The Impact of the Histologic Types of Lung Cancer on CBC-Derived Inflammatory Markers—Current Knowledge and Future Perspectives
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Patients Included in the Study
2.2. Definition of Analyzed Parameters
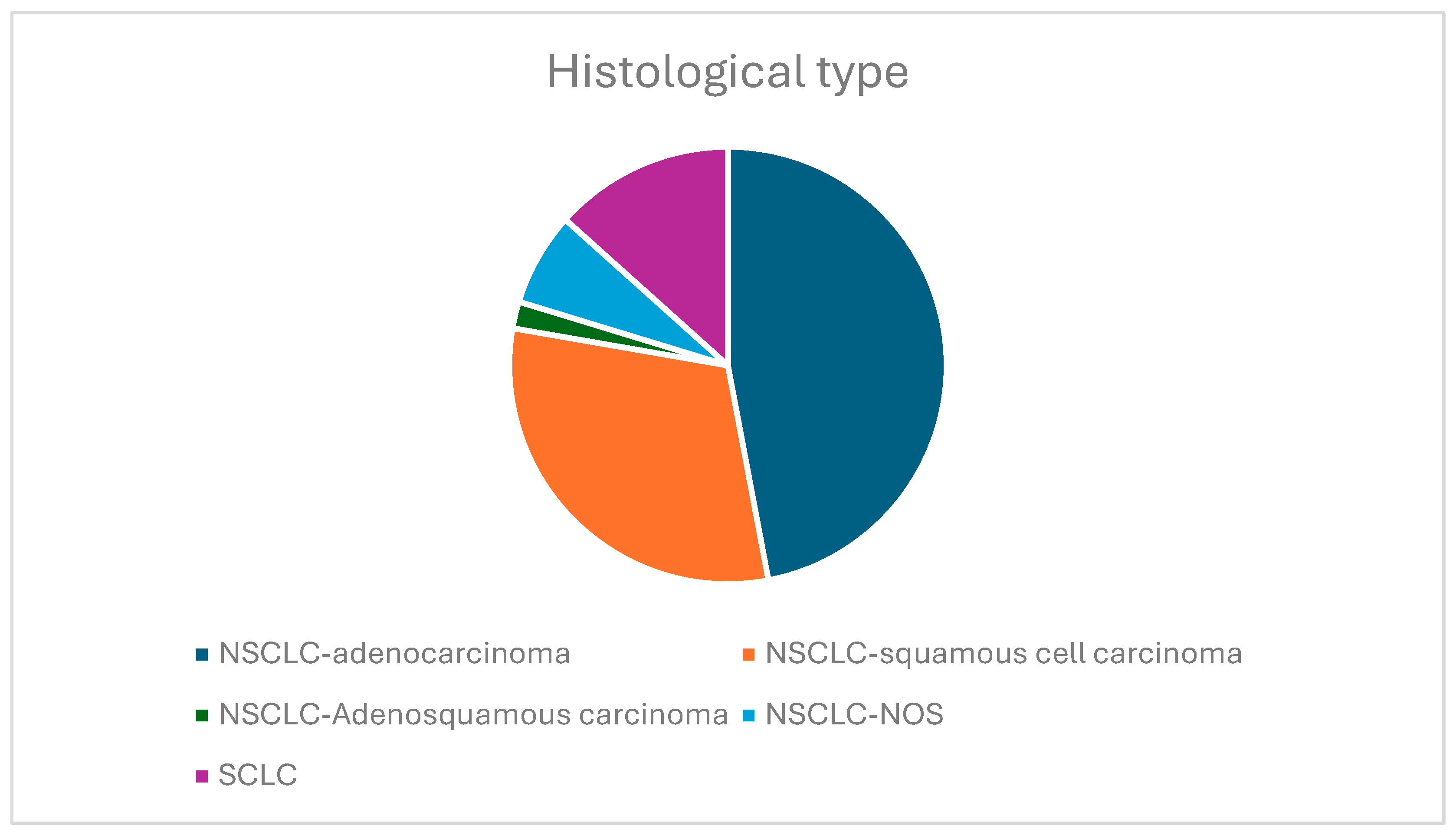
- The histological type of lung carcinoma: NSCLC (adenocarcinoma, squamous cell carcinoma, adenosquamous carcinoma, and NSCLC not otherwise specified (NOS)) and SCLC.Biopsies are needed to identify the histological type of the tumor. In our study group, tissue biopsies were performed, as described below. The primary method used was the transbronchial biopsy (TBLB), using a flexible bronchoscope via the transoral route for central tumors. For tumors that could not be accessed with the aid of bronchoscopy, a percutaneous transthoracic lung biopsy (PTLB), in which a needle was inserted through the patient’s chest wall with the assistance of CT guidance in the suspected area, was also performed for our study population.
