Clinical Applications of the Cone Contrast Test in Ophthalmology and Neurology
Abstract
1. Introduction
2. Comparison of Color Vision Tests
2.1. Pseudoisochromatic Plate (PIP) Tests
2.2. Arrangement Tests
2.3. Anomaloscope
2.4. Computerized Tests
3. Clinical Applications of Cone Contrast Threshold Test
3.1. Congenital Color Vision Deficiencies
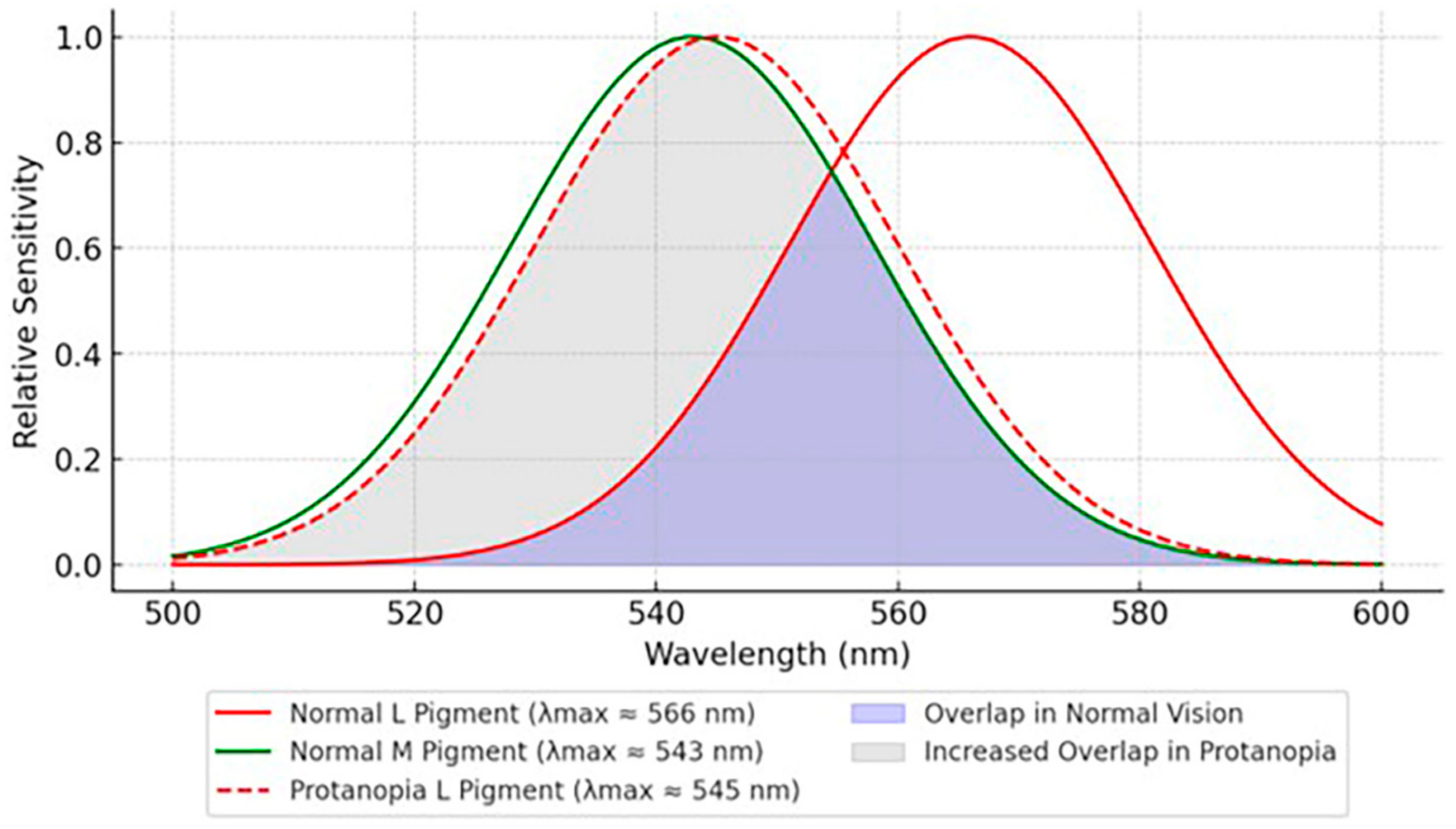
3.1.1. Protanopia/Protanomaly (L-Cone Defect)
3.1.2. Deuteranomalous Trichromats (M-Cone Defect)
3.1.3. Tritanopia/Tritanomaly (S-Cone Defect)
3.1.4. Blue-Cone Monochromacy (L- and M-Cone Defect)
3.1.5. Achromatopsia (Total Color Blindness)
3.2. Cone Dystrophy/Cone–Rod Dystrophy
3.3. Diabetic Retinopathy
3.4. Macular Disorders
3.4.1. Age-Related Macular Degeneration
3.4.2. Occult Macular Dystrophy
3.5. Optic Neuritis
3.6. Glaucoma
3.7. Brain Injury
3.7.1. Traumatic Brain Injury
3.7.2. Non-Traumatic Brain Injury
3.8. Drug Toxicity
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kolb, H. Facts and Figures Concerning the Human Retina. In Webvision: The Organization of the Retina and Visual System; Kolb, H., Fernandez, E., Jones, B., Nelson, R., Eds.; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 1995. [Google Scholar]
- Curcio, C.A.; Sloan, K.R.; Kalina, R.E.; Hendrickson, A.E. Human photoreceptor topography. J. Comp. Neurol. 1990, 292, 497–523. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.W.; Maguire, M.G.; Bressler, N.M.; Glassman, A.R.; Lindblad, A.S.; Ferris, F.L. Visual acuity as an outcome measure in clinical trials of retinal diseases. Ophthalmology 2007, 114, 1804–1809. [Google Scholar] [CrossRef] [PubMed]
- Rabin, J. Cone-specific measures of human color vision. Investig. Ophthalmol. Vis. Sci. 1996, 37, 2771–2774. [Google Scholar]
- Rabin, J. Quantification of color vision with cone contrast sensitivity. Vis. Neurosci. 2004, 21, 483–485. [Google Scholar] [CrossRef]
- Emery, K.J.; Webster, M.A. Individual differences and their implications for color perception. Curr. Opin. Behav. Sci. 2019, 30, 28–33. [Google Scholar] [CrossRef]
- Rabin, J.; Gooch, J.; Ivan, D. Rapid quantification of color vision: The cone contrast test. Investig. Ophthalmol. Vis. Sci. 2011, 52, 816–820. [Google Scholar] [CrossRef]
- Lovell, J.; Rabin, J. A Comparison Between Three Computer-Based Cone Specific Color Vision Tests. Aerosp. Med. Hum. Perform. 2023, 94, 54–58. [Google Scholar] [CrossRef]
- White, K.M.; Livnat, I.; Frambach, C.R.; Doan, J.; Mehta, U.V.; Yuh, C.; Palma, A.M.; Jameson, K.A.; Kenney, M.C.; Mehta, M.C.; et al. Quantitative cone contrast threshold testing in patients with differing pathophysiological mechanisms causing retinal diseases. Int. J. Retin. Vitr. 2023, 9, 9. [Google Scholar] [CrossRef]
- Melamud, A.; Hagstrom, S.; Traboulsi, E. Color vision testing. Ophthalmic Genet. 2004, 25, 159–187. [Google Scholar] [CrossRef]
- Birch, J. Identification of red-green colour deficiency: Sensitivity of the Ishihara and American Optical Company (Hard, Rand and Rittler) pseudo-isochromatic plates to identify slight anomalous trichromatism. Ophthalmic Physiol. Opt. 2010, 30, 667–671. [Google Scholar] [CrossRef]
- Seshadri, J.; Christensen, J.; Lakshminarayanan, V.; Bassi, C.J. Evaluation of the new web-based “Colour Assessment and Diagnosis” test. Optom. Vis. Sci. 2005, 82, 882–885. [Google Scholar] [CrossRef] [PubMed]
- Cole, B.L.; Lian, K.-Y.; Lakkis, C. The new Richmond HRR pseudoisochromatic test for colour vision is better than the Ishihara test. Clin. Exp. Optom. 2006, 89, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Truksa, R.; Fomins, S.; Jansone-Langina, Z.; Tenisa, L. Colour Vision Changes across Lifespan: Insights from FM100 and CAD Tests. Vision 2024, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Marechal, M.; Delbarre, M.; Tesson, J.; Lacambre, C.; Lefebvre, H.; Froussart-Maille, F. Color Vision Tests in Pilots’ Medical Assessments. Aerosp. Med. Hum. Perform. 2018, 89, 737–743. [Google Scholar] [CrossRef]
- Niwa, Y.; Muraki, S.; Naito, F.; Minamikawa, T.; Ohji, M. Evaluation of acquired color vision deficiency in glaucoma using the Rabin cone contrast test. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6686–6690. [Google Scholar] [CrossRef]
- Fujikawa, M.; Muraki, S.; Niwa, Y.; Ohji, M. Evaluation of clinical validity of the Rabin cone contrast test in normal phakic or pseudophakic eyes and severely dichromatic eyes. Acta Ophthalmol. 2018, 96, e164–e167. [Google Scholar] [CrossRef]
