Prostate Cancer—Research Advances in Early Detection
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. International Agency for Research on Cancer. Available online: https://gco.iarc.who.int/media/globocan/factsheets/cancers/27-prostate-fact-sheet.pdf (accessed on 31 March 2025).
- European Commission. Prostate Cancer Burden in EU-27. Available online: https://ecis.jrc.ec.europa.eu/sites/default/files/2023-12/prostate_cancer_En-Nov_2021.pdf (accessed on 31 March 2025).
- American Cancer Society. Prostate Cancer Risk Factors. Available online: https://www.cancer.org/cancer/types/prostate-cancer/causes-risks-prevention/risk-factors.html (accessed on 31 March 2025).
- Al-Fayez, S.; El-Metwally, A. Cigarette smoking and prostate cancer: A systematic review and meta-analysis of prospective cohort studies. Tob. Induc. Dis. 2023, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Khil, H.; Lee, D.H.; Keum, N.; Giovannucci, E.L. Alcohol Consumption and the Risk of Prostate Cancer: A Dose-Response Meta-Analysis. Nutrients 2020, 12, 2188. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force. Prostate Cancer: Screening. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/prostate-cancer-screening (accessed on 31 March 2025).
- Available online: https://uroweb.org/guidelines/prostate-cancer/chapter/diagnostic-evaluation (accessed on 31 March 2025).
- Available online: https://www.annalsofoncology.org/article/S0923-7534(20)39898-7/fulltext (accessed on 31 March 2025).
- Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/prostate-cancer/prostate-cancer-prevention (accessed on 31 March 2025).
- Available online: https://clinicaltrials.gov/ (accessed on 31 March 2025).
- Fudali, K..; Sagan, K.; Kwiatkowska, E.; Kosendiak, A. The impact of the early period of the COVID-19 pandemic on screening programmes of breast, colorectal and cervical cancer. J. Health Inequalities 2023, 9, 37–42. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/study/NCT04782713?titles=Smart%20Prostate%20Specific%20Antigen%20(PSA)%20Screening%20Study%20&rank=1 (accessed on 31 March 2025).
- Available online: https://clinicaltrials.gov/study/NCT04601272?titles=Evaluating%20the%20Shared%20Decision%20Making%20Process%20Scale%20in%20Cancer%20Screening%20Decisions%20&rank=1 (accessed on 31 March 2025).
- Available online: https://clinicaltrials.gov/study/NCT03726320?titles=Trial%20of%20Community%20Health%20Worker-led%20Decision%20Coaching%20&rank=1 (accessed on 31 March 2025).
- Available online: https://clinicaltrials.gov/study/NCT03869216?titles=Fostering%20Shared%20Decision-making%20About%20Prostate%20Cancer%20Screening%20&rank=1 (accessed on 31 March 2025).
- Available online: https://clinicaltrials.gov/study/NCT05886751?titles=Randomized%20Trial%20of%20Trust%20in%20Online%20Videos%20About%20Prostate%20Cancer%20&rank=1 (accessed on 31 March 2025).
- Available online: https://clinicaltrials.gov/study/NCT05331638?titles=Prostate%20Cancer%20Genius%20App%20Education%20and%20Home-based%20PSA%20Screening%20for%20African%20American%20Men%20&rank=1 (accessed on 31 March 2025).
- Available online: https://clinicaltrials.gov/study/NCT05011799?titles=The%20Peer%20Genetic%20Study%20&rank=1 (accessed on 31 March 2025).
- Available online: https://clinicaltrials.gov/study/NCT04902144?titles=Clinical%20Outcomes%20for%20Offering%20Genetic%20Testing%20in%20a%20Tiered%20Approach%20&rank=1 (accessed on 31 March 2025).
- Available online: https://clinicaltrials.gov/study/NCT04763824?titles=KanSurvive:%20Testing%20a%20&rank=1 (accessed on 31 March 2025).
