Techniques for Respiratory Motion-Resolved Magnetic Resonance Imaging of the Chest in Children with Spinal or Chest Deformities: A Comprehensive Overview
Abstract
1. Introduction
2. Methods
Search Strategy and Study Selection
3. Results
3.1. Respiratory Motion-Resolving Techniques
| Moment of Respiration Resolving | Gating Technique | Study |
|---|---|---|
| At acquisition | Breath-holding | Chu et al. [26] |
| Chu et al. [27] | ||
| Breathing with maximal inspiration/expiration | Plathow et al. [8] | |
| Kotani et al. [28] | ||
| Synchronized image acquisition using flow and volume signals from external devices (e.g., pneumotachograph, MR-ABC) | Kondo et al. [21] | |
| At image reconstruction | Motion-gated image reconstruction using chest circumference changes measured by respiratory belts | |
| Estimation of respiratory waveform from navigator echoes | Wachinger et al. [29] | |
| Tibiletti et al. [25] | ||
| Estimation of respiratory waveform from k-space center (DC) signal | Weick et al. [19] | |
| Feng et al. [16,30] | ||
| Chen et al. [31] | ||
| Higano et al. [15] | ||
| Jiang et al. [32] | ||
| Xu et al. [33] | ||
| Miller et al. [34] | ||
| Other advanced techniques that do not generate estimates of respiratory signal. Based on spatial and temporal similarity/continuity, or measurement of the motion vector field. | Baumgartner et al. [35] | |
| Tong et al. [36] | ||
| Hao et al. [37] | ||
| Sun et al. [38] |
3.1.1. Two-Dimensional MRI
3.1.2. Three-Dimensional MRI
Fast 3D Imaging: Motion-Resolved MRI Using DC Signal
Three-Dimensional Ultrashort Echo-Time (UTE) MRI
- Limited field of view excitation: This minimizes motion artifacts and improves visualization of areas of interest.
- Variable density readouts: This involves designing gradient waveforms that adjust sampling density in k-space to improve SNR, especially in short T2* species.
- Radial oversampling: More points are collected, which enhances image quality and reduces blurring.
3.1.3. Four-Dimensional MRI
4. Discussion
Future Directions
5. Conclusions
Funding
Conflicts of Interest
Appendix A
| Year | Study | Strategy | Outcome/s | Strengths | Limitations | MRI Respiratory Function Parameters |
|---|---|---|---|---|---|---|
| 2000 | Kondo et al. [21] * | 2D dynamic MRI | 2D dynamic MRI at specific slices | Good spatial resolution | Patient collaboration is required for performing deep-breathings and breath holds. Manual calculation of distances for the analysis of chest wall motion | Chest wall motion, diaphragm motion and cross sectional areas (at acquired 2d slices) |
| 2004 | Plathow et al. [8] * | |||||
| Kotani et al. [28] * | ||||||
| 2006 | Chu et al. [26] * | |||||
| 2007 | Chu et al. [27] * | |||||
| 2012 | Wachinger et al. [29] | Manifold learning in 2D dynamic MRI | 4D chest MRI | Fully automatic. Good correlation with diaphragm tracking | Manifold learning did not provide a direct differentiation of inspiration and expiration stages | Chest wall motion, diaphragm motion, cross sectional areas (at any slice) and lung volumes for estimation of TC, VC, RV, etc. (see Table 1). |
| 2013 | Baumgartner et al. [35] | Manifold learning in 2D dynamic MRI | 4D chest MRI | Good spatial resolution in the coronal plane | Thick slices | |
| Weick et al. [19] | Breathing motion estimation from DC signal in Cartesian 3D FLASH Sequence | 3D chest MRI at expiration, inspiration and intermediate stages | Retrospective self-gating without the need for patient cooperation (free-breathing) | Longer total acquisition times as multiple measurements were needed to ensure enough data for reconstruction | ||
| 2016 | Feng et al. [30] | XD-GRASP reconstruction | 3D chest MRI at a variable number of breathing stages | Allows for reconstructing additional motion-state dimensions | Challenging selection of the appropriate number of respiratory motion stages: too few may limit motion depiction, while too many can lead to increased aliasing artifacts | |