- Goh, C.; Puah, M.; Toh, Z.H.; Boon, J.; Boey, D.; Tay, R.; Sule, A.A.; Liu, R.; Ong, X.E.; Kalra, A.; et al. Mobile Apps and Visual Function Assessment: A Comprehensive Review of the Latest Advancements. Ophthalmol. Ther. 2025, 14, 23–39. [Google Scholar] [CrossRef]
- Dain, S.J.; Atchison, D.A.; Hovis, J.K.; Boon, M.Y. Lighting for color vision examination in the era of LEDs: The FM100Hue Test. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2020, 37, A122–A132. [Google Scholar] [CrossRef]
- Squire, T.J.; Rodriguez-Carmona, M.; Evans, A.D.; Barbur, J.L. Color vision tests for aviation: Comparison of the anomaloscope and three lantern types. Aviat. Space Environ. Med. 2005, 76, 421–429. [Google Scholar]
- Barbur, J.L.; Rodriguez-Carmona, M. Colour vision requirements in visually demanding occupations. Br. Med. Bull. 2017, 122, 51–77. [Google Scholar] [CrossRef]
- Tsujimura, S.; Shioiri, S.; Hirai, Y.; Yaguchi, H. Selective cone suppression by the L-M- and M-L-cone-opponent mechanisms in the luminance pathway. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 1999, 16, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Simunovic, M.P. Colour vision deficiency. Eye 2010, 24, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Deeb, S.S. Molecular genetics of color-vision deficiencies. Vis. Neurosci. 2004, 21, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Vollrath, D.; Nathans, J.; Davis, R.W. Tandem array of human visual pigment genes at Xq28. Science 1988, 240, 1669–1672. [Google Scholar] [CrossRef]
- Kalloniatis, M.; Luu, C. The Perception of Color. In Webvision: The Organization of the Retina and Visual System; Kolb, H., Fernandez, E., Jones, B., Nelson, R., Eds.; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 1995. [Google Scholar]
- Davidoff, C.; Neitz, M.; Neitz, J. Genetic Testing as a New Standard for Clinical Diagnosis of Color Vision Deficiencies. Transl. Vis. Sci. Technol. 2016, 5, 2. [Google Scholar] [CrossRef]
- Zagui, R.M.B.; Henriques, L.D.; Costa, M.F. Psychophysical assessment of color vision with the Cambridge Color Vision Test in unilateral functional amblyopia. Arq. Bras. Oftalmol. 2025, 88, e2023-0263. [Google Scholar] [CrossRef]
- Koefoed, V.F.; Miles, T.; Cason, J.B.; Troche, R. Colour vision classification-comparing CAD and CIE 143:2001 International recommendations for colour vision requirements in transport. Acta Ophthalmol. 2020, 98, 726–735. [Google Scholar] [CrossRef]
- Almustanyir, A.; Hovis, J.K. Military Research ColorDx and Printed Color Vision Tests. Aerosp. Med. Hum. Perform. 2015, 86, 852–859. [Google Scholar] [CrossRef]
- He, J.C.; Shevell, S.K. Variation in color matching and discrimination among deuteranomalous trichromats: Theoretical implications of small differences in photopigments. Vision Res. 1995, 35, 2579–2588. [Google Scholar] [CrossRef]
- Birch, J. Failure of concordance of the Farnsworth D15 test and the Nagel anomaloscope matching range in anomalous trichromatism. Vis. Neurosci. 2008, 25, 451–453. [Google Scholar] [CrossRef]
- Vingrys, A.J.; Atchison, D.A.; Bowman, K.J. The use of colour difference vectors in diagnosing congenital colour vision deficiencies with the Farnsworth-Munsell 100-hue test. Ophthalmic Physiol. Opt. 1992, 12, 38–45. [Google Scholar] [PubMed]
- Nathans, J.; Piantanida, T.P.; Eddy, R.L.; Shows, T.B.; Hogness, D.S. Molecular genetics of inherited variation in human color vision. Science 1986, 232, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Weitz, C.J.; Miyake, Y.; Shinzato, K.; Montag, E.; Zrenner, E.; Went, L.N.; Nathans, J. Human tritanopia associated with two amino acid substitutions in the blue-sensitive opsin. Am. J. Hum. Genet. 1992, 50, 498–507. [Google Scholar]
- Arden, G.; Gündüz, K.; Perry, S. Color vision testing with a computer graphics system: Preliminary results. Doc. Ophthalmol. Adv. Ophthalmol. 1988, 69, 167–174. [Google Scholar] [CrossRef]
- Garip Kuebler, A.; Halfter, K.; Reznicek, L.; Klingenstein, A.; Priglinger, S.; Rudolph, G.; Hintschich, C. A pathological indicator for dysthyroid optic neuropathy: Tritan color vision deficiency. Graefe’s Arch. Clin. Exp. Ophthalmol. Albrecht Von. Graefes Arch. Klin. Exp. Ophthalmol. 2021, 259, 3421–3426. [Google Scholar] [CrossRef]
- Qiao, S.N.; Zhang, Z.; Ribelayga, C.P.; Zhong, Y.M.; Zhang, D.Q. Multiple cone pathways are involved in photic regulation of retinal dopamine. Sci. Rep. 2016, 6, 28916. [Google Scholar] [CrossRef]