- Available online: https://clinicaltrials.gov/study/NCT04726319?titles=Family%20History%20App%20in%20Personalized%20Medicine%20&rank=1 (accessed on 31 March 2025).
- Available online: https://clinicaltrials.gov/study/NCT04000880?titles=Adapting%20Multiple%20Behavior%20Interventions%20&rank=1 (accessed on 31 March 2025).
- Available online: https://clinicaltrials.gov/study/NCT03749993?titles=Evaluation%20of%20a%20MRI-based%20Prostate%20Cancer%20&rank=1 (accessed on 31 March 2025).
- Available online: https://clinicaltrials.gov/study/NCT04063566?titles=ReIMAGINE%20Prostate%20Cancer%20Screening%20&rank=1 (accessed on 31 March 2025).
- Available online: https://clinicaltrials.gov/study/NCT04175730?titles=Prostate%20Cancer%20Detection%20Screening%20MRI%20Protocol%20&rank=1 (accessed on 31 March 2025).
- Available online: https://clinicaltrials.gov/study/NCT03857477?titles=The%20BARCODE%201%20Study%20&rank=2 (accessed on 31 March 2025).
- Available online: https://clinicaltrials.gov/study/NCT05055843?titles=Novel%20Synthetic%20T2W%20MR%20Imaging%20and%20&rank=1 (accessed on 31 March 2025).
- Available online: https://clinicaltrials.gov/study/NCT05547386?titles=68Ga-PSMA-11%20PET%2FCT%20Screening%20Prior%20to%20177Lu-PSMA-617%20&rank=1 (accessed on 31 March 2025).
- Available online: https://clinicaltrials.gov/study/NCT04843566?titles=PReclude%20Infection%20EVEnts%20With%20No%20Prophylaxis%20Transperineal%20Biopsy%20&rank=1 (accessed on 31 March 2025).
- Available online: https://clinicaltrials.gov/study/NCT04124900?titles=Telomere%20Associated%20Variables&rank=1 (accessed on 31 March 2025).
- Available online: https://clinicaltrials.gov/study/NCT03429244?titles=PSMA-PET%20for%20Biopsy%20and%20Treatment%20Guidance%20in%20Primary%20Prostate%20Cancer%20&rank=1 (accessed on 31 March 2025).
- Available online: https://ctv.veeva.com/study/al18f-psma137-pet-ct-imaging-for-psma-positive-cancer-patients (accessed on 31 March 2025).
- Available online: https://clinicaltrials.gov/study/NCT04176497?titles=PSMA-PET%2FMRI%20Unfavorable-Risk%20Target%20Volume%20Pilot%20Study%20&rank=1 (accessed on 31 March 2025).
- Available online: https://clinicaltrials.gov/study/NCT03927521?titles=Feasibility%20Study%20on%20the%20Use%20of%20PET&rank=2 (accessed on 31 March 2025).
- Available online: https://clinicaltrials.gov/study/NCT04583072?titles=Stockholm3%20Validation%20Study%20in%20a%20Multi-Ethnic%20&rank=1 (accessed on 31 March 2025).
- Available online: https://clinicaltrials.gov/study/NCT04034095?titles=Hormone-na%C3%AFve%20Prostate%20Cancer%20in%20Japan%20&rank=1 (accessed on 31 March 2025).
- Available online: https://clinicaltrials.gov/study/NCT04067960?titles=Pharmacogenomics%20Testing%20in%20the%20Optimal%20Use%20of%20Supportive%20Care%20Medications%20&rank=1 (accessed on 31 March 2025).
