Abstract
Background and Objectives: Rotational atherectomy (RA) is a crucial method for percutaneous coronary intervention (PCI) of heavily calcified coronary lesions. The aim of this study was to compare the clinical outcomes in patients undergoing RA via the radial versus femoral approach. Methods: The Rotational Atherectomy in Calcified Lesions in Korea (ROCK) registry included consecutive patients with severely calcified coronary artery disease who received RA during PCI at nine tertiary centers in Korea. A total of 540 patients who underwent PCI with RA were enrolled between October 2019 and January 2010. We retrospectively investigated the clinical outcomes between the transradial and transfemoral approaches. The primary endpoint was major adverse cardiac and cerebrovascular events (MACCE) within 36 months of follow-up. Results: Of the 540 patients, 248 patients (45.9%) were in the transradial group, and 292 patients (54.1%) were in the transfemoral group. There were no significant differences in MACCE (11.3% vs. 17.8%, adjusted hazard ratio [HR]: 1.520; 95% confidence interval: 0.889–2.600; p = 0.126) and procedural success (97.6% vs. 95.2%, p = 0.145). The occurrence of in-hospital bleeding was numerically higher in the transfemoral group, but the difference was not statistically significant (8 [3.2%] vs. 19 [6.5%], p = 0.081) Conclusions: In this study, the transradial approach did not show a significant difference in clinical outcomes but tended to have lower bleeding events compared to the transfemoral approach. RA via the transradial approach can be a useful vascular access option compared to the transfemoral approach.
1. Introduction
Heavily calcified coronary lesions continue to present substantial difficulties during percutaneous coronary interventions (PCI) due to challenges in lesion crossing, inadequate stent expansion, and a higher risk of procedural complications such as dissection or perforation [1,2]. As life expectancy rises and the population ages, an increasing number of patients are being referred for PCI to manage these complex lesions [3]. Rotational atherectomy (RA), first introduced in 1989, remains an essential method for addressing such heavily calcified and resistant-to-dilation lesions [4].
Both transradial (TR) and transfemoral (TF) approaches are feasible for PCI using rotational atherectomy. In conventional PCI, previous studies have consistently demonstrated better outcomes with the TR approach compared to the TF approach [5,6]. Additionally, the 2023 ESC guidelines recommend radial access as the preferred approach [7]. However, traditionally, the TF approach has been the preferred method for RA, primarily because large-caliber guide catheters are needed to accommodate the atherectomy burrs. The TF approach provides greater support, particularly when using larger burr sizes [8]. However, initially used for plaque debulking, RA has since transitioned into a technique for plaque modification, and the use of large-sized burrs has decreased compared to the past [9]. With advancements in device technology, most burr sizes are now compatible with the TR approach. The TR approach has become increasingly popular in coronary angiography and PCI due to its association with fewer vascular and bleeding complications, as well as enhanced patient comfort and quicker mobilization [5,6].
Most existing studies focus on the general benefits of the TR approach in PCI, such as lower mortality rates and reduced major vascular complications, but few have specifically examined its use in RA [10,11,12]. To the best of our knowledge, there is no data on Asians. Considering that East Asians have a higher bleeding risk compared to Western populations, it is even more important to compare the outcomes and safety of the TR and TF approaches in Asian patients [13]. This study aims to compare the clinical outcomes of patients undergoing RA through the TR versus the TF approach.
2. Methods
2.1. Study Design and Population
From January 2010 to October 2019, this study enrolled 540 patients with heavily calcified coronary artery disease (CAD) who underwent PCI using RA at nine tertiary centers in Korea. The data were derived from the Rotational Atherectomy in Calcified Lesions in Korea (ROCK) registry, approved by the institutional review board of each participating hospital. Patients with severely calcified coronary lesions and significant stenosis (defined as ≥70% of the vessel diameter, or ≥50% for the left main coronary artery) were retrospectively identified through institutional databases. Following angiographic assessment, two lesions were excluded from the registry because the RA procedure could not be carried out. In one case, coronary perforation accompanied by cardiac tamponade occurred prior to the initiation of RA. In the other, advancement of the guidewire across the target lesion was unsuccessful, making RA unfeasible. The lesions were categorized into two groups: transradial approach (n = 248 patients) and transfemoral approach (n = 292 patients). Data collection at each center followed a standardized case report form, capturing demographic and clinical characteristics, procedural details, and follow-up information. Follow-up data covering up to 36 months were obtained from medical records and physician or patient interviews conducted at the time of registry enrollment. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Daejeon St. Mary’s Hospital (Approval Code: DC19REDI0066, approved on 30 July 2019).