- Kumaran, N.; Ripamonti, C.; Kalitzeos, A.; Rubin, G.S.; Bainbridge, J.W.B.; Michaelides, M. Severe Loss of Tritan Color Discrimination in RPE65 Associated Leber Congenital Amaurosis. Investig. Ophthalmol. Vis. Sci. 2018, 59, 85–93. [Google Scholar] [CrossRef]
- Michaelides, M.; Johnson, S.; Simunovic, M.P.; Bradshaw, K.; Holder, G.; Mollon, J.D.; Moore, A.T.; Hunt, D.M. Blue cone monochromatism: A phenotype and genotype assessment with evidence of progressive loss of cone function in older individuals. Eye 2005, 19, 2–10. [Google Scholar] [CrossRef]
- Paramei, G.V.; Oakley, B. Variation of color discrimination across the life span. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2014, 31, A375–A384. [Google Scholar] [CrossRef]
- Shinomori, K.; Panorgias, A.; Werner, J.S. Discrimination thresholds of normal and anomalous trichromats: Model of senescent changes in ocular media density on the Cambridge Colour Test. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2016, 33, A65–A76. [Google Scholar] [CrossRef]
- Grishanin, R.; Vuillemenot, B.; Sharma, P.; Keravala, A.; Greengard, J.; Gelfman, C.; Blumenkrantz, M.; Lawrence, M.; Hu, W.; Kiss, S.; et al. Preclinical Evaluation of ADVM-022, a Novel Gene Therapy Approach to Treating Wet Age-Related Macular Degeneration. Mol. Ther. 2019, 27, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Cideciyan, A.V.; Roman, A.J.; Warner, R.L.; Sumaroka, A.; Wu, V.; Jiang, Y.Y.; Swider, M.; Garafalo, A.V.; Viarbitskaya, I.; Russell, R.C.; et al. Evaluation of Retinal Structure and Visual Function in Blue Cone Monochromacy to Develop Clinical Endpoints for L-opsin Gene Therapy. Int. J. Mol. Sci. 2024, 25, 10639. [Google Scholar] [CrossRef] [PubMed]
- Michalakis, S.; Gerhardt, M.; Rudolph, G.; Priglinger, S.; Priglinger, C. Achromatopsia: Genetics and Gene Therapy. Mol. Diagn. Ther. 2022, 26, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Kohl, S.; Jägle, H.; Wissinger, B.; Zobor, D. Achromatopsia. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Nelli, L. Color Quest: An interactive tool for exploring color palettes and enhancing accessibility in data visualization. PLoS ONE 2024, 19, e0290923. [Google Scholar] [CrossRef]
- Remmer, M.H.; Rastogi, N.; Ranka, M.P.; Ceisler, E.J. Achromatopsia: A review. Curr. Opin. Ophthalmol. 2015, 26, 333–340. [Google Scholar] [CrossRef]
- Michaud, J.L.; Héon, E.; Guilbert, F.; Weill, J.; Puech, B.; Benson, L.; Smallhorn, J.F.; Shuman, C.T.; Buncic, J.R.; Levin, A.V.; et al. Natural history of Alström syndrome in early childhood: Onset with dilated cardiomyopathy. J. Pediatr. 1996, 128, 225–229. [Google Scholar] [CrossRef]
- Kumaran, N.; Pennesi, M.E.; Yang, P.; Trzupek, K.M.; Schlechter, C.; Moore, A.T.; Weleber, R.G.; Michaelides, M. Leber Congenital Amaurosis/Early-Onset Severe Retinal Dystrophy Overview. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Mallick, J.; Devi, L.; Malik, P.K.; Mallick, J. Update on Normal Tension Glaucoma. J. Ophthalmic Vis. Res. 2016, 11, 204–208. [Google Scholar] [CrossRef]
- Hamel, C.P. Cone rod dystrophies. Orphanet J. Rare Dis. 2007, 2, 7. [Google Scholar] [CrossRef]
- Molday, R.S.; Garces, F.A.; Scortecci, J.F.; Molday, L.L. Structure and function of ABCA4 and its role in the visual cycle and Stargardt macular degeneration. Prog. Retin. Eye Res. 2022, 89, 101036. [Google Scholar] [CrossRef]
- Assawachananont, J.; Kim, S.-Y.; Kaya, K.D.; Fariss, R.; Roger, J.E.; Swaroop, A. Cone-rod homeobox CRX controls presynaptic active zone formation in photoreceptors of mammalian retina. Hum. Mol. Genet. 2018, 27, 3555–3567. [Google Scholar] [CrossRef]
- Peshenko, I.V.; Olshevskaya, E.V.; Dizhoor, A.M. GUCY2D mutations in retinal guanylyl cyclase 1 provide biochemical reasons for dominant cone-rod dystrophy but not for stationary night blindness. J. Biol. Chem. 2020, 295, 18301–18315. [Google Scholar] [CrossRef] [PubMed]
- Murga-Zamalloa, C.A.; Atkins, S.J.; Peranen, J.; Swaroop, A.; Khanna, H. Interaction of retinitis pigmentosa GTPase regulator (RPGR) with RAB8A GTPase: Implications for cilia dysfunction and photoreceptor degeneration. Hum. Mol. Genet. 2010, 19, 3591–3598. [Google Scholar] [CrossRef] [PubMed]
- Sarra, G.M.; Stephens, C.; de Alwis, M.; Bainbridge, J.W.; Smith, A.J.; Thrasher, A.J.; Ali, R.R. Gene replacement therapy in the retinal degeneration slow (rds) mouse: The effect on retinal degeneration following partial transduction of the retina. Hum. Mol. Genet. 2001, 10, 2353–2361. [Google Scholar] [CrossRef]