- Carlsson, S.V.; Lilja, H. Perspective on Prostate Cancer Screening. Clin. Chem. 2019, 65, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.C. A discussion on controversies and ethical dilemmas in prostate cancer screening. J. Med. Ethics 2021, 47, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Gulati, R.; Psutka, S.P.; Etzioni, R. Personalized Risks of Over Diagnosis for Screen Detected Prostate Cancer Incorporating Patient Comorbidities: Estimation and Communication. J. Urol. 2019, 202, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Vilson, F.L.; Li, S.; Brooks, J.D.; Eisenberg, M.L. Sudden PSA rise to ≥20 ng/ml and prostate cancer diagnosis in the United States: A population-based study. Prostate 2020, 80, 1438–1443. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Cubero, M.J.; Arance, E.; de Santiago, E.; Sanchez, P.; Sepúlveda, M.R.; Marrero, R.; Lorente, J.A.; Gonzalez-Cabezuelo, J.M.; Cuenca-Lopez, S.; Cozar, J.M.; et al. Follow-Up Biomarkers in the Evolution of Prostate Cancer, Levels of S100A4 as a Detector in Plasma. Int. J. Mol. Sci. 2023, 24, 547. [Google Scholar] [CrossRef]
- Díaz-Fernández, A.; Ryø Jochumsen, M.; Christensen, N.L.; Dalsgaard Sørensen, K.; Bouchelouche, K.; Borre, M.; Holm Vendelbo, M.; Ferapontova, E.E. Liquid-Biopsy Glycan Score Biomarker Accurately Indicates and Stratifies Primary and Metastatic Prostate Cancers. Anal. Chem. 2024, 96, 18815–18823. [Google Scholar] [CrossRef]
- Launer, B.M.; Ellis, T.A.; Scarpato, K.R. A contemporary review: mpMRI in prostate cancer screening and diagnosis. Urol. Oncol. 2025, 43, 15–22. [Google Scholar] [CrossRef]
- Hao, S.; Karlsson, A.; Heintz, E.; Elfström, K.M.; Nordström, T.; Clements, M. Cost-Effectiveness of Magnetic Resonance Imaging in Prostate Cancer Screening: A Microsimulation Study. Value Health 2021, 24, 1763–1772. [Google Scholar] [CrossRef] [PubMed]
- Callender, T.; Emberton, M.; Morris, S.; Pharoah, P.D.P.; Pashayan, N. Benefit, Harm, and Cost-effectiveness Associated with Magnetic Resonance Imaging Before Biopsy in Age-based and Risk-stratified Screening for Prostate Cancer. JAMA Netw. Open 2021, 4, e2037657. [Google Scholar] [CrossRef] [PubMed]
- McCaffery, K.; Nickel, B.; Pickles, K.; Moynihan, R.; Kramer, B.; Barratt, A.; Hersch, J. Resisting recommended treatment for prostate cancer: A qualitative analysis of the lived experience of possible overdiagnosis. BMJ Open 2019, 9, e026960. [Google Scholar] [CrossRef] [PubMed]
- Byrne, L.; Toland, A.E. Polygenic Risk Scores in Prostate Cancer Risk Assessment and Screening. Urol. Clin. N. Am. 2021, 48, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, E.J.; Joffe, S. Ethical Issues in Cancer Genetics. In Holland-Frei Cancer Medicine, 6th ed.; Kufe, D.W., Pollock, R.E., Weichselbaum, R.R., Bast, R.C., Jr., Gansler, T.S., Holland, J.F., Frei, E., Eds.; BC Decker: Hamilton, ON, Canada, 2003. Available online: https://www.ncbi.nlm.nih.gov/books/NBK13460/ (accessed on 5 March 2025).
- Dierks, T.; Heijnsdijk, E.A.M.; Korfage, I.J.; Roobol, M.J.; de Koning, H.J. Informed decision-making based on a leaflet in the context of prostate cancer screening. Patient Educ. Couns 2019, 102, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Drake, B.F.; Lewis-Thames, M.W.; Brown, A.; Rancilio, D.; Hicks, V. An Evaluation of Follow-Up Activities of Participants from an Urban Prostate Cancer Screening Event. Am. J. Men′s Health 2019, 13, 1557988319844353. [Google Scholar] [CrossRef] [PubMed]
- Owens, O.L.; Wooten, N.R.; Tavakoli, A.S. Adaptation and Initial Psychometric Evaluation of an Informed Prostate Cancer Screening Decision Self-Efficacy Scale for African-American Men. J. Racial Ethn. Health Disparities 2020, 7, 746–759. [Google Scholar] [CrossRef] [PubMed]
- Fine, A. Marking a Milestone: The Modernized ClinicalTrials.gov Becomes the Singular Website Experience 2023. Available online: https://nexus.od.nih.gov/all/2024/06/06/marking-a-milestone-the-modernized-clinicaltrials-gov-becomes-the-singular-website-experience/ (accessed on 31 March 2025).