| Tibiletti et al. [25] | Image-based self-gating with 3D UTE imaging | 3D chest MRI at up to 8 breathing stages | Image-based self-gating technique demonstrated significantly better sharpness and diaphragm excursion compared to DC-based self-gating | Maximal temporal resolution is limited and may not be enough to detect rapidly changing irregular breathing patterns | ||
| 2017 | Chen et al. [31] | Manifold learning in k-space data | 2D and 3D chest MRI at as many respiratory positions as the number k-space profiles (2-D) or the number of stacks of k-space profiles (3-D) acquired | Handles both 2D and 3D scenarios | The effectiveness of the method could decrease if there are insufficient profiles at similar respiratory positions, leading to poor reconstruction quality | |
| Tong et al. [36] | Graph-based reconstruction from 2D dynamic MRI | 4D chest MRI | Good spatial and temporal resolution. Tested in patients with chest deformities | Requires manual selection of respiratory stages to delimit different breathing cycles | ||
| Higano et al. [15] * | Breathing motion estimation from DC signal in 3D radial UTE acquisition | 3D chest MRI at end-expiration, near end-expiration, inspiration and near-end inspiration | Removal of bulk motion from DC signal. Short reconstruction times | Accuracy of MRI-measured respiratory rates in neonates needs to be improved | ||
| 2018 | Jiang et al. [32] | 3D Dynamic image navigator from 3D UTE data | 3D chest MRI at a single stage or 4D chest MRI | Robust to irregular breathing patterns | Requires manual identification of the diaphragm. Long reconstruction time using CPU | |
| 2019 | Feng et al. [16] * | XD-GRASP with 3D radial UTE acquisition | 4D chest MRI | Improved image quality, detailed anatomical visualization | Half-spoke acquisition technique to reduce scan time, which affects motion detection and scan efficiency | |
| 2021 | Hao et al. [37] | Optical flux-based reconstruction from 2D dynamic MRI | 4D chest MRI | Good spatial and temporal resolution. Tested in patients with chest deformities. Removal of abnormal breathing cycles. Short reconstruction time. Fully automatic | The cosine function may not be fully suitable to model a normal breathing cycle, as inspiration and expiration phases are not identical in length | |
| 2022 | Sun et al. [38] | Graph-based reconstruction + optical flux for automatic labelling of expiration and inspiration stages | 4D chest MRI | Good spatial and temporal resolution. Tested in patients with chest deformities | Requires manual identification of region of region of interest at the diaphragm | |
| Xu et al. [33] | Oxygen-enhanced lung MRI on 3D UTE acquisition | 3D chest MRI at 24 breathing stages (4 were used for final evaluation) | Increased detail of diaphragm’s edge and lung vessels. | Reduced image quality due to under-sampling, to reduce scan time | ||
| 2023 | Miller et al. [34] | XD-MBDL architecture for 3D UTE MRI | 3D chest MRI at user-selected stage | Short reconstruction time | Lack of a ground truth may challenge the evaluation of image quality and anatomy accuracy |
References
- Weinstein, S.L.; Dolan, L.A.; Cheng, J.C.Y.; Danielsson, A.; Morcuende, J.A. Adolescent idiopathic scoliosis. Lancet 2008, 371, 1527–1537. [Google Scholar] [PubMed]
- Takahashi, S.; Suzuki, N.; Asazuma, T.; Kono, K.; Ono, T.; Toyama, Y. Factors of Thoracic Cage Deformity That Affect Pulmonary Function in Adolescent Idiopathic Thoracic Scoliosis. Spine 2007, 32, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Tsiligiannis, T.; Grivas, T. Pulmonary function in children with idiopathic scoliosis. Scoliosis 2012, 7, 7. [Google Scholar] [CrossRef]