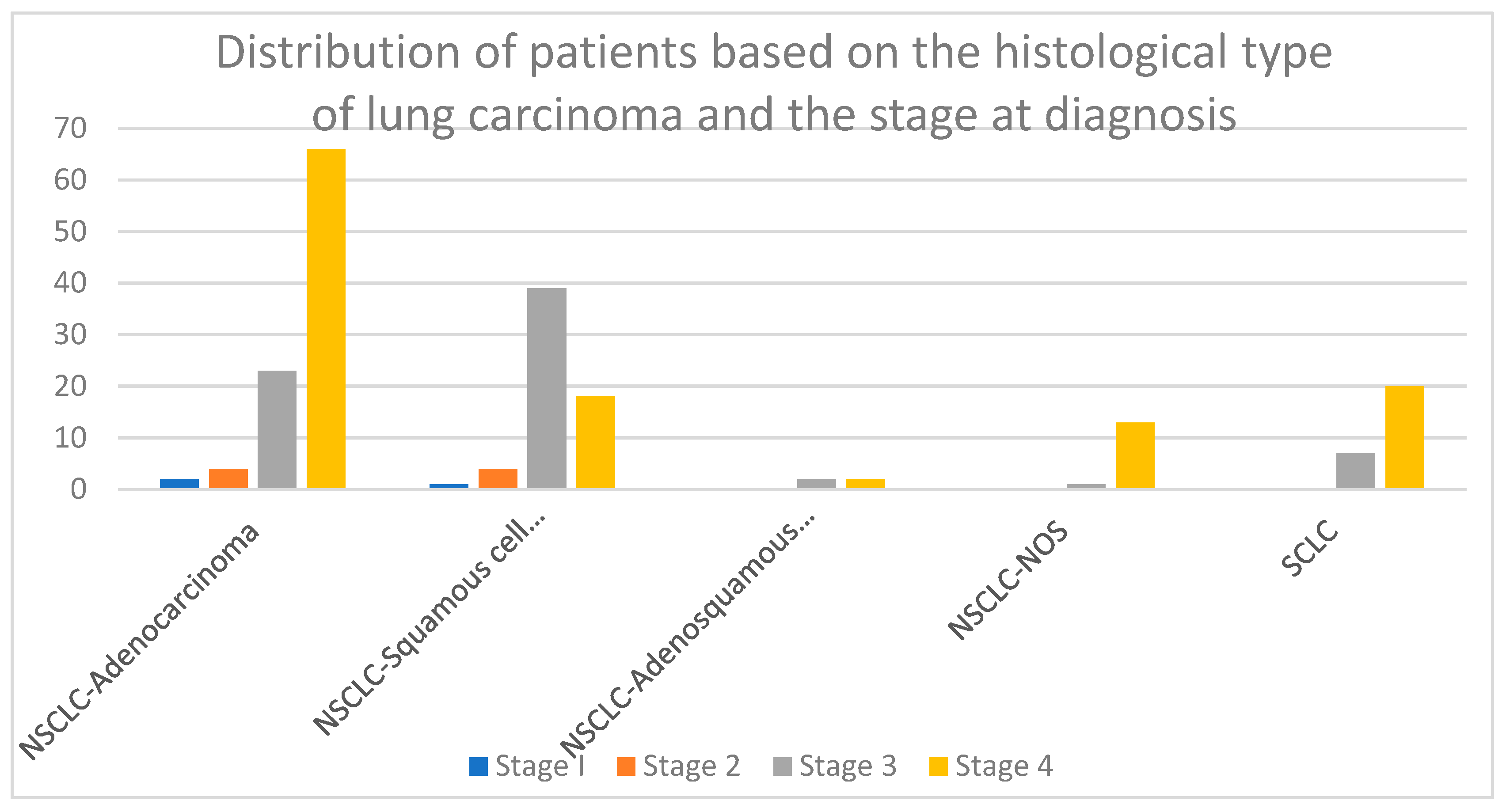
- The stage of the tumor at diagnosis: The patients’ tumoral stage at diagnosis was established taking into consideration the TNM classification of malignant tumors, where T describes the primary tumor site and size, N describes the involvement of the regional lymph nodes, and M describes the presence of distant metastasis. The 8th edition of the TNM grading system was used for the proper tumor staging of the study population, as it is the latest version published in the scientific literature and provides the best characterization of the tumor staging [26].
- Parameters derived from the complete blood count (CBC) of the patients (neutrophils count, lymphocytes count, leucocytes count, monocytes count, platelets count, and eosinophils count)
- The following CBC-derived inflammatory indexes: Neutrophil-to-lymphocyte ratio (NLR); derived neutrophil-to-lymphocyte ratio (d-NLR); monocyte-to-lymphocyte ratio (MLR); platelet-to-lymphocyte ratio (PLR); eosinophil-to-neutrophil ratio (ENR); eosinophil-to-monocyte ratio (EMR); systemic inflammatory index (SII); systemic inflammatory response index (SIRI); and aggregate index of systemic inflammation (AISI). The detailed formulas of the included CBC-derived inflammatory indexes are displayed in Table 1.
- Data regarding the living environment (urban/rural), the gender, the age of the patients at diagnosis, exposure to tobacco smoke, and the presence of COPD as a comorbidity for the current disease
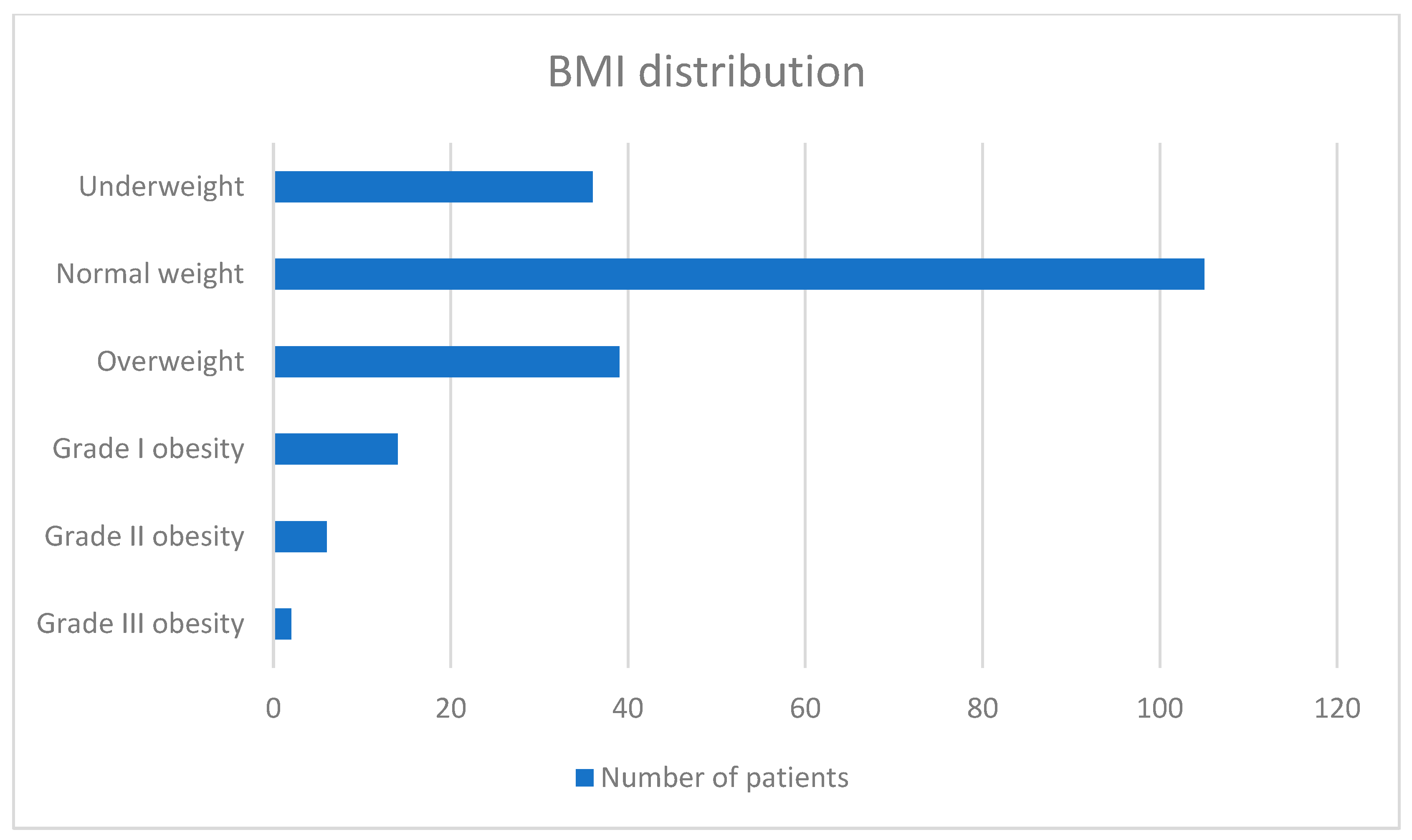
- BMI was calculated using the following formula: BMI = kg/m2. Based on BMI, patients were classified as underweight (BMI < 18.5 kg/m2), normal weight (BMI between 18.5 and 24.99 kg/m2), overweight (BMI between 25 and 29.99 kg/m2), grade I obesity (BMI between 30 and 34.99 kg/m2), grade II obesity (BMI between 35 and 39.99 kg/m2), and grade III obesity (BMI > 40 kg/m2).
