A Comparative Evaluation of the Primary and Secondary Stability of Dental Implants with Progressive and Conventional Thread Designs: A Prospective Non-Interventional Study of 100 Implants in 62 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Design and Population
2.2. The Clinical Protocol
2.3. The Statistical Analysis
3. Results
3.1. Patients’ Demographics, Implant Locations, and Healing Times
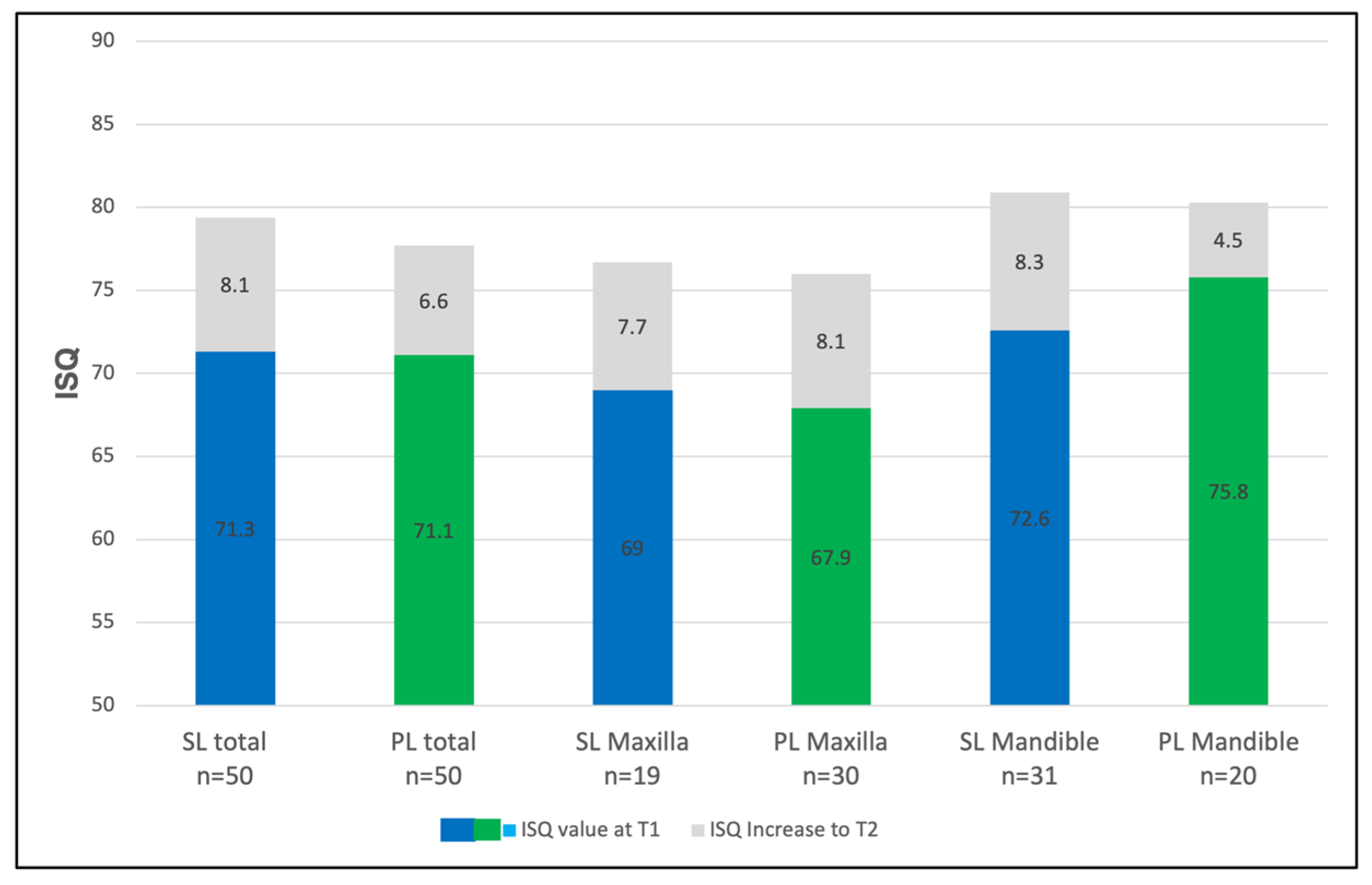
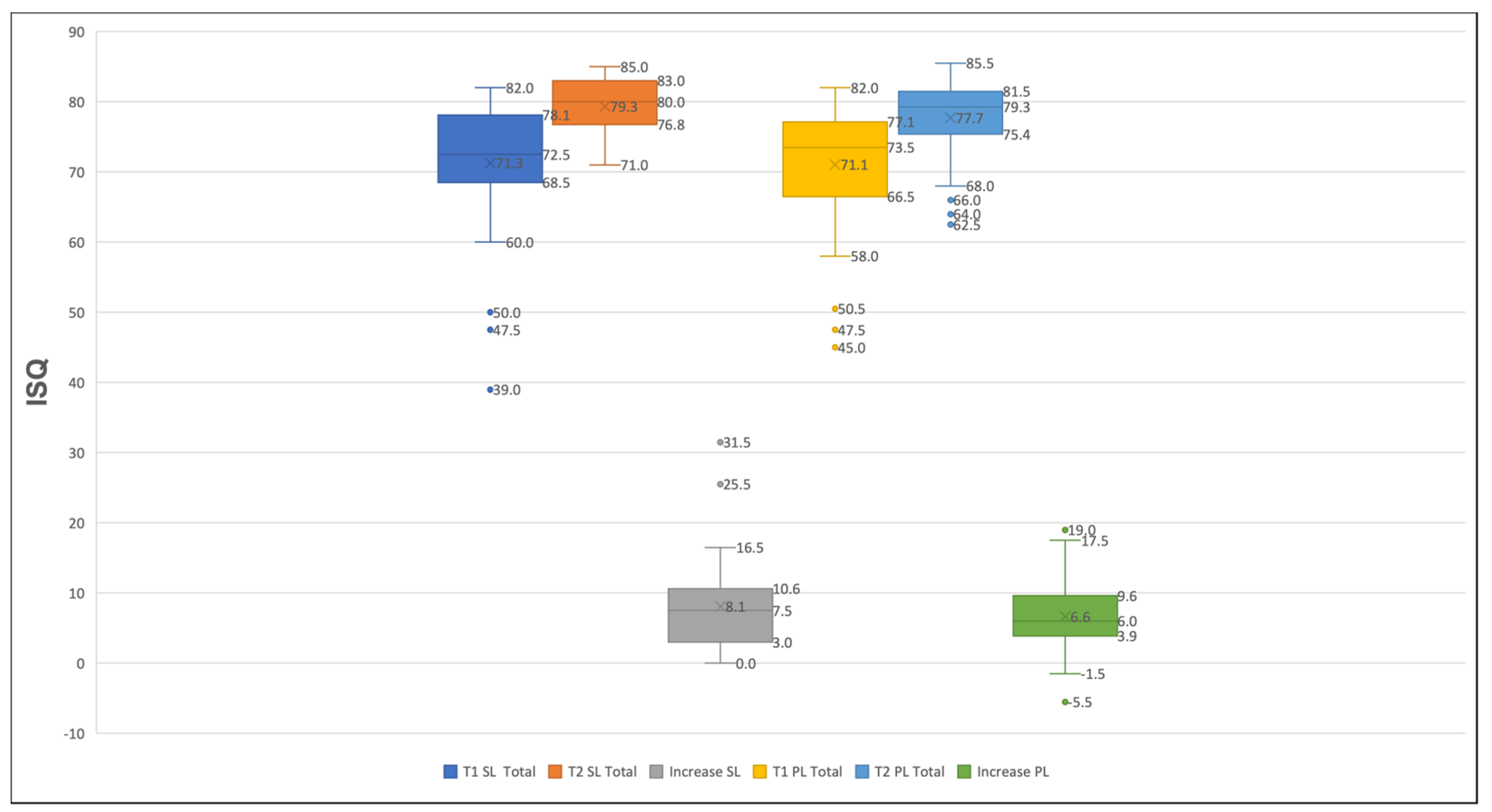
3.2. The Analysis of the Implants’ Stability
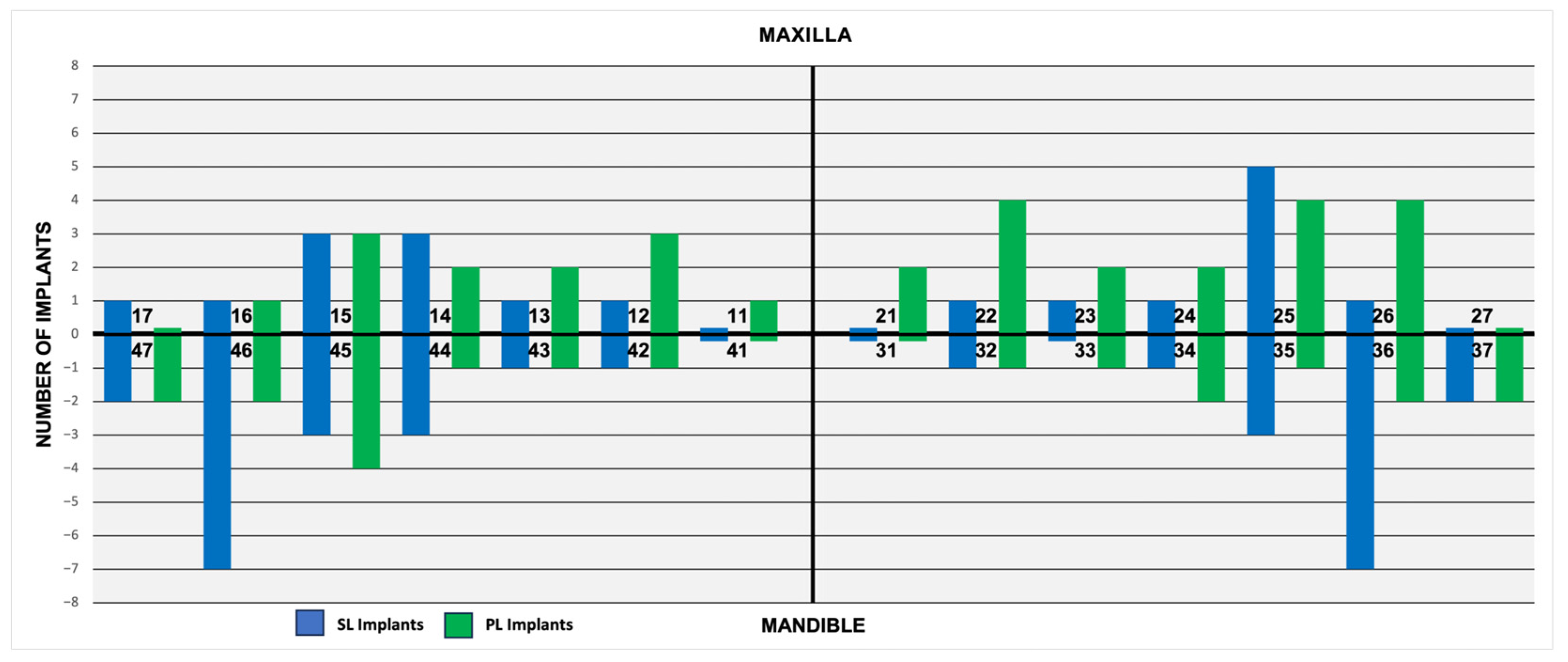
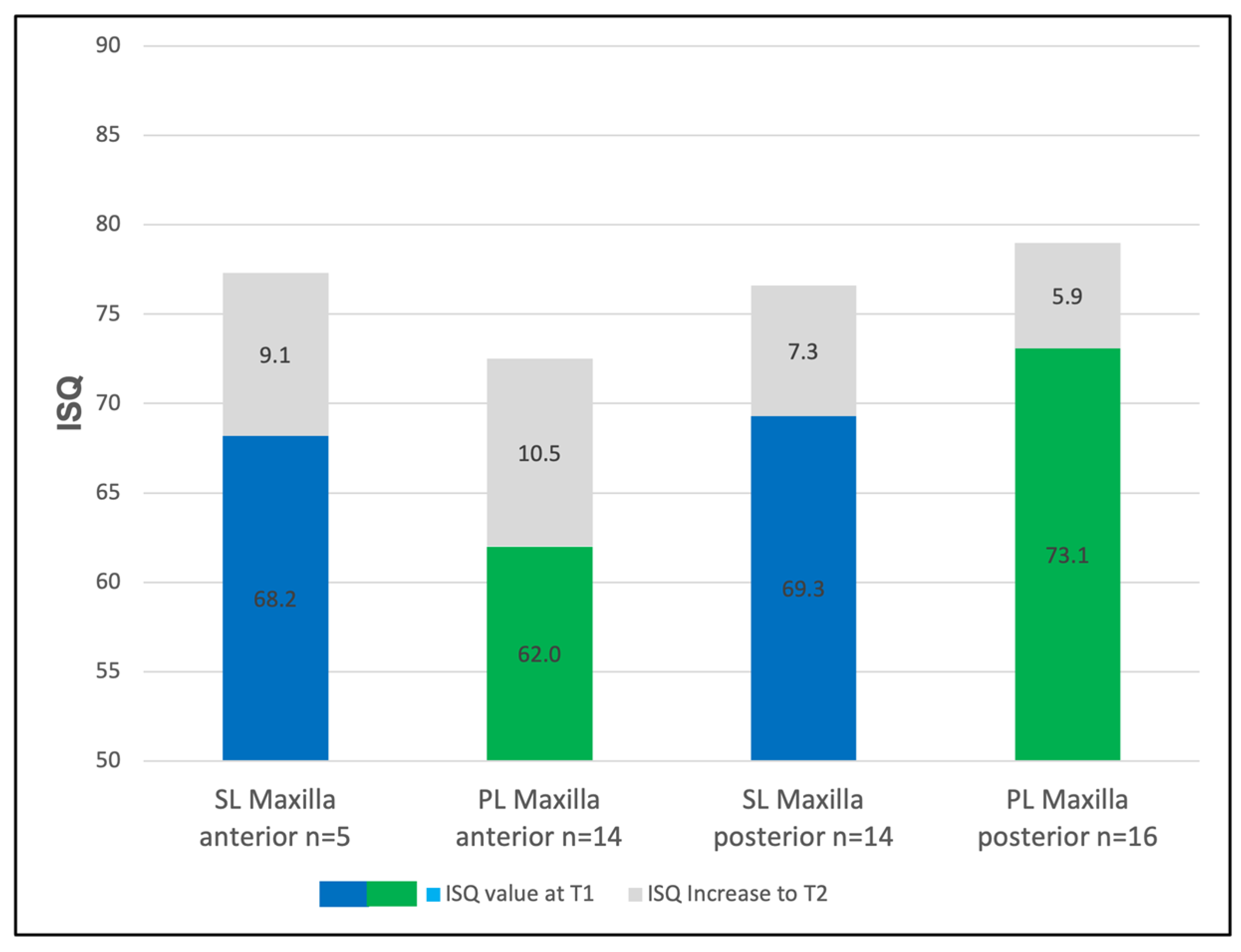
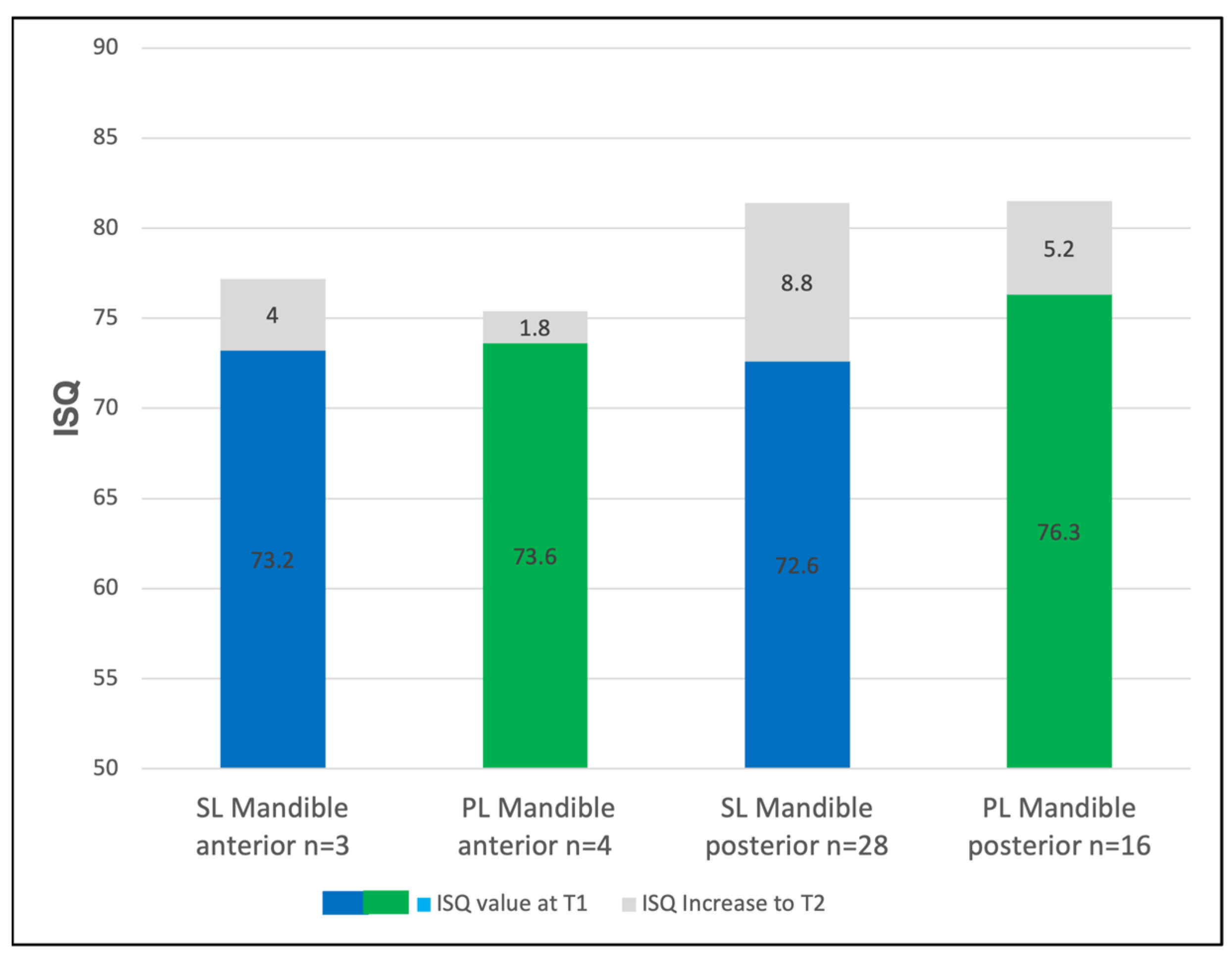
3.3. The Regional Implant Stability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PL | Implants with progressive thread design |
| SL | Implants with conventional thread design |
| RFA | Resonance frequency analysis |
| ISQ | Implant stability quotient |
| T1 | Time point immediately after implant placement |
| T2 | Time point at prosthetic loading |
| CBCT | Cone beam computed tomography |
| HU | Hounsfield units |
| GBR | Guided bone regeneration |
| PS | Primary implant stability |
| SS | Secondary implant stability |
References
- Moraschini, V.; Poubel, L.A.; Ferreira, V.F.; Barboza Edos, S. Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Tey, V.H.S.; Phillips, R.; Tan, K. Five-year retrospective study on success, survival and incidence of complications of single crowns supported by dental implants. Clin. Oral Implants Res. 2017, 28, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Rosa, C.; Bento, V.; Duarte, N.; Sayeg, J.; Santos, T.; Pellizzer, E. Do dental implants installed in different types of bone (I, II, III, IV) have different success rates? A systematic review and meta-analysis. Saudi Dent. J. 2024, 36, 428–442. [Google Scholar] [CrossRef]