- Farrell, J.; Garrido, E.; Vavruch, L.; Schlösser, T.P.C. Thoracic Morphology and Bronchial Narrowing Are Related to Pulmonary Function in Adolescent Idiopathic Scoliosis. J. Bone Jt. Surg. 2021, 103, 2014–2023. [Google Scholar] [CrossRef]
- Tsukahara, K.; Mayer, O.H. Thoracic insufficiency syndrome: Approaches to assessment and management. Paediatr. Respir. Rev. 2022, 44, 78–84. [Google Scholar] [CrossRef]
- Kempen, D.H.R.; Heemskerk, J.L.; Kaçmaz, G.; Altena, M.C.; Reesink, H.J.; Vanhommerig, J.W.; Willigenburg, N.W. Pulmonary function in children and adolescents with untreated idiopathic scoliosis: A systematic review with meta-regression analysis. Spine J. 2022, 22, 1178–1190. [Google Scholar] [CrossRef]
- Cluzel, P.; Similowski, T.; Chartrand-Lefebvre, C.; Zelter, M.; Derenne, J.-P.; Grenier, P.A. Diaphragm and Chest Wall: Assessment of the Inspiratory Pump with MR Imaging—Preliminary Observations. Radiology 2000, 215, 574–583. [Google Scholar] [CrossRef]
- Plathow, C.; Ley, S.; Fink, C.; Puderbach, M.; Heilmann, M.; Zuna, I.; Kauczor, H.-U. Evaluation of Chest Motion and Volumetry During the Breathing Cycle by Dynamic MRI in Healthy Subjects: Comparison with Pulmonary Function Tests. Investig. Radiol. 2004, 39, 202–209. [Google Scholar] [CrossRef]
- Pietton, R.; Bouloussa, H.; Langlais, T.; Taytard, J.; Beydon, N.; Skalli, W.; Vergari, C.; Vialle, R. Estimating pulmonary function after surgery for adolescent idiopathic scoliosis using biplanar radiographs of the chest with 3D reconstruction. Bone Jt. J. 2022, 104-B, 112–119. [Google Scholar] [CrossRef]
- Assi, A.; Karam, M.; Skalli, W.; Vergari, C.; Vialle, R.; Pietton, R.; Bizdikian, A.; Kharrat, K.; Dubousset, J.; Ghanem, I. A Novel Classification of 3D Rib Cage Deformity in Subjects with Adolescent Idiopathic Scoliosis. Clin. Spine Surg. 2021, 34, 331–341. [Google Scholar] [CrossRef]
- Bouloussa, H.; Pietton, R.; Vergari, C.; Haen, T.X.; Skalli, W.; Vialle, R. Biplanar stereoradiography predicts pulmonary function tests in adolescent idiopathic scoliosis: A cross-sectional study. Eur. Spine J. 2019, 28, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Simony, A.; Hansen, E.J.; Christensen, S.B.; Carreon, L.Y.; Andersen, M.O. Incidence of cancer in adolescent idiopathic scoliosis patients treated 25 years previously. Eur. Spine J. 2016, 25, 3366–3370. [Google Scholar] [CrossRef] [PubMed]
- Lollert, A.; Funk, J.; Tietze, N.; Turial, S.; Laudemann, K.; Düber, C.; Staatz, G. Morphologic assessment of thoracic deformities for the preoperative evaluation of pectus excavatum by magnetic resonance imaging. Eur. Radiol. 2015, 25, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Ciet, P.; Tiddens, H.A.W.M.; Wielopolski, P.A.; Wild, J.M.; Lee, E.Y.; Morana, G.; Lequin, M.H. Magnetic resonance imaging in children: Common problems and possible solutions for lung and airways imaging. Pediatr. Radiol. 2015, 45, 1901–1915. [Google Scholar] [CrossRef]
- Higano, N.; Hahn, A.; Tkach, J.; Cao, X.; Walkup, L.; Thomen, R.; Merhar, S.; Kingma, P.; Fain, S.; Woods, J. Retrospective Respiratory Self-Gating and Removal of Bulk Motion in Pulmonary UTE MRI of Neonates and Adults. Magn. Reson. Med. 2017, 77, 1284–1295. [Google Scholar] [CrossRef]
- Feng, L.; Delacoste, J.; Smith, D.; Weissbrot, J.; Flagg, E.; Moore, W.H.; Girvin, F.; Raad, R.; Bhattacharji, P.; Stoffel, D.; et al. Simultaneous Evaluation of Lung Anatomy and Ventilation Using 4D Respiratory-Motion-Resolved Ultrashort Echo Time Sparse MRI. Magn. Reson. Imaging 2019, 49, 411–422. [Google Scholar] [CrossRef]
- Aromataris, E.; Lockwood, C.; Porritt, K.; Pilla, B.; Jordan, Z. (Eds.) JBI Manual for Evidence Synthesis; JBI: North Adelaide, Australia, 2024; Available online: https://synthesismanual.jbi.global (accessed on 6 December 2024).