- Tse, T.; Fain, K.M.; Zarin, D.A. How to avoid common problems when using ClinicalTrials.gov in research: 10 issues to consider. BMJ 2018, 361, k1452. [Google Scholar] [CrossRef] [PubMed]
- Trends and Charts on Registered Studies. Available online: https://clinicaltrials.gov/about-site/trends-charts (accessed on 31 March 2025).
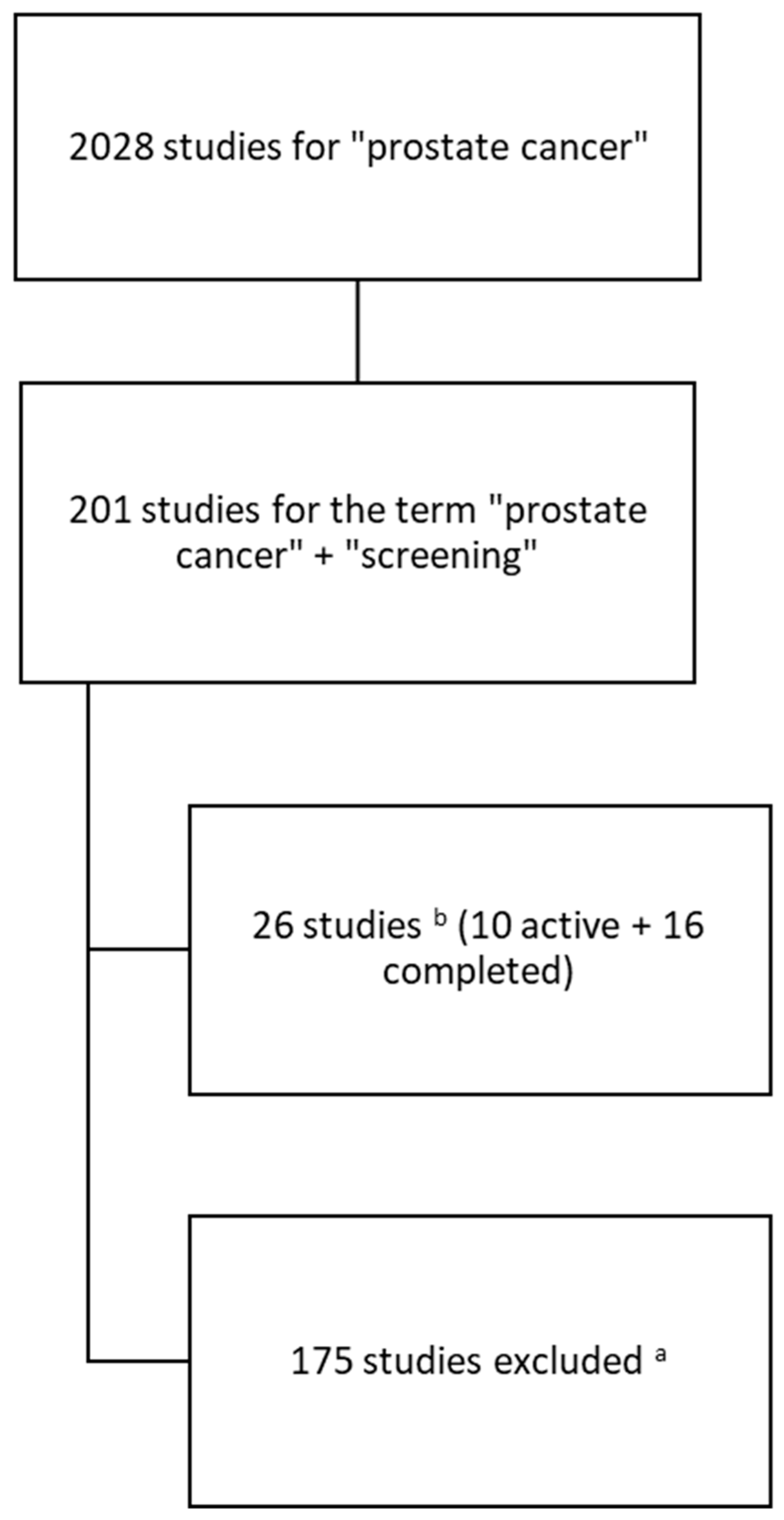
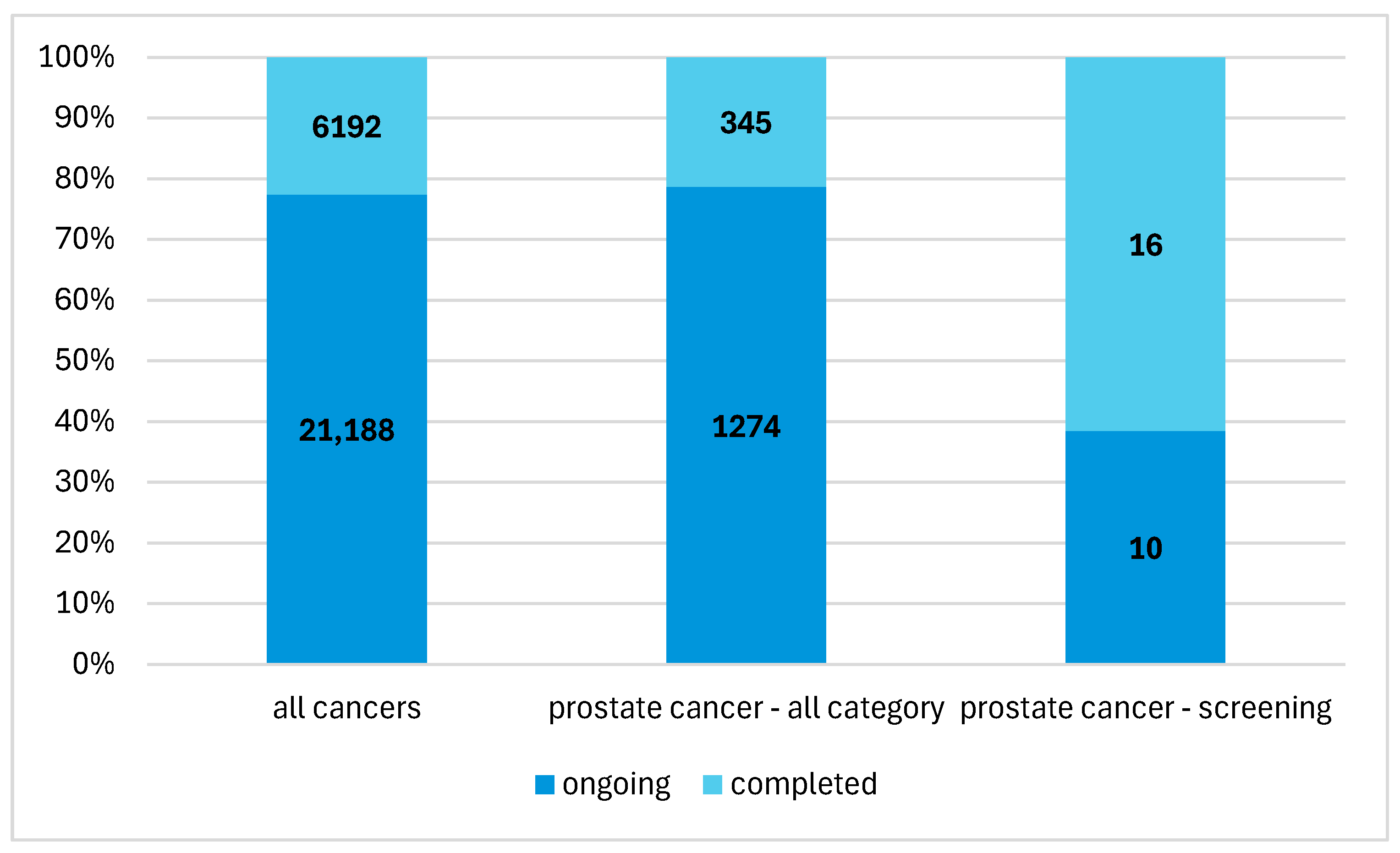
| No. | Group | Title | Years | n | Status | Study | Population |
|---|---|---|---|---|---|---|---|
| 1. | Behavioral | Smart Prostate Specific Antigen (PSA) Screening Study [12] | 2021–2024 | 49 | active | interventional | adult men PSA (\>4.0 ng/mL) |
| 2. | interventions | Evaluating the Shared Decision Making Process Scale in Cancer Screening Decisions [13] | 2020–2021 | 240 | completed | observational | men aged 45–74 |
| 3. | Trial of Community Health Worker-led Decision Coaching [14] | 2019–2023 | 162 | completed | interventional | adult men of African American origin | |
| 4. | Fostering Shared Decision-making About Prostate Cancer Screening Among Clinicians and African American Men [15] | 2020–2023 | 161 | completed | interventional | adult men of African American origin aged at least 40 | |
| 5. | Randomized Trial of Trust in Online Videos About Prostate Cancer [16] | 2021–2022 | 3649 | completed | interventional | adults aged at least 40 | |
| 6. | Prostate Cancer Genius App Education and Home-based PSA Screening for African American Men [17] | 2023–2025 | 80 | active | interventional | adult men of African American origin aged 55–69 | |