- Fouda, A.A.H. The impact of the alveolar bone sites on early implant failure: A systematic review with meta-analysis. J. Korean Assoc. Oral Maxillofac. Surg. 2020, 46, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, S.; Wood, M.C.; Taylor, T.D. Early wound healing around endosseous implants: A review of the literature. Int. J. Oral Maxillofac. Implants 2005, 20, 425–431. [Google Scholar]
- Huang, Y.C.; Huang, Y.C.; Ding, S.J. Primary stability of implant placement and loading related to dental implant materials and designs: A literature review. J. Dent. Sci. 2023, 18, 1467–1476. [Google Scholar] [CrossRef]
- Brunski, J.B. In vivo bone response to biomechanical loading at the bone/dental-implant interface. Adv. Dent. Res. 1999, 13, 99–119. [Google Scholar] [CrossRef]
- Meredith, N. Assessment of implant stability as a prognostic determinant. Int. J. Prosthodont. 1998, 11, 491–501. [Google Scholar]
- Herrero-Climent, M.; Santos-Garcia, R.; Jaramillo-Santos, R.; Romero-Ruiz, M.M.; Fernandez-Palacin, A.; Lazaro-Calvo, P.; Bullon, P.; Rios-Santos, J.V. Assessment of Osstell ISQ’s reliability for implant stability measurement: A cross-sectional clinical study. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e877–e882. [Google Scholar] [CrossRef]
- Abuhussein, H.; Pagni, G.; Rebaudi, A.; Wang, H.L. The effect of thread pattern upon implant osseointegration. Clin. Oral. Implants Res. 2010, 21, 129–136. [Google Scholar] [CrossRef]
- Chambrone, L.; Rincon-Castro, M.V.; Poveda-Marin, A.E.; Diazgranados-Lozano, M.P.; Fajardo-Escolar, C.E.; Bocanegra-Puerta, M.C.; Palma, L.F. Histological healing outcomes at the bone-titanium interface of loaded and unloaded dental implants placed in humans: A systematic review of controlled clinical trials. Int. J. Oral Implantol. 2020, 13, 321–342. [Google Scholar]