- Kolbitsch, C.; Physikalisch-Technische Bundensanstalt. Quantitative MRI. Motion-Compensated MRI. Available online: https://www.ptb.de/cms/en/ptb/fachabteilungen/abt8/fb-81/ag-813/motion-compensated-mri.html (accessed on 3 November 2024).
- Weick, S.; Breuer, F.; Ehses, P.; Völker, M.; Hintze, C.; Biederer, J.; Jakob, P. DC-Gated High Resolution Three-Dimensional Lung Imaging During Free-Breathing. J. Magn. Reson. Imaging 2013, 37, 727–732. [Google Scholar] [CrossRef]
- Molinari, F.; Gaudino, S.; Fink, C.; Corbo, G.M.; Valente, S.; Pirronti, T.; Bonomo, L. Simultaneous Cardiac and Respiratory Synchronization in Oxygen-Enhanced Magnetic Resonance Imaging of the Lung Using a Pneumotachograph for Respiratory Monitoring. Investig. Radiol. 2006, 41, 476–485. [Google Scholar] [CrossRef]
- Kondo, T.; Kobayashi, I.; Taguchi, Y.; Ohta, Y.; Yanagimachi, N. A dynamic analysis of chest wall motions with MRI in healthy young subjects. Respirology 2000, 5, 19–25. [Google Scholar] [CrossRef]
- Arnold, J.F.T.; Mörchel, P.; Glaser, E.; Pracht, E.D.; Jakob, P.M. Lung MRI Using an MR-Compatible Active Breathing Control (MR-ABC). Magn. Reson. Med. 2021, 58, 1092–1098. [Google Scholar] [CrossRef]
- Binks, A.P.; Banzett, R.B.; Duvivier, C. An inexpensive, MRI compatible device to measure tidal volume from chest-wall circumference. Physiol. Meas. 2007, 28, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Ehman, R.L.; Felmlee, J.P. Adaptive Technique for High-Definition MR Imaging of Moving Structures. Radiology 1989, 173, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Tibiletti, M.; Paul, J.; Bianchi, A.; Wundrak, S.; Rottbauer, W.; Stiller, D.; Rasche, V. Multistage Three-Dimensional UTE Lung Imaging by Image-Based Self-Gating. Magn. Reson. Med. 2016, 75, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.C.W.; Li, A.M.; Ng, B.K.W.; Chan, D.F.Y.; Lam, T.; Lam, W.W.M.; Cheng, J.C.Y. Dynamic Magnetic Resonance Imaging in Assessing Lung Volumes, Chest Wall, and Diaphragm Motions in Adolescent Idiopathic Scoliosis Versus Normal Controls. Spine 2006, 31, 2243–2249. [Google Scholar] [CrossRef]
- Chu, W.C.W.; Ng, B.K.W.; Li, A.M.; Lam, T.; Lam, W.W.M.; Cheng, J.C.Y. Dynamic magnetic resonance imaging in assessing lung function in adolescent idiopathic scoliosis: A pilot study of comparison before and after posterior spinal fusion. J. Orthop. Surg. Res. 2007, 2, 20. [Google Scholar] [CrossRef]
- Kotani, T.; Minami, S.; Takahashi, K.; Isobe, K.; Nakata, Y.; Takaso, M.; Inoue, M.; Maruta, T.; Akazawa, T.; Ueda, T.; et al. An Analysis of Chest Wall and Diaphragm Motions in Patients with Idiopathic Scoliosis Using Dynamic Breathing MRI. Spine 2004, 29, 298–302. [Google Scholar] [CrossRef]
- Wachinger, C.; Yigitsoy, M.; Rijkhorst, E.-J.; Navab, N. Manifold learning for image-based breathing gating in ultrasound and MRI. Med. Image Anal. 2012, 16, 806–818. [Google Scholar] [CrossRef]
- Feng, L.; Axel, L.; Chandarana, H.; Block, K.; Sodickson, D.K.; Otazo, R. XD-GRASP: Golden-Angle Radial MRI with Reconstruction of Extra Motion-State Dimensions Using Compressed Sensing. Magn. Reson. Med. 2016, 75, 775–788. [Google Scholar] [CrossRef]
- Chen, X.; Usman, M.; Baumgartner, C.F.; Balfour, D.R.; Marsden, P.K.; Reader, A.J.; Prieto, C.; King, A.P. High-Resolution Self-Gated Dynamic Abdominal MRI Using Manifold Alignment. IEEE Trans. Med. Imaging 2017, 36, 960–971. [Google Scholar] [CrossRef]