2.2. RA Procedure
All RA procedures were performed using the Rotablator™ RA system (Boston Scientific, Marlborough, MA, USA). The procedural techniques and treatment approaches followed those outlined in previous reports [14]. The choice of burr size and other procedural decisions were made by the treating physician, considering the complexity of the anatomy, the patient’s overall clinical condition, and relevant clinical risk factors. During the follow-up period, patient care, including the administration of periprocedural anticoagulation and antiplatelet therapy, adhered to established guidelines and standard medical practices.
2.3. Clinical Outcomes and Definition
The primary outcome was major adverse cardiac and cerebrovascular events (MACCE), defined as a composite of cardiac death, target vessel spontaneous myocardial infarction (TVMI), and target vessel revascularization (TVR). Cardiac death was defined as any mortality resulting from cardiac-related causes, including myocardial infarction, heart failure, arrhythmia, or other cardiovascular complications. Secondary endpoints included all-cause death, cardiac death, any MI, TVMI, TVR, stent thrombosis (ST), cerebrovascular accident (CVA), and total bleeding. Total bleeding was defined as the sum of all bleeding events classified according to the Bleeding Academic Research Consortium (BARC) criteria, including types 1 through 5. Additionally, technical and procedural success, in-hospital events, and periprocedural complications were evaluated. The definitions of the outcomes were consistent with those in the previously cited published report [14].
2.4. Statistical Analysis
Continuous variables were analyzed using the t-test and reported as medians with interquartile ranges or means with standard deviations. Categorical variables were compared using the chi-square test or Fisher’s exact test and presented as frequencies and percentages. Cox proportional hazard models were applied to assess the impact of vascular access on clinical outcomes. Multivariate Cox regression analyses were performed using variables with a p-value < 0.1 in the univariate analyses. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated. Clinical outcomes were estimated using the Kaplan–Meier method and compared with the log-rank test. A p-value < 0.05 was considered statistically significant. The multivariate Cox regression models were adjusted for the following covariates: age, sex, chronic kidney disease (CKD), dialysis, previous coronary artery bypass graft (CABG), peripheral vascular disease (PVD), history of heart failure, left ventricular ejection fraction (EF), hemoglobin level, total cholesterol, and low-density lipoprotein (LDL) cholesterol. All statistical analyses were carried out using Statistical Analysis Software (SAS, version 9.2, SAS Institute, Cary, NC, USA).
3. Results
3.1. Baseline Characteristics
Patients were divided into two groups according to the vascular approach for RA. Among a total of 540 patients, 248 patients (45.9%) were in the TR approach group, and 292 patients (54.1%) were in the TF approach group. The study population flow chart is presented in Figure 1. Table 1 presents a comparison of baseline characteristics between the TR and TF approach groups. The mean age was 72.5 years for the TR group, compared to 70.5 years for the TF group, with a statistically significant difference (p = 0.024). There were no statistically significant differences between the two groups in terms of gender, body mass index (BMI), history of smoking, prevalence of diabetes mellitus (DM), hypertension (HTN), previous PCI, or MI. Comorbidities, including CKD, dialysis, previous CABG, PVD, and the history of heart failure, were more common in the TF approach group. The TF group had lower levels of hemoglobin. The EF of the left ventricle was significantly lower in the TF group (Table 2).
Figure 1.
Study population flow chart. RA = rotational atherectomy; TF = transfemoral; TR = transradial.

Table 1.
Baseline characteristics.

Table 2.
Baseline angiographic features and procedural specifics.
3.2. In-Hospital Events and Procedural Outcomes
Table 3 summarizes the in-hospital events and procedural outcomes between the TF approach group and the TR approach group. There were no significant differences in procedural success between the groups (97.6% vs. 95.2%, p = 0.145). For in-hospital events and procedural outcomes, there were no statistically significant differences except for the insertion of a temporary pacemaker during the procedure (p = 0.007). In-hospital bleeding had a higher tendency in the TF approach group (3.2% vs. 6.5%, p = 0.081).