2.3. Statistical Analysis of Data
3. Results
3.1. General Characteristics of the Study Population
3.2. Histological Types of Lung Carcinoma and Stage at Diagnosis
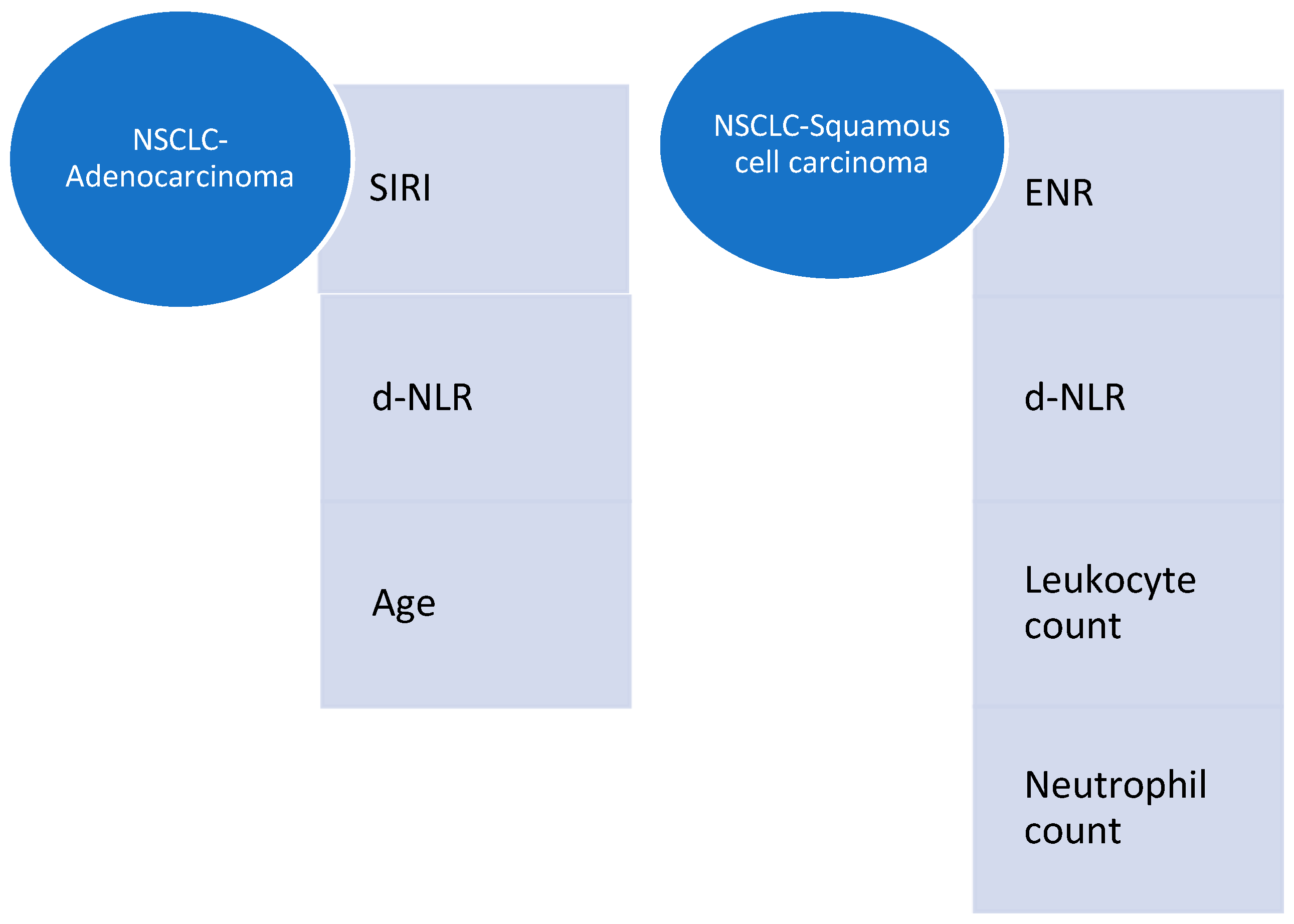
3.3. CBC-Derived Predictors of Severity in Lung Cancer
3.4. A Summary of Correlations Found Between the CBC-Derived Inflammatory Markers, the Stage of the Tumor, and the General Characteristics of the Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBC | complete blood count |
| NLST | National Lung Screening Trial |
| CT | computed tomography |
| SCLC | small-cell lung carcinoma |
| NSCLC | non-small cell lung carcinoma |
| SCCs | lung squamous cell carcinoma |
| LCCs | large cell carcinoma |
| AdCs | adenocarcinoma |
| CIS | carcinoma in situ |
| AAH | atypical adenomatous hyperplasia |
| AIS | adenocarcinoma in situ |
| KRAS | Kirsten Rat Sarcoma viral oncogene |
| EGFR | epidermal growth factor receptor |
| TIME | tumor immune microenvironment |
| IL-6 | Interleukin 6 |
| IL-1 | Interleukin 1 |
| DNA | deoxyribonucleic acid |
| NOS | not-otherwise-specified carcinoma |
| TNM | Tumor, Node, Metastasis |
| NLR | neutrophil-to-lymphocyte ratio |
| d-NLR | derived neutrophil-to-lymphocyte ratio |
| MLR | monocyte-to-lymphocyte ratio |
| PLR | platelet-to-lymphocyte ratio |
| ENR | eosinophil-to-neutrophil ratio |
| EMR | eosinophil-to-monocyte ratio |
| SII | systemic inflammatory index |
| SIRI | systemic inflammatory response index |
| AISI | aggregate index of systemic inflammation |
| COPD | chronic obstructive pulmonary disorder |
| BMI | body mass index |
| ROS | reactive oxygen species |
| MRI | magnetic resonance imaging |
| LDCT | low-dose CT scan |
| ADC | apparent diffusion coefficient |
| DWI | diffusion-weighted imaging |
| ACBS | Aarhus composite biomarker score |
| mGPS | Modified Glasgow Prognostic Score |
| CNG | Combined NLR and Glasgow Prognostic Score |
| PFS | progression-free survival |
| OS | overall survival |
| MdNLR | methylation-derived neutrophil-to-lymphocyte ratio |
| TBLB | transbronchial biopsy |
| PTLB | percutaneous transthoracic biopsy |
| FR+ CTC | folate receptor-positive circulating tumor cell count |
| LC | lymphocyte count |
References
- Mandaliya, H.; Jones, M.; Oldmeadow, C.; Nordman, I.I.C. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): Neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl. Lung Cancer Res. 2019, 8, 886–894. [Google Scholar] [CrossRef] [PubMed]
- da Costa, V.R.; Souza, O.F.; Teixeira, M.R.; Alievi, A.L.; Vigerelli, H.; Araldi, R.P. Inflammasomes driven inflammation in lung cancer revisited: A short review. Explor. Immunol. 2023, 3, 70–81. [Google Scholar] [CrossRef]
- Khusnurrokhman, G.; Wati, F.F. Tumor-promoting inflammation in lung cancer: A literature review. Ann. Med. Surg. 2022, 79, 104022. [Google Scholar] [CrossRef] [PubMed]
- Gasparri, R.; Papale, M.; Sabalic, A.; Catalano, V.; Deleonardis, A.; De Luca, F.; Ranieri, E.; Spaggiari, L. Circulating RKIP and pRKIP in Early-Stage Lung Cancer: Results from a Pilot Study. J. Clin. Med. 2024, 13, 5830. [Google Scholar] [CrossRef]
- Hatabu, H.; Ohno, Y.; Gefter, W.B.; Parraga, G.; Madore, B.; Lee, K.S.; Altes, T.A.; Lynch, D.A.; Mayo, J.R.; Seo, J.B.; et al. Expanding Applications of Pulmonary MRI in the Clinical Evaluation of Lung Disorders: Fleischner Society Position Paper. Radiology 2020, 297, 286–301. [Google Scholar] [CrossRef]
- Bak, S.H.; Kim, C.; Kim, C.H.; Ohno, Y.; Lee, H.Y. Magnetic resonance imaging for lung cancer: A state-of-the-art review. Precis. Futur. Med. 2022, 6, 49–77. [Google Scholar] [CrossRef]
- Monica, V.; Scagliotti, G.V.; Ceppi, P.; Righi, L.; Cambieri, A.; Iacono, M.L.; Saviozzi, S.; Volante, M.; Novello, S.; Papotti, M. Differential Thymidylate Synthase Expression in Different Variants of Large-Cell Carcinoma of the Lung. Clin. Cancer Res. 2009, 15, 7547–7552. [Google Scholar] [CrossRef]
- Ceppi, P.; Volante, M.; Saviozzi, S.; Rapa, I.; Novello, S.; Cambieri, A.; Iacono, M.L.; Cappia, S.; Papotti, M.; Scagliotti, G.V. Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer 2006, 107, 1589–1596. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Muller-Hermelink, H.K.; Harris, C.C. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. WHO/IASLC Classification of Lung and Pleural Tumours; IARC Press: Lyon, France, 2004. [Google Scholar]