- Tharmarajah, B.; Cornish, E.E.; Nguyen, J.; Barnes, E.; Leahy, K.E.; Vaze, A.; Jamieson, R.V.; Grigg, J.R. Hardy-Rand-Rittler colour vision testing in cone and cone-rod dystrophies: Correlation with structural and functional outcome measures. Eye 2025, 39, 527–532. [Google Scholar] [CrossRef]
- Stockman, A.; Henning, G.B.; Michaelides, M.; Moore, A.T.; Webster, A.R.; Cammack, J.; Ripamonti, C. Cone dystrophy with “supernormal” rod ERG: Psychophysical testing shows comparable rod and cone temporal sensitivity losses with no gain in rod function. Investig. Ophthalmol. Vis. Sci. 2014, 55, 832–840. [Google Scholar] [CrossRef]
- Zhen, F.; Zou, T.; Wang, T.; Zhou, Y.; Dong, S.; Zhang, H. Rhodopsin-associated retinal dystrophy: Disease mechanisms and therapeutic strategies. Front. Neurosci. 2023, 17, 1132179. [Google Scholar] [CrossRef]
- Tan, T.E.; Wong, T.Y. Diabetic retinopathy: Looking forward to 2030. Front. Endocrinol. 2022, 13, 1077669. [Google Scholar] [CrossRef]
- Pramanik, S.; Chowdhury, S.; Ganguly, U.; Banerjee, A.; Bhattacharya, B.; Mondal, L.K. Visual contrast sensitivity could be an early marker of diabetic retinopathy. Heliyon 2020, 6, e05336. [Google Scholar] [CrossRef]
- Foote, K.G.; Loumou, P.; Griffin, S.; Qin, J.; Ratnam, K.; Porco, T.C.; Roorda, A.; Duncan, J.L. Relationship Between Foveal Cone Structure and Visual Acuity Measured With Adaptive Optics Scanning Laser Ophthalmoscopy in Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3385–3393. [Google Scholar] [CrossRef]
- Chen, X.D.; Gardner, T.W. A critical review: Psychophysical assessments of diabetic retinopathy. Surv. Ophthalmol. 2021, 66, 213–230. [Google Scholar] [CrossRef]
- Fong, D.S.; Barton, F.B.; Bresnick, G.H. Impaired color vision associated with diabetic retinopathy: Early Treatment Diabetic Retinopathy Study Report No. 15. Am. J. Ophthalmol. 1999, 128, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.C.; Souza, G.S.; Lacerda, E.M.; Nazima, M.T.; Rodrigues, A.R.; Otero, L.M.; Pena, F.P.; Silveira, L.C.; Cortes, M.I. Influence of retinopathy on the achromatic and chromatic vision of patients with type 2 diabetes. BMC Ophthalmol. 2014, 14, 104. [Google Scholar] [CrossRef]
- Ayed, S.; Jeddi, A.; Kallal, Z. Diabetes and color vision disorder detected by the Farnsworth 100 Hue test. Diabetic dyschromatopsia. J. Fr. Ophtalmol. 1990, 13, 506–510. [Google Scholar]
- Ismail, G.M.; Whitaker, D. Early detection of changes in visual function in diabetes mellitus. Ophthalmic Physiol. Opt. 1998, 18, 3–12. [Google Scholar] [CrossRef]
- Feitosa-Santana, C.; Paramei, G.V.; Nishi, M.; Gualtieri, M.; Costa, M.F.; Ventura, D.F. Color vision impairment in type 2 diabetes assessed by the D-15d test and the Cambridge Colour Test. Ophthalmic Physiol. Opt. 2010, 30, 717–723. [Google Scholar] [CrossRef]
- Urani, M.L.; Santos, I.S.; Tirado, V.; Moreno, J.B.; Soberón, S.; Gonzalez-Salinas, R.; Morales-Canton, V.; Quiroz-Mercado, H. Evaluation of cone function in diabetic retinopathy with and without macular edema using the rabin cone contrast test. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2647. [Google Scholar]
- Hovis, J.K.; Ramaswamy, S.; Anderson, M. Repeatability indices for the Adams D-15 test for colour-normal and colour-defective adults. Clin. Exp. Optom. 2004, 87, 326–333. [Google Scholar] [CrossRef]
- Marchesi, N.; Capierri, M.; Pascale, A.; Barbieri, A. Different Therapeutic Approaches for Dry and Wet AMD. Int. J. Mol. Sci. 2024, 25, 13053. [Google Scholar] [CrossRef]
- Okano, K.; Maeda, A.; Chen, Y.; Chauhan, V.; Tang, J.; Palczewska, G.; Sakai, T.; Tsuneoka, H.; Palczewski, K.; Maeda, T. Retinal cone and rod photoreceptor cells exhibit differential susceptibility to light-induced damage. J. Neurochem. 2012, 121, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Decleva, D.; Vidal, K.S.; Kreuz, A.C.; de Menezes, P.; Ventura, D.F. Alterations of color vision and pupillary light responses in age-related macular degeneration. Front. Aging Neurosci. 2022, 14, 933453. [Google Scholar] [CrossRef]
- Vemala, R.; Sivaprasad, S.; Barbur, J.L. Detection of Early Loss of Color Vision in Age-Related Macular Degeneration-With Emphasis on Drusen and Reticular Pseudodrusen. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO247–BIO254. [Google Scholar] [CrossRef] [PubMed]
- Midena, E.; Degli Angeli, C.; Blarzino, M.C.; Valenti, M.; Segato, T. Macular function impairment in eyes with early age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 1997, 38, 469–477. [Google Scholar]