- Jiang, W.; Ong, F.; Johnson, K.; Nagle, S.; Hope, T.; Lustig, M.; Larson, P. Motion Robust High Resolution 3D Free-Breathing Pulmonary MRI Using Dynamic 3D Image Self-Navigator. Magn. Reson. Med. 2018, 79, 2954–2967. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, J.; Nan, Z.; Meersmann, T.; Wang, C. Free-Breathing Phase-Resolved Oxygen-Enhanced Pulmonary MRI Based on 3D Stack-of-Stars UTE Sequence. Sensors 2022, 22, 3270. [Google Scholar] [CrossRef] [PubMed]
- Miller, Z.; Johnson, K.M. Motion compensated self supervised deep learning for highly accelerated 3D Ultrashort Echo Time Pulmonary MRI. Magn. Reson. Med. 2023, 89, 2361–2375. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, C.F.; Kolbitsch, C.; McClelland, J.R.; Rueckert, D.; King, A.P. Groupwise Simultaneous Manifold Alignment for High-Resolution Dynamic MR Imaging of Respiratory Motion. In Information Processing in Medical Imaging; Gee, J.C., Joshi, S., Pohl, K.M., Wells, W.M., Zöllei, L., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2013; Volume 7917, pp. 232–243. ISBN 978-3-642-38867-5. Available online: http://link.springer.com/10.1007/978-3-642-38868-2_20 (accessed on 2 July 2024).
- Tong, Y.; Udupa, J.K.; Ciesielski, K.C.; Wu, C.; McDonough, J.M.; Mong, D.A.; Campbell, R.M. Retrospective 4D MR image construction from free-breathing slice Acquisitions: A novel graph-based approach. Med. Image Anal. 2017, 35, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Udupa, J.K.; Tong, Y.; Wu, C.; Li, H.; McDonough, J.M.; Lott, C.; Qiu, C.; Galagedera, N.; Anari, J.B.; et al. OFx: A method of 4D image construction from free-breathing non-gated MRI slice acquisitions of the thorax via optical flux. Med. Image Anal. 2021, 72, 102088. [Google Scholar] [CrossRef]
- Sun, C.; Udupa, J.K.; Tong, Y.; Wu, C.; Guo, S.; McDonough, J.M.; Torigian, D.A.; Cahill, P.J. A Minimally Interactive Method for Labeling Respiratory Phases in Free-Breathing Thoracic Dynamic MRI for Constructing 4D Images. IEEE Trans. Biomed. Eng. 2022, 69, 1424–1434. [Google Scholar] [CrossRef]
- Loyd, H.M.; String, S.T.; DuBois, A.B. Radiographic and Plethysmographic Determination of Total Lung Capacity. Radiology 1966, 86, 7–14. [Google Scholar] [CrossRef]
- Glover, G.H.; Pauly, J.M. Projection Reconstruction Techniques for Reduction of Motion Effects in MRI. Magn. Reson. Med. 1992, 28, 275–289. [Google Scholar] [CrossRef]
- Johnson, K.M.; Fain, S.B.; Schiebler, M.L.; Nagle, S. Optimized 3D ultrashort echo time pulmonary MRI. Magn. Reson. Med. 2013, 70, 1241–1250. [Google Scholar] [CrossRef]
- Hao, Y.; Udupa, J.K.; Tong, Y.; Wu, C.; Li, H.; McDonough, J.M.; Torigian, D.A.; Cahill, P.J. 4D image construction from free-breathing MRI slice acquisitions of the thorax based on a concept of flux. In Proceedings of the Medical Imaging 2020: Physics of Medical Imaging, Houston, TX, USA, 16–19 February 2020; Bosmans, H., Chen, G.-H., Eds.; SPIE: Houston, TX, USA, 2020; p. 91. Available online: https://www.spiedigitallibrary.org/conference-proceedings-of-spie/11312/2550040/4D-image-construction-from-free-breathing-MRI-slice-acquisitions-of/10.1117/12.2550040.full (accessed on 23 October 2024).