- Travis, W.D.; Brambilla, E.; Noguchi, M.; Nicholson, A.G.; Geisinger, K.R.; Yatabe, Y.; Yankelewitz, D. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J. Thorac. Oncol. 2011, 6, 244–285. [Google Scholar] [CrossRef]
- Wistuba, I.I.; Gazdar, A.F. LUNG CANCER PRENEOPLASIA. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 331–348. [Google Scholar] [CrossRef]
- Lantuéjoul, S.; Salameire, D.; Salon, C.; Brambilla, E. Pulmonary preneoplasia—Sequential molecular carcinogenetic events. Histopathology 2009, 54, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Naunheim, K.S.; Taylor, J.R.; Skosey, C.; Hoffman, P.C.; Ferguson, M.K.; Golomb, H.M.; Little, A.G. Adenosquamous lung carcinoma: Clinical characteristics, treatment, and prognosis. Ann. Thorac. Surg. 1987, 44, 462–466. [Google Scholar] [CrossRef]
- Ben, Y.; Yu, H.; Wang, Z.; Miao, Q.; Ren, H.; Zhang, Z.; Li, Z. Adenosquamous lung carcinoma: Clinical characteristics, surgical treament and prognosis. Chin. Med. Sci. J. 2000, 15, 238–240. [Google Scholar]
- Wistuba, I.I.; Berry, J.; Behrens, C.; Maitra, A.; Shivapurkar, N.; Milchgrub, S.; Gazdar, A.F. Molecular changes in the bronchial epithe-lium of patients with small cell lung cancer. Clin. Cancer Res. 2000, 6, 2604–2610. [Google Scholar] [PubMed]
- Badovinac, S.; Korsic, M.; Mursic, D.; Samarzija, M.; Cucevic, B.; Roglic, M.; Jakopovic, M. Cancer-related inflammation as predicting tool for treatment outcome in locally advanced and metastatic non-small cell lung cancer. J. Thorac. Dis. 2016, 8, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Wang, H.; Zheng, Q.; Li, S.; Liu, J.; Huang, J.; Tang, J.; Meng, X. Add fuel to the fire: Inflammation and immune response in lung cancer combined with COVID-19. Front. Immunol. 2023, 14, 1174184. [Google Scholar] [CrossRef]
- Tan, Z.; Xue, H.; Sun, Y.; Zhang, C.; Song, Y.; Qi, Y. The Role of Tumor Inflammatory Microenvironment in Lung Cancer. Front. Pharmacol. 2021, 12, 688625. [Google Scholar] [CrossRef]
- Dubinett, S.M. (Ed.) Inflammation and Lung Cancer; Springer: New York, NY, USA, 2015; 212p. [Google Scholar]
- Sang, J.; Ye, X. Potential biomarkers for predicting immune response and outcomes in lung cancer patients undergoing thermal ablation. Front. Immunol. 2023, 14, 1268331. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Zhang, S.; Liu, Y.; Ma, L.; Zhu, J.; Cheng, Y. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. Clin. Lab. Anal. 2019, 33, e22964. [Google Scholar] [CrossRef]
- Gaur, P.; Bhattacharya, S.; Kant, S.; Kushwaha, R.A.; Garg, R.; Singh, G.; Pandey, S. Association of inflammatory biomarkers with lung cancer in North Indian population. Afr. Health Sci. 2019, 19, 2147. [Google Scholar] [CrossRef]
- Winther-Larsen, A.; Aggerholm-Pedersen, N.; Sandfeld-Paulsen, B. Inflammation-scores as prognostic markers of overall survival in lung cancer: A register-based study of 6,210 Danish lung cancer patients. BMC Cancer 2022, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.J.; Belani, C.P. Diversity and heterogeneity of immune states in non-small cell lung cancer and small cell lung cancer. PLoS ONE 2021, 16, e0260988. [Google Scholar] [CrossRef]
- Grieshober, L.; Graw, S.; Barnett, M.J.; Goodman, G.E.; Chen, C.; Koestler, D.C.; Marsit, C.J.; Doherty, J.A. Pre-diagnosis neutrophil-to-lymphocyte ratio and mortality in individuals who develop lung cancer. Cancer Causes Control 2021, 32, 1227–1236. [Google Scholar] [CrossRef]
- Detterbeck, F.C. The eighth edition TNM stage classification for lung cancer: What does it mean on main street? J. Thorac. Cardiovasc. Surg. 2018, 155, 356–359. [Google Scholar] [CrossRef]
- Guo, B.; Liu, X.; Si, Q.; Zhang, D.; Li, M.; Li, X.; Zhao, Y.; Hu, F.; Zhang, M.; Liu, Y.; et al. Associations of CBC-Derived inflammatory indicators with sarcopenia and mortality in adults: Evidence from Nhanes 1999∼2006. BMC Geriatr. 2024, 24, 432. [Google Scholar] [CrossRef]