Table 3.
In-hospital events and procedural outcomes.
3.3. Clinical Outcomes
Figure 2 shows the Kaplan–Meier curve for clinical outcomes during follow-up. During the 36-month follow-up period, MACCE showed a statistically significant difference between the two groups with a p-value of 0.032. No significant differences were observed in CD, TVMI, or TVR. However, in the multivariate analysis of MACCE at 36 months, there were no significant differences between the TF approach group and the TR approach group (unadjusted HR: 1.643, 95% CI: 1.038–2.601, p = 0.034; adjusted HR: 1.520, 95% CI: 0.889–2.600, p = 0.126) (Table 4). The secondary endpoints also did not show significant differences (all-cause death: unadjusted HR: 1.124, 95% CI: 0.627–2.013, p = 0.695; adjusted HR: 1.230, 95% CI: 0.610–2.482, p = 0.562; any MI: unadjusted HR: 1.639, 95% CI: 0.654–4.108, p = 0.292; adjusted HR: 1.601, 95% CI: 0.536–4.786, p = 0.400; TLR: unadjusted HR: 1.706, 95% CI: 0.873–3.334, p = 0.118; adjusted HR: 1.245, 95% CI: 0.582–2.663, p = 0.572; ST: unadjusted HR: 2.163, 95% CI: 0.420–11.151, p = 0.356; adjusted HR: 2.224, 95% CI: 0.343–14.414, p = 0.402; CVA: unadjusted HR: 1.301, 95% CI: 0.367–4.610, p = 0.684; adjusted HR: 1.479, 95% CI: 0.230–9.512, p = 0.681; total bleeding: unadjusted HR: 1.280, 95% CI: 0.632–2.592, p = 0.493; adjusted HR: 1.214, 95% CI: 0.526–2.802, p = 0.649).
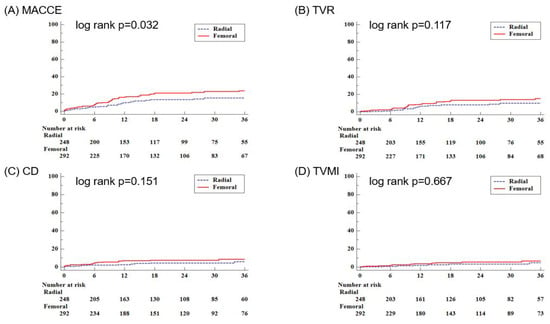
Figure 2.
The Kaplan–Meier curves for clinical outcomes during the follow-up period, including the primary outcome of MACCE and its components. CD = cardiac death; MACCE = major adverse cardiac and cerebrovascular events; TVMI = target vessel spontaneous myocardial infarction; TVR = target vessel revascularization.

Table 4.
Clinical outcomes of the TR versus the TF approach.
4. Discussion
The main findings of this study are as follows: (1) The TR approach demonstrated comparable outcomes to the TF approach in terms of MACCE. (2) In-hospital events and procedural outcomes showed no statistically significant differences between the two groups; however, in-hospital bleeding had a higher tendency in the TF approach group.
PCI performed via the TR approach has been shown to reduce vascular and bleeding complications while improving clinical outcomes in a wide range of patients with coronary artery disease compared to the TF approach [5,6]. Consequently, it has been recommended as the preferred access site in the most recent European guidelines on myocardial revascularization [7]. With the aging population, heavily calcified coronary lesions are becoming more common, resulting in a growing need for RA in these cases [4,15]. The TR approach is used less frequently in complex PCI procedures, such as those requiring RA, compared to the general PCI population, due to the need to accommodate larger guiding catheters and provide greater backup support [8,16]. However, the TF approach is associated with a higher risk of bleeding complications, a concern that is particularly pronounced in East Asian populations, where the risk of bleeding often outweighs the risk of ischemic events [13]. This elevated bleeding risk associated with the TF approach may be one of the factors contributing to hesitancy in adopting RA, despite its necessity for managing severely calcified lesions [17,18]. Confirming that the TR approach in RA does not result in significant differences in clinical outcomes would highlight its advantage in reducing bleeding risks. Furthermore, this finding suggests the possibility of the TR approach becoming a safe and efficient vascular access strategy in RA as well. In this context, distal radial access (DRA) is also gaining attention as a feasible and safe option for RA. Recent studies have demonstrated its applicability in complex procedures such as complex, high-risk indicated PCI, with potential advantages including reduced bleeding and enhanced patient comfort. Moreover, DRA may even facilitate radial artery occlusion recanalization, thus preserving long-term vascular access. While our registry did not specifically distinguish between conventional and distal radial access, this technique represents a promising advancement in access strategies for high-risk indicated PCI [19].