- Feigl, B.; Ojha, G.; Hides, L.; Zele, A.J. Melanopsin-Driven Pupil Response and Light Exposure in Non-seasonal Major Depressive Disorder. Front. Neurol. 2018, 9, 764. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.H.; Sharma, T. Occult Macular Dystrophy. Adv. Exp. Med. Biol. 2018, 1085, 103–104. [Google Scholar]
- Nakanishi, A.; Ueno, S.; Kawano, K.; Ito, Y.; Kominami, T.; Yasuda, S.; Kondo, M.; Tsunoda, K.; Iwata, T.; Terasaki, H. Pathologic Changes of Cone Photoreceptors in Eyes With Occult Macular Dystrophy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7243–7249. [Google Scholar] [CrossRef]
- Huchzermeyer, C.; Fars, J.; Kremers, J.; Kuhlewein, L.; Kempf, M.; Ott, S.; Stingl, K.; Stingl, K. Photoreceptor-Specific Temporal Contrast Sensitivities in RP1L1-Associated Occult Macular Dystrophy. Investig. Ophthalmol. Vis. Sci. 2023, 64, 33. [Google Scholar] [CrossRef]
- Nakamura, N.; Tsunoda, K.; Mizuno, Y.; Usui, T.; Hatase, T.; Ueno, S.; Kuniyoshi, K.; Hayashi, T.; Katagiri, S.; Kondo, M.; et al. Clinical Stages of Occult Macular Dystrophy Based on Optical Coherence Tomographic Findings. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4691–4700. [Google Scholar] [CrossRef]
- Guier, C.P.; Stokkermans, T.J. Optic Neuritis. In StatPearls; StatPearls Publishing: Treasure Island, CA, USA, 2025. [Google Scholar]
- Newman, N.J. The Optic Neuritis Treatment Trial. Ophthalmology 2020, 127, S172–S173. [Google Scholar] [CrossRef]
- Beck, R.W.; Cleary, P.A.; Backlund, J.C. The course of visual recovery after optic neuritis. Experience of the Optic Neuritis Treatment Trial. Ophthalmology 1994, 101, 1771–1778. [Google Scholar] [CrossRef]
- Trobe, J.D.; Beck, R.W.; Moke, P.S.; Cleary, P.A. Contrast sensitivity and other vision tests in the optic neuritis treatment trial. Am. J. Ophthalmol. 1996, 121, 547–553. [Google Scholar] [CrossRef]
- Evangelou, N.; Konz, D.; Esiri, M.M.; Smith, S.; Palace, J.; Matthews, P.M. Size-selective neuronal changes in the anterior optic pathways suggest a differential susceptibility to injury in multiple sclerosis. Brain 2001, 124, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Oberwahrenbrock, T.; Ringelstein, M.; Jentschke, S.; Deuschle, K.; Klumbies, K.; Bellmann-Strobl, J.; Harmel, J.; Ruprecht, K.; Schippling, S.; Hartung, H.P.; et al. Retinal ganglion cell and inner plexiform layer thinning in clinically isolated syndrome. Mult. Scler. 2013, 19, 1887–1895. [Google Scholar] [CrossRef] [PubMed]
- Levin, N.; Devereux, M.; Bick, A.; Baker, N.; Green, A. Color perception impairment following optic neuritis and its association with retinal atrophy. J. Neurol. 2019, 266, 1160–1166. [Google Scholar] [CrossRef]
- Dietze, J.; Blair, K.; Zeppieri, M.; Havens, S.J. Glaucoma. In StatPearls; StatPearls Publishing: Treasure Island, CA, USA, 2025. [Google Scholar]
- Gedde, S.J.; Vinod, K.; Wright, M.M.; Muir, K.W.; Lind, J.T.; Chen, P.P.; Li, T.; Mansberger, S.L. Primary Open-Angle Glaucoma Preferred Practice Pattern®. Ophthalmology 2021, 128, P71–P150. [Google Scholar] [CrossRef]
- Stein, J.D.; Khawaja, A.P.; Weizer, J.S. Glaucoma in Adults-Screening, Diagnosis, and Management: A Review. JAMA 2021, 325, 164–174. [Google Scholar] [CrossRef]
- Papaconstantinou, D.; Georgalas, I.; Kalantzis, G.; Karmiris, E.; Koutsandrea, C.; Diagourtas, A.; Ladas, I.; Georgopoulos, G. Acquired color vision and visual field defects in patients with ocular hypertension and early glaucoma. Clin. Ophthalmol. 2009, 3, 251–257. [Google Scholar]
- Ouchi, J.; Kunikata, H.; Omodaka, K.; Sato, H.; Sato, H.; Ito, A.; Aizawa, N.; Tanaka, Y.; Ichikawa, K.; Nakazawa, T. Color visual acuity in preperimetric glaucoma and open-angle glaucoma. PLoS ONE 2019, 14, e0215290. [Google Scholar] [CrossRef]
- Nork, T.M. Acquired color vision loss and a possible mechanism of ganglion cell death in glaucoma. Trans. Am. Ophthalmol. Soc. 2000, 98, 331–363. [Google Scholar]
- Adams, A.J.; Rodic, R.; Husted, R.; Stamper, R. Spectral sensitivity and color discrimination changes in glaucoma and glaucoma-suspect patients. Investig. Ophthalmol. Vis. Sci. 1982, 23, 516–524. [Google Scholar]
- Castelo-Branco, M.; Faria, P.; Forjaz, V.; Kozak, L.R.; Azevedo, H. Simultaneous comparison of relative damage to chromatic pathways in ocular hypertension and glaucoma: Correlation with clinical measures. Investig. Ophthalmol. Vis. Sci. 2004, 45, 499–505. [Google Scholar] [CrossRef]