- Sammons, R.L.; Lumbikanonda, N.; Gross, M.; Cantzler, P. Comparison of osteoblast spreading on microstructured dental implant surfaces and cell behaviour in an explant model of osseointegration. A scanning electron microscopic study. Clin. Oral Implants Res. 2005, 16, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Falco, A.; Berardini, M.; Trisi, P. Correlation Between Implant Geometry, Implant Surface, Insertion Torque, and Primary Stability: In Vitro Biomechanical Analysis. Int. J. Oral Maxillofac. Implants 2018, 33, 824–830. [Google Scholar] [CrossRef]
- Kreve, S.; Ferreira, I.; da Costa Valente, M.L.; Dos Reis, A.C. Relationship between dental implant macro-design and osseointegration: A systematic review. Oral Maxillofac. Surg. 2024, 28, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Waechter, J.; Madruga, M.M.; Carmo Filho, L.C.D.; Leite, F.R.M.; Schinestsck, A.R.; Faot, F. Comparison between tapered and cylindrical implants in the posterior regions of the mandible: A prospective, randomized, split-mouth clinical trial focusing on implant stability changes during early healing. Clin. Implant Dent. Relat. Res. 2017, 19, 733–741. [Google Scholar] [CrossRef]
- Supachaiyakit, P.; Serichetaphongse, P.; Chengprapakorn, W. The influence of implant design on implant stability in low-density bone under guided surgical template in inexperienced surgeons: A pilot randomized controlled clinical trial using resonance frequency analysis. Clin. Implant Dent. Relat. Res. 2022, 24, 444–454. [Google Scholar] [CrossRef]
- Steigenga, J.T.; al-Shammari, K.F.; Nociti, F.H.; Misch, C.E.; Wang, H.L. Dental implant design and its relationship to long-term implant success. Implant Dent. 2003, 12, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Shiota, M.; Fujii, M.; Shimogishi, M.; Munakata, M. Effects of implant thread design on primary stability-a comparison between single- and double-threaded implants in an artificial bone model. Int. J. Implant Dent. 2020, 6, 42. [Google Scholar] [CrossRef]