Our study demonstrated that performing RA via the TR approach resulted in clinical outcomes comparable to those of the TF approach, with a tendency for lower in-hospital bleeding. These findings support the broader use of TR with RA even in Asian populations. Similar results have also been reported in previous studies conducted in Western populations [20,21,22,23]. A meta-analysis demonstrated that the TR approach for RA of calcified native coronary lesions is associated with a lower incidence of access site bleeding, while achieving comparable procedural success, procedural time, all-cause mortality, and major adverse cardiac events compared to the TF approach [20]. Similarly, a study found that the TR approach was associated with a significantly lower risk of bleeding complications without compromising angiographic success or long-term efficacy compared to the TF approach [21]. Also, small single-center observational studies showed that the TR approach was associated with similar procedural success but lower risk for in-hospital bleeding and vascular complications compared to the TF approach [22,23]. Our findings are in line with those of previous studies.
In our study, the incidence of MACCE in the TF group was nearly twice as high as in the TR group. This may be explained by the higher prevalence of comorbidities in the TF group, including CKD, dialysis, PAD, and a previous CABG, as well as lower EF, a greater total number of stents, and longer stent lengths and procedural time. These findings suggest that the TF group may have had more severe lesions and included higher-risk patients. As a result, when statistically adjusted for these factors, no significant difference was observed between the two groups. Also, it is possible that the TR approach with RA was performed by operators with more experience in RA, a procedure known to be highly operator-dependent [4,24]. Additionally, changes in RA strategies and improvement of devices may have contributed to the lack of statistical differences in MACCE. Initially used for removing plaque bulk, RA has since transitioned into a technique for modifying plaque [25,26]. Thus, the utilization of 2.0 mm burrs, which require a 7 Fr guiding catheter, has significantly decreased. With the improvement of sheathless guiding catheters and slender sheaths that have smaller external diameters, rotational atherectomy using larger burr sizes can now be safely performed through the radial artery [27]. Thus, the utilization of 2.0 mm burrs, which require a 7 Fr guiding catheter, has significantly decreased. Even in cases where a 7 Fr catheter is required, performing RA with all burr sizes via the radial approach remains feasible [28]. In our study as well, there was no statistically significant difference in the burr sizes used between the TR and TF approaches (1.50 ± 0.20 vs. 1.50 ± 0.20, p = 0.367). Given these developments, the heterogeneity of atherosclerotic disease across different vascular beds is an important consideration. Recent studies have emphasized that plaque morphology, vulnerability, and clinical expression vary depending on the anatomical location of the lesion [29]. Such differences support the need for individualized interventional strategies in complex coronary artery disease. RA, as a lesion-specific tool, is particularly well suited to address these diverse pathophysiologic presentations.
In the case of bleeding events during RA, several previous studies comparing the TR and TF approaches have demonstrated that bleeding events were less frequent with the TR approach [21,30]. However, in our study, the total bleeding events did not differ between the two groups. This can be attributed to several reasons. There was a higher proportion of patients on direct oral anticoagulants (DOACs) in the TR group. Additionally, our study was conducted more recently compared to previous studies that demonstrated differences in bleeding events, during which advancements in closure devices have significantly improved hemostasis [31]. Similarly, increased physician experience with hemostasis, driven by the growing use of larger sheaths for procedures such as transcatheter aortic valve implantation, may have contributed. Lastly, our analysis included not only procedure-related bleeding events but also non-procedure-related bleeding events. In addition to bleeding outcomes, periprocedural MI is known to be associated with adverse outcomes and may be considered a potential risk during RA, particularly in complex coronary lesions. However, in our study, the incidence of periprocedural MI was low in both groups. This finding supports the safety of the RA technique even in the setting of complex coronary lesions [32].