- Rauscher, F.G.; Chisholm, C.M.; Edgar, D.F.; Barbur, J.L. Assessment of novel binocular colour, motion and contrast tests in glaucoma. Cell Tissue Res. 2013, 353, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Cutillas, M.; Edgar, D.F.; Sahraie, A. Acquired colour vision defects in glaucoma-their detection and clinical significance. Br. J. Ophthalmol. 1999, 83, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control. CDC grand rounds: Reducing severe traumatic brain injury in the United States. MMWR. Morb. Mortal. Wkly. Rep. 2013, 62, 549–552. [Google Scholar]
- Capizzi, A.; Woo, J.; Verduzco-Gutierrez, M. Traumatic Brain Injury: An Overview of Epidemiology, Pathophysiology, and Medical Management. Med. Clin. North Am. 2020, 104, 213–238. [Google Scholar] [CrossRef]
- Yamamoto, S.; Levin, H.S.; Prough, D.S. Mild, moderate and severe: Terminology implications for clinical and experimental traumatic brain injury. Curr. Opin. Neurol. 2018, 31, 672–680. [Google Scholar] [CrossRef]
- Ng, S.Y.; Lee, A.Y.W. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front. Cell. Neurosci. 2019, 13, 528. [Google Scholar] [CrossRef]
- Silverberg, N.D.; Iaccarino, M.A.; Panenka, W.J.; Iverson, G.L.; McCulloch, K.L.; Dams-O’Connor, K.; Reed, N.; McCrea, M.; American Congress of Rehabilitation Medicine Brain Injury Interdisciplinary Special Interest Group Mild TBI Task Force. Management of Concussion and Mild Traumatic Brain Injury: A Synthesis of Practice Guidelines. Arch. Phys. Med. Rehabil. 2020, 101, 382–393. [Google Scholar] [CrossRef]
- Golden, K.; Borsi, L.; Sterling, A.; Giacino, J.T. Recovery after moderate to severe TBI and factors influencing functional outcome: What you need to know. J. Trauma Acute Care Surg. 2024, 97, 343–355. [Google Scholar] [CrossRef]
- Andelic, N.; Bautz-Holter, E.; Ronning, P.; Olafsen, K.; Sigurdardottir, S.; Schanke, A.-K.; Sveen, U.; Tornas, S.; Sandhaug, M.; Roe, C. Does an early onset and continuous chain of rehabilitation improve the long-term functional outcome of patients with severe traumatic brain injury? J. Neurotrauma 2012, 29, 66–74. [Google Scholar] [CrossRef]
- Hayashi, S.; Kamo, T.; Momosaki, R. Effectiveness of early rehabilitation interventions in patients with traumatic brain injury using a large database. PM R J. Inj. Funct. Rehabil. 2024, 17, 170–177. [Google Scholar] [CrossRef]
- Königs, M.; Beurskens, E.A.; Snoep, L.; Scherder, E.J.; Oosterlaan, J. Effects of Timing and Intensity of Neurorehabilitation on Functional Outcome After Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2018, 99, 1149–1159.e1141. [Google Scholar] [CrossRef] [PubMed]
- Capó-Aponte, J.E.; Jorgensen-Wagers, K.L.; Sosa, J.A.; Walsh, D.V.; Goodrich, G.L.; Temme, L.A.; Riggs, D.W. Visual Dysfunctions at Different Stages after Blast and Non-blast Mild Traumatic Brain Injury. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2017, 94, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Barnett, B.P.; Singman, E.L. Vision concerns after mild traumatic brain injury. Curr. Treat. Options Neurol. 2015, 17, 329. [Google Scholar] [CrossRef] [PubMed]
- Tukur, H.N.; Uwishema, O.; Sheikhah, D.; Akbay, H.; Chehab, T.E.; Wellington, J. The impact of traumatic brain injury on visual processing: A neuro-ophthalmological perspective. Postgrad. Med. J. 2024, qgae188. [Google Scholar] [CrossRef]
- Ventura, R.E.; Balcer, L.J.; Galetta, S.L. The neuro-ophthalmology of head trauma. Lancet Neurol. 2014, 13, 1006–1016. [Google Scholar] [CrossRef]
- Fox, S.M.; Koons, P.; Dang, S.H. Vision Rehabilitation After Traumatic Brain Injury. Phys. Med. Rehabil. Clin. N. Am. 2019, 30, 171–188. [Google Scholar] [CrossRef]
- Munk, A.H.; Starup, E.B.; Lambon Ralph, M.A.; Leff, A.P.; Starrfelt, R.; Robotham, R.J. Colour perception deficits after posterior stroke: Not so rare after all? Cortex A J. Devoted Study Nerv. Syst. Behav. 2023, 159, 118–130. [Google Scholar] [CrossRef]
- Rasdall, M.A.; Cho, C.; Stahl, A.N.; Tovar, D.A.; Lavin, P.; Kerley, C.I.; Chen, Q.; Ji, X.; Colyer, M.H.; Groves, L.; et al. Primary Visual Pathway Changes in Individuals With Chronic Mild Traumatic Brain Injury. JAMA Ophthalmol. 2025, 143, 33–42. [Google Scholar] [CrossRef]
- Saliman, N.H.; Belli, A.; Blanch, R.J. Afferent Visual Manifestations of Traumatic Brain Injury. J. Neurotrauma 2021, 38, 2778–2789. [Google Scholar] [CrossRef]
- Nicolau da Costa, L.R.; Sousa, J.B.; Brito, F.A.C.; Igarashi, Y.; Gomes, J.M.S.; Lobão, C.A.; Costa, M.F.; Miquilini, L.; Souza, G.S. Color discrimination in fixed saturation level of patients with acute traumatic injury. Front. Neurol. 2024, 15, 1363167. [Google Scholar] [CrossRef]