| 7. | The Peer Genetic Study [18] | 2020–2024 | 149 | active | interventional | adult men of African American origin aged 35–69 | |
| 8. | Clinical Outcomes for Offering Genetic Testing in a Tiered Approach [19] | 2020–2021 | 6 | completed | interventional | adults aged 18–65 | |
| 9. | KanSurvive: Testing a Model for Improving Cancer Survivorship Care in Rural Practice [20] | 2020–2023 | 267 | completed | interventional | adults aged 18–75 | |
| 10. | Family History App in Personalized Medicine [21] | 2021–2024 | 627 | active | interventional | adults aged 30–69 | |
| 11. | Adapting Multiple Behavior Interventions That Effectively Improve Cancer Survivor Health [22] | 2020–2024 | 603 | active | interventional | people aged at least 50 | |
| 12. | Diagnostic | Evaluation of an MRI-based Prostate Cancer Screening Program [23] | 2019–2023 | 241 | completed | interventional | adult men |
| 13. | tests | ReIMAGINE Prostate Cancer Screening [24] | 2019–2021 | 309 | completed | interventional | adult men |
| 14. | Prostate Cancer Detection Screening MRI Protocol [25] | 2019–2020 | 48 | completed | interventional | men aged 18–80 | |
| 15. | The BARCODE 1 Study (Full Study): The Use of Genetic Profiling to Guide Prostate Cancer Targeted Screening [26] | 2019–2028 | 4700 | active | observational | men aged 55–69 | |
| 16. | Novel Synthetic T2W MR Imaging and Spin Parameter Mapping Techniques for Screening Prostate Cancer [27] | 2021–2025 | 33 | active | interventional | men aged at least 18 | |