This study has several limitations. The main limitation of this study is its retrospective design and the non-randomized selection of the approach sites. While multivariate Cox regression analysis was employed to minimize confounding variables, the possibility of residual confounders influencing the results cannot be fully excluded. Additionally, operator bias based on their prior experience with either the TR or TF approach may have affected the outcomes to some extent [33]. Furthermore, the relatively small sample size reduces the statistical power to detect significant differences. Lastly, the lack of follow-up evaluation, such as coronary angiography, may have led to an underestimation of post-procedure event rates.
Author Contributions
Conceptualization, K.K. and J.J.; methodology, J.J. and S.-H.H.; software, J.-H.J.; validation, K.K., J.J., S.-H.H. and K.L.; formal analysis, J.J. and S.-H.H.; investigation, K.K. and J.J.; resources, K.-D.Y., K.-W.M. and D.M.; data curation, S.-H.H., K.L., S.-N.L., W.-Y.J., I.-J.C., J.-H.L. (Jae-Hwan Lee), J.-H.L. (Jang-Hoon Lee), S.-W.L., K.-H.Y., H.-J.L. and S.-R.L.; writing—original draft preparation, K.K.; writing—review and editing, J.J. and K.L.; visualization, K.K.; supervision, J.J. and K.L.; project administration, K.K., J.J. and K.L.; funding acquisition, none. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (IRB) of Daejeon St. Mary’s Hospital (Approval Code: DC19REDI0066, approved on 30 July 2019).
Informed Consent Statement
Patient consent was waived due to the retrospective design of the study.
Data Availability Statement
Data are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Madhavan, M.V.; Tarigopula, M.; Mintz, G.S.; Maehara, A.; Stone, G.W.; Genereux, P. Coronary artery calcification: Pathogenesis and prognostic implications. J. Am. Coll. Cardiol. 2014, 63, 1703–1714. [Google Scholar] [CrossRef] [PubMed]
- Vavuranakis, M.; Toutouzas, K.; Stefanadis, C.; Chrisohou, C.; Markou, D.; Toutouzas, P. Stent deployment in calcified lesions: Can we overcome calcific restraint with high-pressure balloon inflations? Catheter. Cardiovasc. Interv. 2001, 52, 164–172. [Google Scholar] [CrossRef]
- Barbato, E.; Carrie, D.; Dardas, P.; Fajadet, J.; Gaul, G.; Haude, M.; Khashaba, A.; Koch, K.; Meyer-Gessner, M.; Palazuelos, J.; et al. European expert consensus on rotational atherectomy. EuroIntervention 2015, 11, 30–36. [Google Scholar] [CrossRef]
- Cavusoglu, E.; Kini, A.S.; Marmur, J.D.; Sharma, S.K. Current status of rotational atherectomy. Catheter. Cardiovasc. Interv. 2004, 62, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Jolly, S.S.; Yusuf, S.; Cairns, J.; Niemela, K.; Xavier, D.; Widimsky, P.; Budaj, A.; Niemela, M.; Valentin, V.; Lewis, B.S.; et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): A randomised, parallel group, multicentre trial. Lancet 2011, 377, 1409–1420. [Google Scholar] [CrossRef]
- Valgimigli, M.; Gagnor, A.; Calabro, P.; Frigoli, E.; Leonardi, S.; Zaro, T.; Rubartelli, P.; Briguori, C.; Ando, G.; Repetto, A.; et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: A randomised multicentre trial. Lancet 2015, 385, 2465–2476. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Sharma, S.K.; Tomey, M.I.; Teirstein, P.S.; Kini, A.S.; Reitman, A.B.; Lee, A.C.; Genereux, P.; Chambers, J.W.; Grines, C.L.; Himmelstein, S.I.; et al. North American Expert Review of Rotational Atherectomy. Circ. Cardiovasc. Interv. 2019, 12, e007448. [Google Scholar] [CrossRef]