- Wei, J.; Brown, L.; Gao, B.; Nagrial, A.; da Silva, I.P. P1.21-08 Eosinophil to Neutrophil Ratio Predicts Efficacy in Patients Receiving Immunotherapy in Metastatic Non-small Cell Lung Cancer (mNSCLC). J. Thorac. Oncol. 2023, 18, S237. [Google Scholar] [CrossRef]
- Chen, X.; Huang, W.; Zhao, L.; Li, Y.; Wang, L.; Mo, F.; Guo, W. Relationship Between the Eosinophil/Monocyte Ratio and Prognosis in Decompensated Heart Failure: A Retrospective Study. J. Inflamm. Res. 2021, 14, 4687–4696. [Google Scholar] [CrossRef]
- Tiucă, O.M.; Morariu, S.H.; Mariean, C.R.; Tiucă, R.A.; Nicolescu, A.C.; Cotoi, O.S. Impact of Blood-Count-Derived Inflammatory Markers in Psoriatic Disease Progression. Life 2024, 14, 114. [Google Scholar] [CrossRef]
- Zhou, D.; Yang, H.; Zeng, L.; Yang, W.; Guo, F.; Cui, W.; Chen, C.; Zhao, J.; Wu, S.; Yang, N.; et al. Calculated inflammatory markers derived from complete blood count results, along with routine laboratory and clinical data, predict treatment failure of acute peritonitis in chronic peritoneal dialysis patients. Ren. Fail. 2023, 45, 2179856. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Zhu, K.; Shi, B.; Yin, Y.; Zhu, J.; Yue, D.; Zhang, B.; Wang, C. Prognostic Significance of Combination of Preoperative Platelet Count and Neutrophil-Lymphocyte Ratio (COP-NLR) in Patients with Non-Small Cell Lung Cancer: Based on a Large Cohort Study. PLoS ONE 2015, 10, e0126496. [Google Scholar] [CrossRef]
- Pinato, D.J.; Shiner, R.J.; Seckl, M.J.; Stebbing, J.; Sharma, R.; Mauri, F.A. Prognostic performance of inflammation-based prognostic indices in primary operable non-small cell lung cancer. Br. J. Cancer 2014, 110, 1930–1935. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Shimizu, T.; Ayabe, T.; Yonei, A.; Onitsuka, T. Preoperative neutrophil to lymphocyte ratio as a prognostic predictor after curative resection for non-small cell lung cancer. Anticancer Res. 2011, 31, 2995–2998. [Google Scholar] [PubMed]
- Fest, J.; Ruiter, R.; Mulder, M.; Groot Koerkamp, B.; Ikram, M.A.; Stricker, B.H.; van Eijck, C.H. The systemic immune-inflammation index is associated with an increased risk of incident cancer—A population-based cohort study. Int. J. Cancer 2020, 146, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Capone, M.; Giannarelli, D.; Mallardo, D.; Madonna, G.; Festino, L.; Grimaldi, A.M.; Vanella, V.; Simeone, E.; Paone, M.; Palmieri, G.; et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J. Immunother. Cancer 2018, 6, 74. [Google Scholar] [CrossRef]
- Putzu, C.; Cortinovis, D.L.; Colonese, F.; Canova, S.; Carru, C.; Zinellu, A.; Paliogiannis, P. Blood cell count indexes as predictors of outcomes in advanced non-small-cell lung cancer patients treated with Nivolumab. Cancer Immunol. Immunother. 2018, 67, 1349–1353. [Google Scholar] [CrossRef]
- Ogata, T.; Satake, H.; Ogata, M.; Hatachi, Y.; Inoue, K.; Hamada, M.; Yasui, H. Neutrophil-to-lymphocyte ratio as a predictive or prognostic factor for gastric cancer treated with nivolumab: A multicenter retrospective study. Oncotarget 2018, 9, 34520–34527. [Google Scholar] [CrossRef]
- Takeda, T.; Takeuchi, M.; Saitoh, M.; Takeda, S. Neutrophil-to-lymphocyte ratio after four weeks of nivolumab admin-istration as a predictive marker in patients with pretreated non-small-cell lung cancer. Thorac. Cancer 2018, 9, 1291–1299. [Google Scholar] [CrossRef]
- Cupp, M.A.; Cariolou, M.; Tzoulaki, I.; Aune, D.; Evangelou, E.; Berlanga-Taylor, A.J. Neutrophil to lymphocyte ratio and cancer prognosis: An umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020, 18, 360. [Google Scholar] [CrossRef]
- Wuhao, H.; Shengguang, W.; Hua, Z.; Bin, Z.; Changli, W. Prognostic significance of combined fibrinogen concentration and neutrophil-to-lymphocyte ratio in patients with resectable non-small cell lung cancer. Cancer Biol. Med. 2018, 15, 88–96. [Google Scholar] [CrossRef]
- Kuang, Z.; Miao, J.; Zhang, X. Serum albumin and derived neutrophil-to-lymphocyte ratio are potential predictive biomarkers for immune checkpoint inhibitors in small cell lung cancer. Front. Immunol. 2024, 15, 1327449. [Google Scholar] [CrossRef]