- Rakasevic, D.; Lazic, Z.; Soldatovic, I.; Scepanovic, M.; Gabric, D. Influence of titanium implant macrodesign on peri-implantitis occurrence: A cross-sectional study. Clin. Oral Investig. 2022, 26, 5237–5246. [Google Scholar] [CrossRef]
- Misch, C.E. Density of bone: Effect on treatment plans, surgical approach, healing, and progressive boen loading. Int. J. Oral Implantol. 1990, 6, 23–31. [Google Scholar]
- Selvaraj, A.; Jain, R.K.; Nagi, R.; Balasubramaniam, A. Correlation between gray values of cone-beam computed tomograms and Hounsfield units of computed tomograms: A systematic review and meta-analysis. Imaging Sci. Dent. 2022, 52, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Balleri, P.; Cozzolino, A.; Ghelli, L.; Momicchioli, G.; Varriale, A. Stability measurements of osseointegrated implants using Osstell in partially edentulous jaws after 1 year of loading: A pilot study. Clin. Implant Dent. Relat. Res. 2002, 4, 128–132. [Google Scholar] [CrossRef]
- da Rocha Ferreira, J.J.; Machado, L.F.M. Insertion Torque Value and Implant Stability Quotient: Separate Evaluation and Correlation for Different Clinical Parameters. Int. J. Oral Maxillofac. Implants 2022, 37, 812–822. [Google Scholar] [CrossRef]
- O’Sullivan, D.; Sennerby, L.; Meredith, N. Measurements comparing the initial stability of five designs of dental implants: A human cadaver study. Clin. Implant Dent. Relat. Res. 2000, 2, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Kido, H.; Schulz, E.E.; Kumar, A.; Lozada, J.; Saha, S. Implant diameter and bone density: Effect on initial stability and pull-out resistance. J. Oral Implantol. 1997, 23, 163–169. [Google Scholar]