- Mota, P.; de Belder, A.; Leitao-Marques, A. Rotational atherectomy: Technical update. Rev. Port. Cardiol. 2015, 34, 271–278. [Google Scholar] [CrossRef]
- Kotowycz, M.A.; Khan, S.Q.; Freixa, X.; Ivanov, J.; Seidelin, P.H.; Overgaard, C.B.; Dzavik, V. Rotational atherectomy through the radial artery is associated with similar procedural success when compared with the transfemoral route. Coron. Artery Dis. 2015, 26, 254–258. [Google Scholar] [CrossRef]
- Watt, J.; Austin, D.; Mackay, D.; Nolan, J.; Oldroyd, K.G. Radial Versus Femoral Access for Rotational Atherectomy: A UK Observational Study of 8622 Patients. Circ. Cardiovasc. Interv. 2017, 10, e005311. [Google Scholar] [CrossRef]
- Watt, J.; Oldroyd, K.G. Radial versus femoral approach for high-speed rotational atherectomy. Catheter. Cardiovasc. Interv. 2009, 74, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Iijima, R. Implications and characteristics of high bleeding risk in East Asian patients undergoing percutaneous coronary intervention: Start with what is right rather than what is acceptable. J. Cardiol. 2021, 78, 91–98. [Google Scholar] [CrossRef]
- Lee, K.; Jung, J.H.; Lee, M.; Kim, D.W.; Park, M.W.; Choi, I.J.; Lee, J.H.; Lee, J.H.; Lee, S.R.; Lee, P.H.; et al. Clinical Outcome of Rotational Atherectomy in Calcified Lesions in Korea-ROCK Registry. Medicina 2021, 57, 694. [Google Scholar] [CrossRef] [PubMed]
- Camnitz, W.M.; Keeley, E.C. Heavily calcified coronary arteries: The bane of an interventionalist’s existence. J. Interv. Cardiol. 2010, 23, 254–255. [Google Scholar] [CrossRef]
- Kinnaird, T.; Cockburn, J.; Gallagher, S.; Choudhury, A.; Sirker, A.; Ludman, P.; de Belder, M.; Copt, S.; Mamas, M.; de Belder, A. Temporal changes in radial access use, associates and outcomes in patients undergoing PCI using rotational atherectomy between 2007 and 2014: Results from the British Cardiovascular Intervention Society national database. Am. Heart J. 2018, 198, 46–54. [Google Scholar] [CrossRef]
- Ramsdale, D.R.; Morris, J.L. If Rotablator is useful, why don’t we use it? Heart 1997, 78 (Suppl. 2), 36–37. [Google Scholar] [CrossRef] [PubMed]
- Genereux, P.; Madhavan, M.V.; Mintz, G.S.; Maehara, A.; Kirtane, A.J.; Palmerini, T.; Tarigopula, M.; McAndrew, T.; Lansky, A.J.; Mehran, R.; et al. Relation between coronary calcium and major bleeding after percutaneous coronary intervention in acute coronary syndromes (from the Acute Catheterization and Urgent Intervention Triage Strategy and Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction Trials). Am. J. Cardiol. 2014, 113, 930–935. [Google Scholar]
- Achim, A.; Kakonyi, K.; Jambrik, Z.; Olajos, D.; Nemes, A.; Bertrand, O.F.; Ruzsa, Z. Distal Radial Artery Access for Recanalization of Radial Artery Occlusion and Repeat Intervention: A Single Center Experience. J. Clin. Med. 2022, 11, 6916. [Google Scholar] [CrossRef]
- Khan, A.A.; Panchal, H.B.; Zaidi, S.I.M.; Papireddy, M.R.; Mukherjee, D.; Cohen, M.G.; Banerjee, S.; Rao, S.V.; Pancholy, S.; Paul, T.K. Safety and efficacy of radial versus femoral access for rotational Atherectomy: A systematic review and meta-analysis. Cardiovasc. Revasc. Med. 2019, 20, 241–247. [Google Scholar] [CrossRef]
- Desta, L.; Jurga, J.; Volz, S.; Omerovic, E.; Ulvenstam, A.; Zwackman, S.; Pagonis, C.; Calle, F.; Olivecrona, G.K.; Persson, J.; et al. Transradial versus trans-femoral access site in high-speed rotational atherectomy in Sweden. Int. J. Cardiol. 2022, 352, 45–51. [Google Scholar] [CrossRef]