- Zhao, N.; Ruan, M.; Koestler, D.; Lu, J.; Marsit, C.; Kelsey, K.; Platz, E.; Michaud, D.S. Abstract LB086: Methylation-derived neutrophil-to-lymphocyte ratio and lung cancer risk and survival. Cancer Res. 2021, 81 (Suppl. 13), LB086. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Xu, R.; Lu, T.; Zhao, J.; Zhang, P.; Qu, L.; Zhang, S.; Guo, J.; Zhang, L. The MLR, NLR, PLR and D-dimer are associated with clinical outcome in lung cancer patients treated with surgery. BMC Pulm. Med. 2022, 22, 104. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, F.R.; Spreafico, A.; Novello, S.; Wood, M.D.; Simms, L.; Papotti, M. The Prognostic and Predictive Role of Histology in Advanced Non-small Cell Lung Cancer: A Literature Review. J. Thorac. Oncol. 2008, 3, 1468–1481. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.; Dahlberg, S.E.; Schiller, J.H.; Johnson, D.H. Does histology predict survival of advanced non-small cell lung cancer patients treated with platin-based chemotherapy? An analysis of the Eastern Cooperative Oncology Group Study E1594. Lung Cancer 2013, 81, 47–52. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.; Patil, P.; Thakkannavar, S.; Pujari, V. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121. [Google Scholar] [CrossRef]
- Mazzella, A.; Orlandi, R.; Maiorca, S.; Uslenghi, C.; Chiari, M.; Bertolaccini, L.; Casiraghi, M.; Iacono, G.L.; Girelli, L.; Spaggiari, L. How General and Inflammatory Status Impacts on the Prognosis of Patients Affected by Lung Cancer: State of the Art. Biomedicines 2024, 12, 1554. [Google Scholar] [CrossRef]
- Łochowski, M.; Chałubińska-Fendler, J.; Zawadzka, I.; Łochowska, B.; Rębowski, M.; Brzeziński, D.; Kozak, J. The Prognostic Significance of Preoperative Platelet-to-Lymphocyte and Neutrophil-to-Lymphocyte Ratios in Patients Operated for Non-Small Cell Lung Cancer. Cancer Manag. Res. 2021, 13, 7795–7802. [Google Scholar] [CrossRef]
- Alves, A.; Dias, M.; Campainha, S.; Barroso, A. Peripheral blood eosinophilia may be a prognostic biomarker in non-small cell lung cancer patients treated with immunotherapy. J. Thorac. Dis. 2021, 13, 2716–2727. [Google Scholar] [CrossRef]
- Ownby, H.E.; Roi, L.D.; Isenberg, R.R.; Brennan, M.J. Peripheral lymphocyte and eosinophil counts as indicators of prognosis in primary breast cancer. Cancer 2006, 52, 126–130. [Google Scholar] [CrossRef]
- Prizment, A.E.; Anderson, K.E.; Visvanathan, K.; Folsom, A.R. Inverse Association of Eosinophil Count with Colorectal Cancer Incidence: Atherosclerosis Risk in Communities Study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1861–1864. [Google Scholar] [CrossRef]
- Khakwani, A.; Rich, A.L.; Powell, H.A.; Tata, L.J.; Stanley, R.A.; Baldwin, D.R.; Duffy, J.P.; Hubbard, R.B. Lung cancer survival in England: Trends in non-small-cell lung cancer survival over the duration of the National Lung Cancer Audit. Br. J. Cancer 2013, 109, 2058–2065. [Google Scholar] [CrossRef] [PubMed]
- Polański, J.; Chabowski, M.; Jankowska-Polańska, B.; Janczak, D.; Rosinczuk, J. Histological subtype of lung cancer affects acceptance of illness, severity of pain, and quality of life. J. Pain Res. 2018, 11, 727–733. [Google Scholar] [CrossRef]
- Visbal, A.L.; Williams, B.A.; Nichols, F.C., III; Marks, R.S.; Jett, J.R.; Aubry, M.C.; Yang, P. Gender differences in non–small-cell lung cancer survival: An analysis of 4,618 patients diagnosed between 1997 and 2002. Ann. Thorac. Surg. 2004, 78, 209–215. [Google Scholar] [CrossRef]
- Lam, V.K.; Bentzen, S.M.; Mohindra, P.; Nichols, E.M.; Bhooshan, N.; Vyfhuis, M.; Scilla, K.A.; Feigenberg, S.J.; Edelman, M.J.; Feliciano, J.L. Obesity is associated with long-term improved survival in definitively treated locally advanced non-small cell lung cancer (NSCLC). Lung Cancer 2017, 104, 52–57. [Google Scholar] [CrossRef]
- Hervochon, R.; Bobbio, A.; Guinet, C.; Mansuet-Lupo, A.; Rabbat, A.; Régnard, J.-F.; Roche, N.; Damotte, D.; Iannelli, A.; Alifano, M. Body Mass Index and Total Psoas Area Affect Outcomes in Patients Undergoing Pneumonectomy for Cancer. Ann. Thorac. Surg. 2017, 103, 287–295. [Google Scholar] [CrossRef]