- Bischof, M.; Nedir, R.; Szmukler-Moncler, S.; Bernard, J.P.; Samson, J. Implant stability measurement of delayed and immediately loaded implants during healing. Clin. Oral Implants Res. 2004, 15, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Degidi, M.; Daprile, G.; Piattelli, A. Primary stability determination by means of insertion torque and RFA in a sample of 4135 implants. Clin. Implant Dent. Relat. Res. 2012, 14, 501–507. [Google Scholar] [CrossRef]
- Aparicio, C.; Lang, N.P.; Rangert, B. Validity and clinical significance of biomechanical testing of implant/bone interface. Clin. Oral Implants Res. 2006, 17 (Suppl. S2), 2–7. [Google Scholar] [CrossRef]
- de Carvalho Formiga, M.; da Silva, H.D.P.; Ghiraldini, B.; Siroma, R.S.; Ardelean, L.C.; Piattelli, A.; Shibli, J.A. Effects of Osseodensification on Primary Stability of Cylindrical and Conical Implants-An Ex Vivo Study. J. Clin. Med. 2023, 12, 3736. [Google Scholar] [CrossRef]
- Olmedo-Gaya, M.V.; Romero-Olid, M.N.; Ocaña-Peinado, F.M.; Vallecillo-Rivas, M.; Vallecillo, C.; Reyes-Botella, C. Influence of different surgical techniques on primary implant stability in the posterior maxilla: A randomized controlled clinical trial. Clin. Oral Investig. 2023, 27, 3499–3508. [Google Scholar] [CrossRef]
- Vaddamanu, S.K.; Saini, R.S.; Vyas, R.; Kanji, M.A.; Alshadidi, A.A.F.; Hafedh, S.; Cicciu, M.; Minervini, G. A comparative study on bone density before and after implant placement using osseodensification technique: A clinical evaluation. Int. J. Implant Dent. 2024, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Lima Monteiro, F.; Moreira, C.L.; Galego Arias Pecorari, V.; Cardona Orth, C.; Joly, J.C.; Peruzzo, D. Biomechanical and histomorphometric analysis of osseodensification drilling versus conventional technique: A systematic review and meta-analysis. Quintessence Int. 2024, 55, 212–222. [Google Scholar] [PubMed]
- Mohamed, S.H.; Seenivasan, M.K. Comparison of Osteoblastic Activity Around Endosseous Implants Placed with Osseodensification and Adaptive Osteotomy Techniques—A Split-Mouth Prospective Case-Control Study. Int. J. Oral Maxillofac. Implants 2023, 38, 136–141. [Google Scholar] [CrossRef]