- Kubler, P.; Zimoch, W.; Kosowski, M.; Tomasiewicz, B.; Telichowski, A.; Reczuch, K. In patients undergoing percutaneous coronary intervention with rotational atherectomy radial access is safer and as efficient as femoral access. J. Interv. Cardiol. 2018, 31, 471–477. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Wu, X.Q.; Ye, F.; Chen, S.L. Advantages of Transradial Rotational Atherectomy versus Transfemoral Approach in Elderly Patients with Hard-Handling Calcified Coronary Lesions—A Single Center Experience. Acta Cardiol. Sin. 2018, 34, 464–471. [Google Scholar] [PubMed]
- Kim, J.Y.; Yoon, J. Transradial approach as a default route in coronary artery interventions. Korean Circ. J. 2011, 41, 1–8. [Google Scholar] [CrossRef]
- Whitlow, P.L.; Bass, T.A.; Kipperman, R.M.; Sharaf, B.L.; Ho, K.K.; Cutlip, D.E.; Zhang, Y.; Kuntz, R.E.; Williams, D.O.; Lasorda, D.M.; et al. Results of the study to determine rotablator and transluminal angioplasty strategy (STRATAS). Am. J. Cardiol. 2001, 87, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Safian, R.D.; Feldman, T.; Muller, D.W.; Mason, D.; Schreiber, T.; Haik, B.; Mooney, M.; O’Neill, W.W. Coronary angioplasty and Rotablator atherectomy trial (CARAT): Immediate and late results of a prospective multicenter randomized trial. Catheter. Cardiovasc. Interv. 2001, 53, 213–220. [Google Scholar] [CrossRef]
- Allali, A.; Abdel-Wahab, M.; Elbasha, K.; Mankerious, N.; Traboulsi, H.; Kastrati, A.; El-Mawardy, M.; Hemetsberger, R.; Sulimov, D.S.; Neumann, F.J.; et al. Rotational atherectomy of calcified coronary lesions: Current practice and insights from two randomized trials. Clin. Res. Cardiol. 2023, 112, 1143–1163. [Google Scholar] [CrossRef]
- Kassimis, G.; Patel, N.; Kharbanda, R.K.; Channon, K.M.; Banning, A.P. High-speed rotational atherectomy using the radial artery approach and a sheathless guide: A single-centre comparison with the “conventional” femoral approach. EuroIntervention 2014, 10, 694–699. [Google Scholar] [CrossRef]
- Achim, A.; Peter, O.A.; Cocoi, M.; Serban, A.; Mot, S.; Dadarlat-Pop, A.; Nemes, A.; Ruzsa, Z. Correlation between Coronary Artery Disease with Other Arterial Systems: Similar, Albeit Separate, Underlying Pathophysiologic Mechanisms. J. Cardiovasc. Dev. Dis. 2023, 10, 210. [Google Scholar] [CrossRef]
- Yin, W.H.; Tseng, C.K.; Tsao, T.P.; Jen, H.L.; Huang, W.P.; Huang, C.L.; Wang, J.J.; Young, M.S. Transradial versus transfemoral rotablation for heavily calcified coronary lesions in contemporary drug-eluting stent era. J. Geriatr. Cardiol. 2015, 12, 489–496. [Google Scholar]
- Sheth, R.A.; Walker, T.G.; Saad, W.E.; Dariushnia, S.R.; Ganguli, S.; Hogan, M.J.; Hohenwalter, E.J.; Kalva, S.P.; Rajan, D.K.; Stokes, L.S.; et al. Quality improvement guidelines for vascular access and closure device use. J. Vasc. Interv. Radiol. 2014, 25, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Armillotta, M.; Bergamaschi, L.; Paolisso, P.; Belmonte, M.; Angeli, F.; Sansonetti, A.; Stefanizzi, A.; Bertolini, D.; Bodega, F.; Amicone, S.; et al. Prognostic Relevance of Type 4a Myocardial Infarction and Periprocedural Myocardial Injury in Patients with Non-ST-Segment-Elevation Myocardial Infarction. Circulation 2025, 151, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, R.A.; Townsend, J.C.; Bonnema, D.D.; Patel, C.A.; Gibbons, M.T.; Todoran, T.M.; Nielsen, C.D.; Powers, E.R.; Steinberg, D.H. Comparison of percutaneous coronary intervention safety before and during the establishment of a transradial program at a teaching hospital. Am. J. Cardiol. 2012, 109, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).