- Georgakopoulou, V.; Lempesis, I.; Trakas, N.; Sklapani, P.; He, Y.; Spandidos, D. Lung cancer and obesity: A contentious re-lationship (Review). Oncol. Rep. 2024, 52, 158. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, S. Body mass index and lung cancer risk in never smokers: A meta-analysis. BMC Cancer 2018, 18, 635. [Google Scholar] [CrossRef]
- Yu, D.; Zheng, W.; Johansson, M.; Lan, Q.; Park, Y.; White, E.; Matthews, C.E.; Sawada, N.; Gao, Y.-T.; Robien, K.; et al. Overall and Central Obesity and Risk of Lung Cancer: A Pooled Analysis. JNCI J. Natl. Cancer Inst. 2018, 110, 831–842. [Google Scholar] [CrossRef]
- Miracle, C.E.; McCallister, C.L.; Egleton, R.D.; Salisbury, T.B. Mechanisms by which obesity regulates inflammation and anti-tumor immunity in cancer. Biochem. Biophys. Res. Commun. 2024, 733, 150437. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, D.; Lee, J.; Jeon, Y.J.; Park, S.Y.; Cho, J.H.; Cho, J. Association of Obesity and Skeletal Muscle with Postoperative Survival in Non–Small Cell Lung Cancer. Radiology 2025, 314, e241507. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Assumpção, J.A.F.; Pasquarelli-Do-Nascimento, G.; Duarte, M.S.V.; Bonamino, M.H.; Magalhães, K.G. The ambiguous role of obesity in oncology by promoting cancer but boosting antitumor immunotherapy. J. Biomed. Sci. 2022, 29, 12. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Dong, T.; Li, S.; Zhang, Y.; Han, Z.; Liu, M.; Dong, W.; Hong, Z.; Fu, M.; Zhang, H. A Bibliometric Analysis of Comorbidity of COPD and Lung Cancer: Research Status and Future Directions. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 3049–3065. [Google Scholar] [CrossRef]
- Ma, A.; Wang, G.; Du, Y.; Guo, W.; Guo, J.; Hu, Y.; Bai, D.; Huang, H.; Zhuang, L.; Chen, J.; et al. The clinical relevance of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in chronic obstructive pulmonary disease with lung cancer. Front. Oncol. 2022, 12, 902955. [Google Scholar] [CrossRef]
- Wasswa-Kintu, S.; Gan, W.Q.; Man, S.F.P.; Pare, P.D.; Sin, D.D. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: A systematic review and meta-analysis. Thorax 2005, 60, 570–575. [Google Scholar] [CrossRef]
- Wang, H.; Liu, L.; Yan, J.; Ma, W.; Du, Y.; Zhang, T. Folate receptor-positive circulating tumor cell count, lymphocyte count and derived neutrophil-to- lymphocyte ratio for diagnosing lung cancer relapse. Front. Oncol. 2023, 12, 1097816. [Google Scholar] [CrossRef]


| Parameter | Formula |
|---|---|
| Neutrophil-to-lymphocyte ratio (NLR) | Neutrophil count/lymphocyte count [×103/μL] [1] |
| Derived-neutrophil-to-lymphocyte ratio (d-NLR) | Neutrophil count/(WBC − neutrophil count) [×103/μL] [27] |
| Monocyte-to-lymphocyte ratio (MLR) | Monocyte count/lymphocyte count [×103/μL] [27] |
| Platelet-to-lymphocyte ratio (PLR) | Platelet count/lymphocyte count [×103/μL] [1] |
| Eosinophil-to-neutrophil ratio (ENR) | Eosinophil count/neutrophil count [×103/μL] [28] |
| Eosinophil-to-monocyte ratio (EMR) | Eosinophil count/monocyte count [×103/μL] [29] |
| Systemic inflammatory index (SII) | (Neutrophil count × platelet count)/lymphocyte count [×103/μL] [19] |
| Systemic inflammatory response index (SIRI) | (Neutrophil count × monocyte count)/lymphocyte count [×103/μL] [27] |
| Aggregate index of systemic inflammation (AISI) | (Neutrophil count × monocyte count × platelet count)/lymphocyte count [×103/μL] [30] |
| Parameters | N (Absolute Count) | N (Percentage %) |
|---|---|---|
| ALL N = 202 patients | ||
| AGE—mean: 66.62 ± 8.34 | ||
| <50 years | 4 | 1.98% |
| 50–59 years | 37 | 18.32% |
| 60–69 years | 83 | 41.09% |
| 70–79 years | 71 | 35.15% |
| ≥80 years | 7 | 3.46% |
| Gender | ||
| MALE | 150 | 74.25% |
| FEMALE | 52 | 25.75% |
| Living environment | ||
| RURAL | 118 | 58.42% |
| URBAN | 84 | 41.58% |
| BMI—a median of numeric values (when available): 24 [23.328–24.653] | ||
| <18.5 | 36 | 17.82% |
| 18.5–24.99 | 105 | 51.98% |
| 25–29.99 | 39 | 19.30% |
| 30–34.99 | 14 | 6.94% |
| 35–39.99 | 6 | 2.97% |
| >40 | 2 | 0.99% |
| Associated COPD | ||
| YES | 81 | 40.1% |
| NO | 121 | 59.9% |