- Insua, A.; Monje, A.; Wang, H.L.; Miron, R.J. Basis of bone metabolism around dental implants during osseointegration and peri-implant bone loss. J. Biomed. Mater. Res. A 2017, 105, 2075–2089. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Chrcanovic, B.; Ostman, P.O.; Sennerby, L. Initial and long-term crestal bone responses to modern dental implants. Periodontol 2000 2017, 73, 41–50. [Google Scholar] [CrossRef]
- Amghar-Maach, S.; Sanchez-Torres, A.; Camps-Font, O.; Gay-Escoda, C. Piezoelectric surgery versus conventional drilling for implant site preparation: A meta-analysis. J. Prosthodont. Res. 2018, 62, 391–396. [Google Scholar] [CrossRef]
- Trisi, P.; Berardini, M.; Falco, A.; Podaliri Vulpiani, M. New Osseodensification Implant Site Preparation Method to Increase Bone Density in Low-Density Bone: In Vivo Evaluation in Sheep. Implant Dent. 2016, 25, 24–31. [Google Scholar] [CrossRef]
- Ho, D.S.; Yeung, S.C.; Zee, K.Y.; Curtis, B.; Hell, P.; Tumuluri, V. Clinical and radiographic evaluation of NobelActive(TM) dental implants. Clin. Oral Implants Res. 2013, 24, 297–304. [Google Scholar] [CrossRef]
- Kim, J.H.; Lim, Y.J.; Kim, B.; Lee, J. How Do Parameters of Implant Primary Stability Correspond with CT-Evaluated Bone Quality in the Posterior Maxilla? A Correlation Analysis. Materials 2021, 14, 270. [Google Scholar] [CrossRef]
- Rabel, A.; Kohler, S.G.; Schmidt-Westhausen, A.M. Clinical study on the primary stability of two dental implant systems with resonance frequency analysis. Clin. Oral Investig. 2007, 11, 257–265. [Google Scholar] [CrossRef]