- Tong, Y.; Udupa, J.K.; Hao, Y.; Xie, L.; McDonough, J.; Wu, C.; Lott, C.; Clark, A.; Anari, J.B.; Torigian, D.A.; et al. QdMRI: A system for a comprehensive analysis of thoracic dynamics via dynamic MRI. In Proceedings of the Medical Imaging 2022: Image-Guided Procedures, Robotic Interventions, and Modeling, San Diego, CA, USA, 20–23 February 2022; Linte, C.A., Siewerdsen, J.H., Eds.; SPIE: San Diego, CA, USA, 2022; p. 43. Available online: https://www.spiedigitallibrary.org/conference-proceedings-of-spie/12034/2612117/QdMRI--a-system-for-a-comprehensive-analysis-of-thoracic/10.1117/12.2612117.full (accessed on 2 November 2024).
- Tong, Y.; Udupa, J.K.; McDonough, J.M.; Wu, C.; Xie, L.; Rajapakse, C.S.; Gogel, S.; Sarkar, S.; Mayer, O.H.; Anari, J.B.; et al. Characterizing Lung Parenchymal Aeration via Standardized Signal Intensity from Free-breathing 4D Dynamic MRI in Phantoms, Healthy Children, and Pediatric Patients with Thoracic Insufficiency Syndrome. Radiol. Cardiothorac. Imaging 2024, 6, e230262. [Google Scholar] [CrossRef]
- Tong, Y.; Udupa, J.K.; Wileyto, E.P.; Wu, C.; McDonough, J.M.; Capraro, A.; Mayer, O.H.; Torigian, D.A.; Campbell, R.M. Quantitative dynamic MRI (QdMRI) volumetric analysis of pediatric patients with thoracic insufficiency syndrome. In Proceedings of the Medical Imaging 2018: Biomedical