| 17. | 68Ga-PSMA-11 PET/CT Screening Prior to 177Lu-PSMA-617 Therapy for Patients With Metastatic Castrate Resistant Prostate Cancer [28] | 2022–2023 | 163 | completed | interventional | men aged at least 18 | |
| 18. | PReclude Infection EVEnts With No Prophylaxis Transperineal Biopsy [29] | 2021–2024 | 738 | completed | interventional | men aged at least 18 | |
| 19. | Telomere Associated Variables (TAVs) in Prostate Cancer [30] | 2019–2023 | 509 | completed | observational | adult men referred for a biopsy based on PSA test results | |
| 20. | PSMA-PET for Biopsy and Treatment Guidance in Primary Prostate Cancer [31] | 2019–2022 | 36 | completed | interventional | men aged at least 18 | |
| 21. | [Al18F] PSMA137 PET/CT Imaging for PSMA-Positive Cancer Patients [32] | 2021–2024 | 20 | active | interventional | men aged at least 18 | |
| 22. | PSMA-PET/MRI Unfavorable-Risk Target Volume Pilot Study [33] | 2020–2028 | 9 | active | interventional | men aged at least 18 | |
| 23. | Feasibility Study on the Use of PET-MRI/68Ga-PSMA Imaging for HIFU-focal Treatment in the Event of Recurrent Prostate Cancer After Radiotherapy—a PSMA Study [34] | 2020–2020 | 11 | completed | interventional | men aged at least 50 | |
| 24. | Stockholm3 Validation Study in a Multi-Ethnic Cohort [35] | 2019–2023 | 2152 | completed | observational | men aged 45–75 | |
| 25. | A Registry Study to Observe Clinical Outcomes of Participants With High-risk Metastatic Hormone-naïve Prostate Cancer in Japan [36] | 2019–2025 | 979 | active | observational | men aged at least 20 with diagnosed hormone-dependent prostate cancer | |