- Kästel, I.; de Quincey, G.; Neugebauer, J.; Sader, R.; Gehrke, P. Does the manual insertion torque of smartpegs affect the outcome of implant stability quotients (ISQ) during resonance frequency analysis (RFA)? Int. J. Implant Dent. 2019, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Antequera-Diaz, R.; Quesada-García, M.P.; Vallecillo, C.; Vallecillo-Rivas, M.; Muñoz-Soto, E.; Olmedo-Gaya, M.V. Intra- and inter-operator concordance of the resonance frequency analysis. A cross-sectional and prospective clinical study. Clin. Oral Investig. 2022, 26, 6521–6530. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, J.E.; Lobbezoo, F.; Visscher, C.M.; Wismeijer, D.; Naeije, M. Reliability and validity of the instrumental assessment of implant stability in dry human mandibles. J. Oral Rehabil. 2009, 36, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 1—Review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 2004, 17, 536–543. [Google Scholar]
- Becker, W.; Hujoel, P.; Becker, B.E. Resonance frequency analysis: Comparing two clinical instruments. Clin. Implant Dent. Relat. Res. 2018, 20, 308–312. [Google Scholar] [CrossRef]
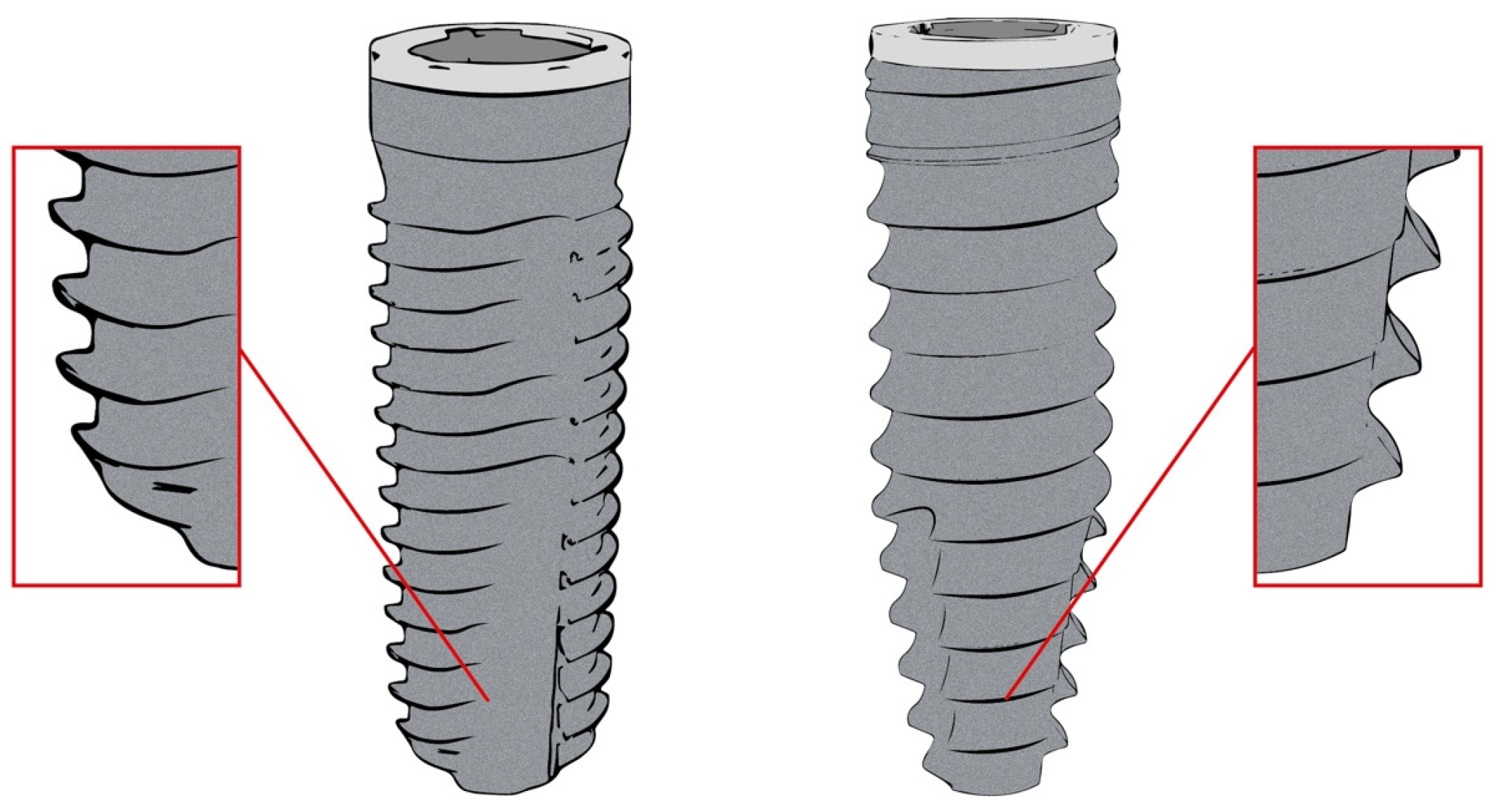
| Implant Dimensions | 3.3 × 11 | 3.3 × 13 | 3.8 × 9 | 3.8 × 11 | 3.8 × 13 | 4.3 × 9 | 4.3 × 11 | 4.3 × 13 | Total n= |
|---|---|---|---|---|---|---|---|---|---|
| n = SL 1 | - | - | 6 | 4 | 10 | 10 | 18 | 2 | 50 |
| n = PL 2 | 2 | 2 | 1 | 15 | 11 | 6 | 12 | 1 | 50 |
| Bone Density | SL Implants 1 | PL Implants 2 |
|---|---|---|
| D1 | 0 | 0 |
| D2 | 0 | 0 |
| D3 | 31 | 34 |
| D4 | 17 | 11 |
| D5 | 1 | 0 |
| Not evaluable | 1 | 5 |
| Total | 50 | 50 |
| Bone Density (Hounsfield Units) | SL Implants 1 | PL Implants 2 |
|---|---|---|
| D5 (HU < 150) | 75.0 (n = 1) | (n = 0) |
| D4 (HU 150–350) | 78.2 (n = 17) | 78.8 (n = 11) |
| D3 (HU 351–850) | 80.5 (n = 32) | 79 (n = 34) |
| Implant Type and Diameter | Anterior Maxilla | Posterior Maxilla | Anterior Mandible | Posterior Mandible |
|---|---|---|---|---|
| SL 1 D3.8 mm | 78.2 (n = 3) | 74.8 (n = 6) | 77.2 (n = 3) | 79.8 (n = 8) |
| SL 1 D4.3 mm | 76.0 (n = 2) | 77.9 (n = 8) | (n = 0) | 82.0 (n = 20) |