Applications in Molecular, Structural, and Functional Imaging, Houston, TX, USA, 11–13 February 2018; Gimi, B., Krol, A., Eds.; SPIE: Houston, TX, USA, 2018; p. 5. Available online: https://www.spiedigitallibrary.org/conference-proceedings-of-spie/10578/2294048/Quantitative-dynamic-MRI-QdMRI-volumetric-analysis-of-pediatric-patients-with/10.1117/12.2294048.full (accessed on 3 November 2024).
- Tong, Y.; Udupa, J.K.; McDonough, J.M.; Wileyto, E.P.; Capraro, A.; Wu, C.; Ho, S.; Galagedera, N.; Talwar, D.; Mayer, O.H.; et al. Quantitative Dynamic Thoracic MRI: Application to Thoracic Insufficiency Syndrome in Pediatric Patients. Radiology 2019, 292, 206–213. [Google Scholar] [CrossRef]
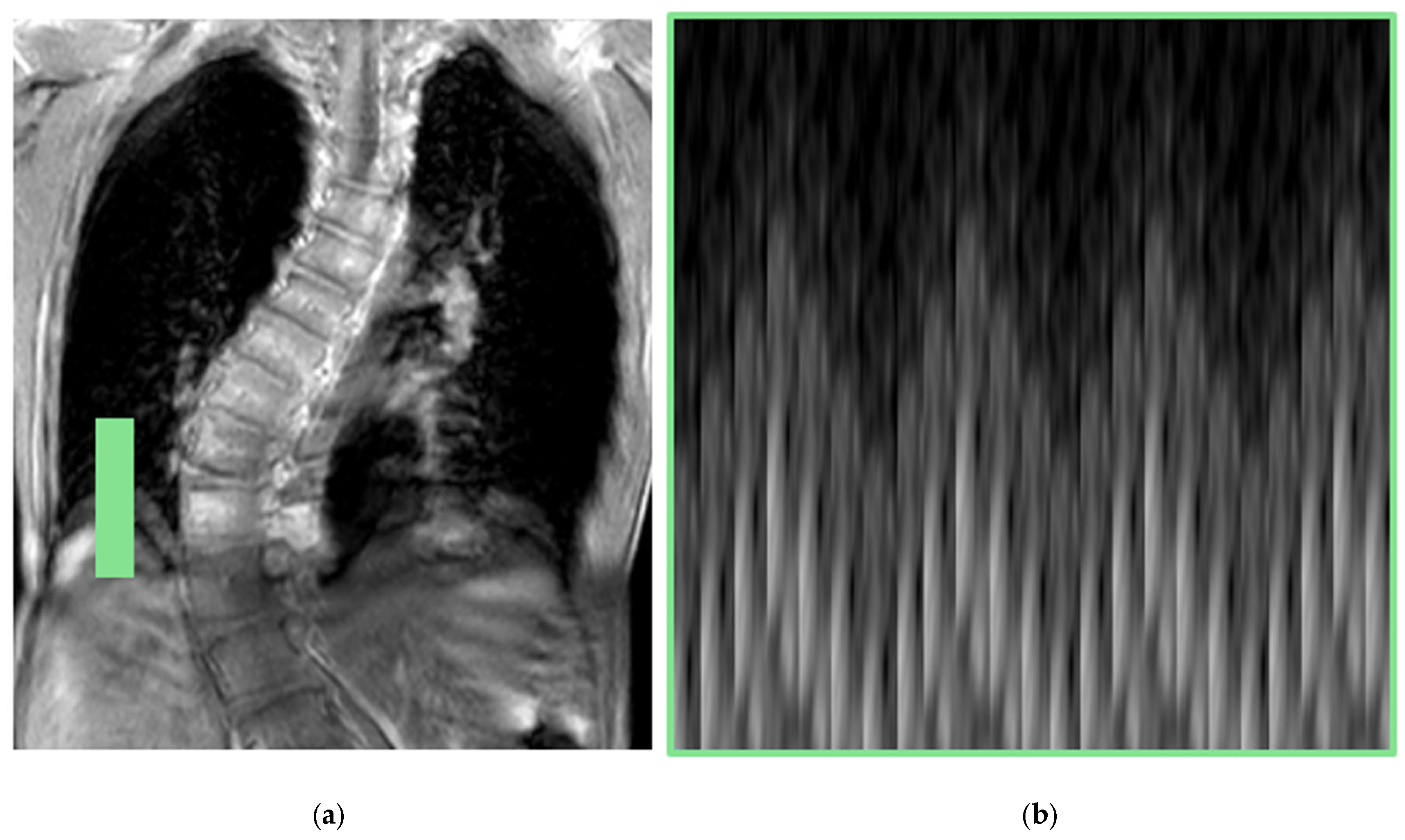
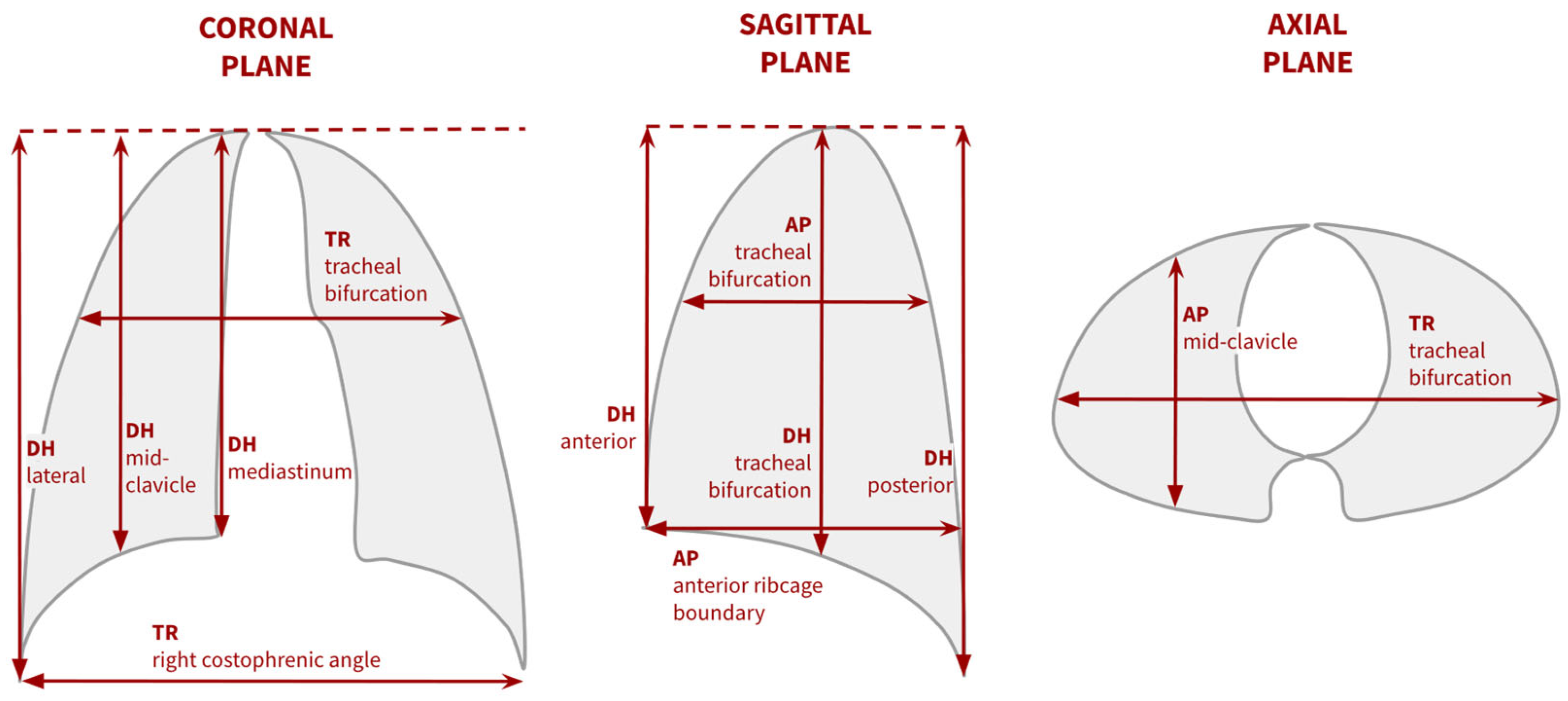

| Image Type | Advantages | Disadvantages |
|---|---|---|
| 2D | High spatial resolution. High temporal resolution in dynamic acquisitions. | Visualization limited to specific slices. |
| 3D | Visualization of complete chest volumes. | In dynamic acquisitions, temporal resolution is achieved at the expense of spatial resolution. |
| 4D | Visualization of complete chest volumes with high spatio-temporal resolution. | Reconstruction requires advanced techniques with high computational burden. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias-Martínez, P.; Lafranca, P.P.G.; Mohamed Hoesein, F.A.A.; Vincken, K.; Schlösser, T.P.C. Techniques for Respiratory Motion-Resolved Magnetic Resonance Imaging of the Chest in Children with Spinal or Chest Deformities: A Comprehensive Overview. J. Clin. Med. 2025, 14, 2916. https://doi.org/10.3390/jcm14092916
Arias-Martínez P, Lafranca PPG, Mohamed Hoesein FAA, Vincken K, Schlösser TPC. Techniques for Respiratory Motion-Resolved Magnetic Resonance Imaging of the Chest in Children with Spinal or Chest Deformities: A Comprehensive Overview. Journal of Clinical Medicine. 2025; 14(9):2916. https://doi.org/10.3390/jcm14092916
Chicago/Turabian StyleArias-Martínez, Paula, Peter P. G. Lafranca, Firdaus A. A. Mohamed Hoesein, Koen Vincken, and Tom P. C. Schlösser. 2025. "Techniques for Respiratory Motion-Resolved Magnetic Resonance Imaging of the Chest in Children with Spinal or Chest Deformities: A Comprehensive Overview" Journal of Clinical Medicine 14, no. 9: 2916. https://doi.org/10.3390/jcm14092916
APA StyleArias-Martínez, P., Lafranca, P. P. G., Mohamed Hoesein, F. A. A., Vincken, K., & Schlösser, T. P. C. (2025). Techniques for Respiratory Motion-Resolved Magnetic Resonance Imaging of the Chest in Children with Spinal or Chest Deformities: A Comprehensive Overview. Journal of Clinical Medicine, 14(9), 2916. https://doi.org/10.3390/jcm14092916