| Smoking | ||
| YES | 175 | 86.63% |
| NO | 27 | 13.37% |
| Factor | p-Value | 95% CI | logOR | Overall Predictive Value |
|---|---|---|---|---|
| NSCLC patients | ||||
| Leukocyte count | 0.01 | 0.004632–0.03408 | 0.019 | p = 0.0034 |
| Neutrophil count | 0.011 | 0.003436–0.02620 | 0.014 | |
| Male gender | 0.014 | 0.02728–0.2383 | 0.13 | |
| NSCLC–Adenocarcinoma | ||||
| SIRI | 0.005 | 0.06960–0.01266 | −0.04 | p = 0.0004 |
| d-NLR | 0.039 | 0.003848–0.1457 | 0.074 | |
| Age | 0.004 | 0.03392–0.006669 | −0.02 | |
| Squamous cell carcinoma | ||||
| d-NLR | 0.02 | 0.01410–0.1869 | 0.1 | p= 0.0441 |
| ENR | 0.0004 | −9.3075–−1.7811 | −5.5 | |
| Leukocyte count | 0.03 | 0.01301–0.2771 | 0.15 | |
| Neutrophil count | 0.013 | −0.3996–−0.05337 | −0.23 | |
| SCLC | ||||
| AISI | <0.0001 | −0.002430–0.001229 | −0.00183 | p = 0.0001 |
| SII | <0.0001 | 0.001185–0.002034 | 0.00161 | |
| SIRI | <0.0001 | 0.5917–1.1510 | 0.8713 | |
| d-NLR | <0.001 | −1.3360–−0.7728 | −1.0544 | |
| EMR | 0.004 | 0.9183–3.8487 | 2.3835 | |
| ENR | 0.004 | −48.5515–−11.9517 | −30.25 | |
| MLR | 0.001 | −8.1070–−2.7492 | −5.4281 | |
| COPD | 0.0001 | −1.0162–−0.4747 | −0.7454 | |
| Male gender | 0.0002 | −0.8465–−0.3574 | −0.602 | |
| Leukocyte count | <0.001 | 0.3202–0.5954 | 0.4578 | |
| Lymphocyte count | 0.0007 | −1.2249–−0.4424 | −0.8336 | |
| Neutrophil count | <0.001 | −0.6009–−0.3776 | −0.4892 | |
| Platelets count | 0.042 | −0.003261–−0.00007213 | −0.00167 | |
| NSCLC–Adenocarcinoma | |||
| Parameter | Correlation | p-Value | Correlation Coefficient |
| SII | Smoking | p = 0.0328 | 0.219 |
| d-NLR | Smoking | p = 0.0127 | 0.255 |
| PLR | Stage | p = 0.0266 | 0.227 |
| Age | Gender | p = 0.0037 | 0.295 |
| Stage | p = 0.0096 | −0.264 | |
| Gender | Age | p = 0.0037 | 0.295 |
| Stage | PLR | p = 0.0266 | 0.227 |
| Age | p = 0.0096 | −0.264 | |
| BMI | Platelets | p = 0.0268 | −0.228 |
| Smoking | COPD | p = 0.0425 | 0.209 |
| d-NLR | p = 0.0127 | 0.255 | |
| NLR | p = 0.0443 | 0.207 | |
| SII | p = 0.0328 | 0.219 | |
| COPD | Smoking | p = 0.0425 | 0.209 |
| NSCLC-Squamous cell carcinoma | |||
| BMI | COPD | p = 0.0301 | 0.273 |
| d-NLR | p = 0.0382 | −0.262 | |
| Smoking | None | - | - |
| COPD | BMI | p = 0.0301 | 0.273 |
| NSCLC–Adenosquamous carcinoma | |||
| Gender | Living environment | p < 0.0001 | 1 |
| Smoking | None | - | - |
| COPD | None | - | - |
| Living environment | Gender | p < 0.0001 | 1 |
| NSCLC–NOS | |||
| Gender | Smoking | p = 0.0075 | 0.679 |
| BMI | SII | p = 0.0425 | −0.548 |
| Smoking | Gender | p = 0.0075 | 0.679 |
| COPD | None | - | - |
| SCLC | |||
| Gender | Smoking | p = 0.0413 | 0.395 |
| Smoking | Gender | p = 0.0413 | 0.395 |
| COPD | None | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariean, C.R.; Tiucă, O.M.; Mariean, A.; Szekely, T.-B.; Niculescu, R.; Sabau, A.H.; Al-Akel, C.F.; Cotoi, O.S. The Impact of the Histologic Types of Lung Cancer on CBC-Derived Inflammatory Markers—Current Knowledge and Future Perspectives. J. Clin. Med. 2025, 14, 3038. https://doi.org/10.3390/jcm14093038
Mariean CR, Tiucă OM, Mariean A, Szekely T-B, Niculescu R, Sabau AH, Al-Akel CF, Cotoi OS. The Impact of the Histologic Types of Lung Cancer on CBC-Derived Inflammatory Markers—Current Knowledge and Future Perspectives. Journal of Clinical Medicine. 2025; 14(9):3038. https://doi.org/10.3390/jcm14093038
Chicago/Turabian StyleMariean, Claudia Raluca, Oana Mirela Tiucă, Alexandru Mariean, Tiberiu-Bogdan Szekely, Raluca Niculescu, Adrian Horatiu Sabau, Cristina Flavia Al-Akel, and Ovidiu Simion Cotoi. 2025. "The Impact of the Histologic Types of Lung Cancer on CBC-Derived Inflammatory Markers—Current Knowledge and Future Perspectives" Journal of Clinical Medicine 14, no. 9: 3038. https://doi.org/10.3390/jcm14093038
APA StyleMariean, C. R., Tiucă, O. M., Mariean, A., Szekely, T.-B., Niculescu, R., Sabau, A. H., Al-Akel, C. F., & Cotoi, O. S. (2025). The Impact of the Histologic Types of Lung Cancer on CBC-Derived Inflammatory Markers—Current Knowledge and Future Perspectives. Journal of Clinical Medicine, 14(9), 3038. https://doi.org/10.3390/jcm14093038