- Clark, J.; Hasselfeld, K.; Bigsby, K.; Divine, J. Colored Glasses to Mitigate Photophobia Symptoms Posttraumatic Brain Injury. J. Athl. Train. 2017, 52, 725–729. [Google Scholar] [CrossRef]
- Smaakjær, P.; Wachner, L.G.; Rasmussen, R.S. Vision therapy improves binocular visual dysfunction in patients with mild traumatic brain injury. Neurol. Res. 2022, 44, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Dutca, L.M.; Stasheff, S.F.; Hedberg-Buenz, A.; Rudd, D.S.; Batra, N.; Blodi, F.R.; Yorek, M.S.; Yin, T.; Shankar, M.; Herlein, J.A.; et al. Early Detection of Subclinical Visual Damage After Blast-Mediated TBI Enables Prevention of Chronic Visual Deficit by Treatment With P7C3-S243. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8330–8341. [Google Scholar] [CrossRef] [PubMed]
- Ventura, R.E.; Jancuska, J.M.; Balcer, L.J.; Galetta, S.L. Diagnostic tests for concussion: Is vision part of the puzzle? J. Neuroophthalmol. 2015, 35, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Lammers, N.A.; Van den Berg, N.S.; Lugtmeijer, S.; Smits, A.R.; Pinto, Y.; de Haan, E.H.F.; Visual Brain Group. Mid-range visual deficits after stroke: Prevalence and co-occurrence. PLoS ONE 2022, 17, e0262886. [Google Scholar] [CrossRef]
- Larjavaara, S.; Mäntylä, R.; Salminen, T.; Haapasalo, H.; Raitanen, J.; Jääskeläinen, J.; Auvinen, A. Incidence of gliomas by anatomic location. Neuro-Oncol. 2007, 9, 319–325. [Google Scholar] [CrossRef]
- Jaeger, W.; Krastel, H.; Braun, S. Cerebral achromatopsia (symptoms, course, differential diagnosis and examination strategy). II. Klin. Monatsblatter Fur Augenheilkd. 1989, 194, 32–36. [Google Scholar] [CrossRef]
- Kawas, C.H.; Corrada, M.M.; Brookmeyer, R.; Morrison, A.; Resnick, S.M.; Zonderman, A.B.; Arenberg, D. Visual memory predicts Alzheimer’s disease more than a decade before diagnosis. Neurology 2003, 60, 1089–1093. [Google Scholar] [CrossRef]
- Cabrera DeBuc, D.; Somfai, G.M.; Arthur, E.; Kostic, M.; Oropesa, S.; Mendoza Santiesteban, C. Investigating Multimodal Diagnostic Eye Biomarkers of Cognitive Impairment by Measuring Vascular and Neurogenic Changes in the Retina. Front. Physiol. 2018, 9, 1721. [Google Scholar] [CrossRef]
- Ageed, A.; Aslam, M.D.; El Haouari, S. Acquired Dyschromatopsia and Its Link to Drug Toxicity. Cureus 2024, 16, e76190. [Google Scholar] [CrossRef]
- Cabral, R.T.S.; Klumb, E.M.; Couto, M.; Carneiro, S. Evaluation of toxic retinopathy caused by antimalarial medications with spectral domain optical coherence tomography. Arq. Bras. Oftalmol. 2019, 82, 12–17. [Google Scholar] [CrossRef]
- Jorge, A.; Ung, C.; Young, L.H.; Melles, R.B.; Choi, H.K. Hydroxychloroquine retinopathy-implications of research advances for rheumatology care. Nat. Rev. Rheumatol. 2018, 14, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Michaelides, M.; Stover, N.B.; Francis, P.J.; Weleber, R.G. Retinal toxicity associated with hydroxychloroquine and chloroquine: Risk factors, screening, and progression despite cessation of therapy. Arch. Ophthalmol. 2011, 129, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Pasadhika, S.; Fishman, G.A. Effects of chronic exposure to hydroxychloroquine or chloroquine on inner retinal structures. Eye 2010, 24, 340–346. [Google Scholar] [CrossRef]
- Vu, B.L.; Easterbrook, M.; Hovis, J.K. Detection of color vision defects in chloroquine retinopathy. Ophthalmology 1999, 106, 1799–1803. [Google Scholar] [CrossRef]
- Oishi, A.; Miyamoto, K.; Kashii, S.; Yoshimura, N. Photopsia as a manifestation of digitalis toxicity. Can. J. Ophthalmol. 2006, 41, 603–604. [Google Scholar] [CrossRef]
- Rietbrock, N.; Alken, R.G. Color vision deficiencies: A common sign of intoxication in chronically digoxin-treated patients. J. Cardiovasc. Pharmacol. 1980, 2, 93–99. [Google Scholar] [CrossRef]
- Wolin, M.J. Digoxin visual toxicity with therapeutic blood levels of digoxin. Am. J. Ophthalmol. 1998, 125, 406–407. [Google Scholar] [CrossRef]
- Lawrenson, J.G.; Kelly, C.; Lawrenson, A.L.; Birch, J. Acquired colour vision deficiency in patients receiving digoxin maintenance therapy. Br. J. Ophthalmol. 2002, 86, 1259–1261. [Google Scholar] [CrossRef][Green Version]
- Garg, P.; Garg, R.; Prasad, R.; Mishra, A.K. A prospective study of ocular toxicity in patients receiving ethambutol as a part of directly observed treatment strategy therapy. Lung India 2015, 32, 16–19. [Google Scholar] [CrossRef]