| PL 2 D3.3 mm | 69.8 (n = 4) | (n = 0) | (n = 0) | (n = 0) |
| PL 2 D3.8 mm | 73.7 (n = 10) | 80.2 (n = 6) | 75.4 (n = 4) | 80.0 (n = 7) |
| PL 2 D4.3 mm | (n = 0) | 78.3 (n = 10) | (n = 0) | 82.7 (n = 9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seidel, D.; Neugebauer, J.; Dhom, G.; Weinhold, O.; Zimmermann, K.-P.; Sader, R.; Weigl, P.; Gehrke, P. A Comparative Evaluation of the Primary and Secondary Stability of Dental Implants with Progressive and Conventional Thread Designs: A Prospective Non-Interventional Study of 100 Implants in 62 Patients. J. Clin. Med. 2025, 14, 3040. https://doi.org/10.3390/jcm14093040
Seidel D, Neugebauer J, Dhom G, Weinhold O, Zimmermann K-P, Sader R, Weigl P, Gehrke P. A Comparative Evaluation of the Primary and Secondary Stability of Dental Implants with Progressive and Conventional Thread Designs: A Prospective Non-Interventional Study of 100 Implants in 62 Patients. Journal of Clinical Medicine. 2025; 14(9):3040. https://doi.org/10.3390/jcm14093040
Chicago/Turabian StyleSeidel, Daniel, Jörg Neugebauer, Günter Dhom, Octavio Weinhold, Kai-Peter Zimmermann, Robert Sader, Paul Weigl, and Peter Gehrke. 2025. "A Comparative Evaluation of the Primary and Secondary Stability of Dental Implants with Progressive and Conventional Thread Designs: A Prospective Non-Interventional Study of 100 Implants in 62 Patients" Journal of Clinical Medicine 14, no. 9: 3040. https://doi.org/10.3390/jcm14093040
APA StyleSeidel, D., Neugebauer, J., Dhom, G., Weinhold, O., Zimmermann, K.-P., Sader, R., Weigl, P., & Gehrke, P. (2025). A Comparative Evaluation of the Primary and Secondary Stability of Dental Implants with Progressive and Conventional Thread Designs: A Prospective Non-Interventional Study of 100 Implants in 62 Patients. Journal of Clinical Medicine, 14(9), 3040. https://doi.org/10.3390/jcm14093040






