovery after radical cystectomy program in a prospective multicenter study: Compliance and key components for success. World J. Urol. 2020, 38, 3121–3129. [Google Scholar] [CrossRef]
- Schiavina, R.; Droghetti, M.; Bianchi, L.; Ercolino, A.; Chessa, F.; Casablanca, C.; Piazza, P.; Mottaran, A.; Recenti, D.; Salvador, M.; et al. The robotic approach improves the outcomes of ERAS protocol after radical cystectomy: A prospective case-control analysis. Urol. Oncol. 2021, 39, 833.e1–833.e8. [Google Scholar] [CrossRef] [PubMed]
- Ripollés-Melchor, J.; Ramírez-Rodríguez, J.M.; Casans-Francés, R.; Aldecoa, C.; Abad-Motos, A.; Logroño-Egea, M.; García-Erce, J.A.; Camps-Cervantes, Á.; Ferrando-Ortolá, C.; de la Rica, A.S.; et al. Association Between Use of Enhanced Recovery After Surgery Protocol and Postoperative Complications in Colorectal Surgery: The Postoperative Outcomes Within Enhanced Recovery After Surgery Protocol (POWER) Study. JAMA Surg. 2019, 154, 725–736. [Google Scholar] [CrossRef]
- Pang, Q.; Duan, L.; Jiang, Y.; Liu, H. Oncologic and long-term outcomes of enhanced recovery after surgery in cancer surgeries—A systematic review. World J. Surg. Oncol. 2021, 19, 191. [Google Scholar] [CrossRef]
- Ma, R.; Sheybaee Moghaddam, F.; Ghoreifi, A.; Ladi-Seyedian, S.; Cai, J.; Miranda, G.; Aron, M.; Schuckman, A.; Desai, M.; Gill, I.; et al. The effect of enhanced recovery after surgery on oncologic outcome following radical cystectomy for urothelial bladder carcinoma. Surg. Oncol. 2024, 54, 102061. [Google Scholar] [CrossRef]
- Tanneru, K.; Jazayeri, S.B.; Kumar, J.; Alam, M.U.; Norez, D.; Nguyen, S.; Bazargani, S.; Ganapathi, H.P.; Bandyk, M.; Marino, R.; et al. Intracorporeal versus extracorporeal urinary diversion following robot-assisted radical cystectomy: A meta-analysis, cumulative analysis, and systematic review. J. Robot. Surg. 2021, 15, 321–333. [Google Scholar] [CrossRef]
- Mastroianni, R.; Tuderti, G.; Ferriero, M.; Anceschi, U.; Bove, A.M.; Brassetti, A.; D’Annunzio, S.; Misuraca, L.; Torregiani, G.; Covotta, M.; et al. Robot-assisted Radical Cystectomy with Totally Intracorporeal Urinary Diversion Versus Open Radical Cystectomy: 3-Year Outcomes from a Randomised Controlled Trial. Eur. Urol. 2024, 85, 422–430. [Google Scholar] [CrossRef]
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 2102–2114. [Google Scholar] [CrossRef]

| ERAS Item | Achievement Rate |
|---|---|
| Training regarding performing urostomy care/self-catheterization | 70% |
| Explanation of medication use and nutritional management | 63% |
| Avoidance of oral bowel preparation | 99% |
| Avoidance of preoperative long-acting sedatives | 99% |
| Carbohydrate loading | 48% |
| Long-term use of prophylaxis to avoid thromboembolism | 99% |
| Goal-directed perioperative fluid management | 44% |
| Prevention of intraoperative hypothermia | 79% |
| Use of epidural analgesia | 95% |
| Avoidance of nasogastric intubation | 91% |
| Pain management with opioid-sparing analgesics | 86% |