| 26. | Pharmacogenomics Testing in the Optimal Use of Supportive Care Medications in Stage III-IV Cancer [37] | 2019–2022 | 197 | completed | interventional | patients in stage III or IV of cancer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czerw, A.; Deptała, A.; Głowacka, M.; Partyka, O.; Pajewska, M.; Czerw, N.; Badowska-Kozakiewicz, A.; Sygit, K.; Kopczyński, Z.; Czarnywojtek, P.; et al. Prostate Cancer—Research Advances in Early Detection. J. Clin. Med. 2025, 14, 3067. https://doi.org/10.3390/jcm14093067
Czerw A, Deptała A, Głowacka M, Partyka O, Pajewska M, Czerw N, Badowska-Kozakiewicz A, Sygit K, Kopczyński Z, Czarnywojtek P, et al. Prostate Cancer—Research Advances in Early Detection. Journal of Clinical Medicine. 2025; 14(9):3067. https://doi.org/10.3390/jcm14093067
Chicago/Turabian StyleCzerw, Aleksandra, Andrzej Deptała, Mariola Głowacka, Olga Partyka, Monika Pajewska, Natalia Czerw, Anna Badowska-Kozakiewicz, Katarzyna Sygit, Zygmunt Kopczyński, Piotr Czarnywojtek, and et al. 2025. "Prostate Cancer—Research Advances in Early Detection" Journal of Clinical Medicine 14, no. 9: 3067. https://doi.org/10.3390/jcm14093067
APA StyleCzerw, A., Deptała, A., Głowacka, M., Partyka, O., Pajewska, M., Czerw, N., Badowska-Kozakiewicz, A., Sygit, K., Kopczyński, Z., Czarnywojtek, P., Gąska, I., Kaczmarski, M., Banaś, T., Grochans, E., Grochans, S., Cybulska, A. M., Schneider-Matyka, D., Bandurska, E., Ciećko, W., ... Kozlowski, R. (2025). Prostate Cancer—Research Advances in Early Detection. Journal of Clinical Medicine, 14(9), 3067. https://doi.org/10.3390/jcm14093067