- Wang, M.Y.; Sadun, A.A. Drug-related mitochondrial optic neuropathies. J. Neuroophthalmol. 2013, 33, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Koul, P.A. Ocular toxicity with ethambutol therapy: Timely recaution. Lung India 2015, 32, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kaimbo, W.K.; Bifuko, Z.A.; Longo, M.B.; Dralands, L.; Missotten, L. Color vision in 42 Congolese patients with tuberculosis receiving ethambutol treatment. Bull. Soc. Belge. Ophtalmol. 2002, 284, 57–61. [Google Scholar]
- Azzouni, F.; Abu Samra, K. Are phosphodiesterase type 5 inhibitors associated with vision-threatening adverse events? A critical analysis and review of the literature. J. Sex. Med. 2011, 8, 2894–2903. [Google Scholar] [CrossRef]
- Luu, J.K.; Chappelow, A.V.; McCulley, T.J.; Marmor, M.F. Acute effects of sildenafil on the electroretinogram and multifocal electroretinogram. Am. J. Ophthalmol. 2001, 132, 388–394. [Google Scholar] [CrossRef]
- Iizuka, T.; Kawamorita, T.; Handa, T.; Ishikawa, H. Cone contrast test-HD: Sensitivity and specificity in red-green dichromacy and the impact of age. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2023, 40, 849–858. [Google Scholar] [CrossRef]
- Reeves, A. Five problems with color constancy metrics: Discussion. J. Opt. Soc. Am. A 2025, 42, B118–B123. [Google Scholar] [CrossRef]
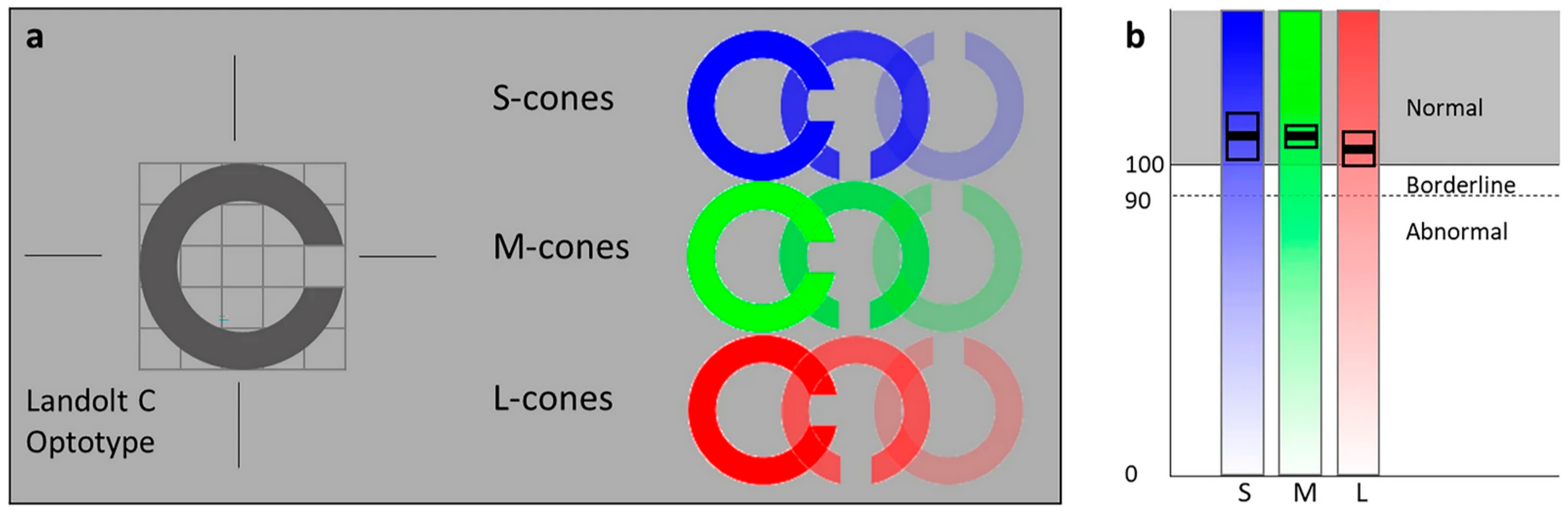
| Name | Time to Complete (Minutes) | Cost | Sensitivity | Specificity |
|---|---|---|---|---|
| Detect | ||||
| Ishihara plates [11,12] | 2–4 | USD 550 * | 98.4% | 100% |
| HRR [11,12,13] | 5–10 | USD 610 * | 87–98% | 33–100% |
| Classify | ||||
| Anomaloscope | 15–20 | USD 18,000 | 100% | 100% |
| FM-100 [12,14] | 10–15 | USD 1150 * | 81.3–100% | 93–95.4% |
| D15 [15] | 2–10 | USD 550 * | 58% | 100% |
| Quantify | ||||
| CAD [14] | 12–30 | USD 9000 | 100% | 100% |
| Cambridge Color Test | 8–15 | USD 30,000 ** | *** | *** |
| CCT [5,7,16,17] | 4–6 | USD 4000 to 10,000 | 100% | 100% |
| Deficiency | Cone(s) Affected | Inheritance | Prevalence |
|---|---|---|---|
| Anomalous trichromacy | |||
| Protanomaly | Red | XLR | 1.08% |
| Deuteranomaly | Green | XLR | 4.63% |
| Tritanomaly | Blue | AD | See tritanopia * |
| Dichromacy | |||
| Protanopia | Red | XLR | 1.01% |
| Deuteranopia | Green | XLR | 1.27% |
| Tritanopia | Blue | AD | 1 in 500 |
| Monochromacy | |||
| Green-cone monochromacy | Red and blue | Dual XLR and AD # | ≤1 in 1,000,000 |
| Red-cone monochromacy | Green and blue | Dual XLR and AD # | ≤1 in 1,000,000 |
| Blue-cone monochromacy | Red and green | XLR | 1 in 100,000 |
| Rod monochromacy and incomplete achromatopsia | Red, green, and blue | AR | 1 in 33,000–50,000 |
| Condition | Proposed CCT Use | Performance Metrics |
|---|---|---|
| Congenital CVDs | Diagnostic tool | Large-scale validation studies with anomaloscope; 100% sensitivity/specificity |
| CRD | Monitoring progressive cone dysfunction | * |
| Glaucoma | Detection of early S-/M-cone loss | Scores correlate with OCT and VF loss |
| DR | Detection of early neuroretinal dysfunction | Scores correlate with OCT [70] |
| AMD | Functional assessment alongside OCT | Scores correlate with OCT and gradings by retinal specialists [9] |
| OMD | Functional confirmation in normal-appearing retina | * |
| ON | Quantification of post-inflammatory changes | Scores correlate with OCT and gradings by retinal specialists [88] |
| MS | Detection of subclinical visual pathway involvement | Scores correlate with diagnosis by specialists [9] |
| Brain injury | Assessment of visual processing disruption | * |
| Drug toxicity | Early detection of cone dysfunction | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raju, P.; Yu, M. Clinical Applications of the Cone Contrast Test in Ophthalmology and Neurology. J. Clin. Med. 2025, 14, 3079. https://doi.org/10.3390/jcm14093079
Raju P, Yu M. Clinical Applications of the Cone Contrast Test in Ophthalmology and Neurology. Journal of Clinical Medicine. 2025; 14(9):3079. https://doi.org/10.3390/jcm14093079
Chicago/Turabian StyleRaju, Priya, and Minzhong Yu. 2025. "Clinical Applications of the Cone Contrast Test in Ophthalmology and Neurology" Journal of Clinical Medicine 14, no. 9: 3079. https://doi.org/10.3390/jcm14093079
APA StyleRaju, P., & Yu, M. (2025). Clinical Applications of the Cone Contrast Test in Ophthalmology and Neurology. Journal of Clinical Medicine, 14(9), 3079. https://doi.org/10.3390/jcm14093079