| Prevention of paralytic ileus | 89% |
| Prevention of nausea and vomiting | 76% |
| Early ambulation within 24 h postoperatively | 60% |
| Early return to an oral diet | 65% |
| Adherence to <12 ERAS Items (n = 41) | Adherence to ≥12 ERAS Items (n = 39) | p | |
|---|---|---|---|
| Median age, years (IQR) | 70 (58–74) | 68 (65–73) | 0.2953 |
| Median BMI, kg/m2 (IQR) | 23.9 (21.1–26.0) | 23.9 (22.3–25.0) | 0.9102 |
| Sex, no. (%) | 0.5526 | ||
| Male | 35 (44) | 35 (44) | |
| Female | 6 (7) | 4 (5) | |
| Charlson comorbidity index, no. (%) | 0.5908 | ||
| 0 | 17 (21) | 22 (28) | |
| 1–2 | 23 (29) | 16 (20) | |
| ≥3 | 1 (1) | 1 (1) | |
| Clinical stage, no. (%) | 0.2310 | ||
| cT1–2 | 31 (39) | 31 (39) | |
| cT3–4 | 10 (12) | 8 (10) | |
| Neoadjuvant chemotherapy, no. (%) | 0.9105 | ||
| None | 7 (9) | 8 (10) | |
| 1 | 8 (10) | 7 (9) | |
| ≥2 | 26 (32) | 24 (30) |
| Adherence to <12 ERAS Items (n = 41) | Adherence to ≥12 ERAS Items (n = 39) | p | |
|---|---|---|---|
| Operative time (min), median (IQR) | 519 (453–613) | 375 (321–438) | 0.0001 * |
| Blood loss (mL), median (IQR) | 310 (190–550) | 232 (155–466) | 0.1569 |
| Urinary diversion type, no. (%) | 0.0013 * | ||
| ECUD IC | 8 (10) | 0 (0) | |
| ECUD NB | 15 (19) | 6 (8) | |
| ICUD IC | 18 (22) | 30 (37) | |
| ICUD NB | 0 (0) | 3 (4) | |
| Hospital LOS (day), median (IQR) | 24 (19–30) | 19 (15–25) | 0.0133 * |
| Complications, no. (%) | 0.0154 * | ||
| Grade 1–2 | 8 (10) | 11 (14) | |
| Grade ≥3 | 13 (16) | 2 (3) | |
| Postoperative ileus, no. (%) | 11 (14) | 4 (5) | 0.0425 * |
| Pathological T stage, no. (%) | 0.5516 | ||
| pT0–1 | 20 (25) | 24 (30) | |
| pT2–4 | 21 (26) | 15 (19) | |
| Adjuvant chemotherapy, no. (%) | 7 (9) | 8 (6) | 0.8374 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitamura, K.; Miyoshi, Y.; Ieda, T.; China, T.; Shimizu, F.; Horie, S.; Muto, S. Impact of the Enhanced Recovery After Surgery Protocol on the Perioperative Outcomes of Robot-Assisted Radical Cystectomy. J. Clin. Med. 2025, 14, 3082. https://doi.org/10.3390/jcm14093082
Kitamura K, Miyoshi Y, Ieda T, China T, Shimizu F, Horie S, Muto S. Impact of the Enhanced Recovery After Surgery Protocol on the Perioperative Outcomes of Robot-Assisted Radical Cystectomy. Journal of Clinical Medicine. 2025; 14(9):3082. https://doi.org/10.3390/jcm14093082
Chicago/Turabian StyleKitamura, Kosuke, Yuto Miyoshi, Takeshi Ieda, Toshiyuki China, Fumitaka Shimizu, Shigeo Horie, and Satoru Muto. 2025. "Impact of the Enhanced Recovery After Surgery Protocol on the Perioperative Outcomes of Robot-Assisted Radical Cystectomy" Journal of Clinical Medicine 14, no. 9: 3082. https://doi.org/10.3390/jcm14093082
APA StyleKitamura, K., Miyoshi, Y., Ieda, T., China, T., Shimizu, F., Horie, S., & Muto, S. (2025). Impact of the Enhanced Recovery After Surgery Protocol on the Perioperative Outcomes of Robot-Assisted Radical Cystectomy. Journal of Clinical Medicine, 14(9), 3082. https://doi.org/10.3390/jcm14093082






