Retrospective Analysis of Cement Extravasation Rates in Vertebroplasty, Kyphoplasty, and Bone Tumor Radiofrequency Ablation
Abstract
1. Introduction
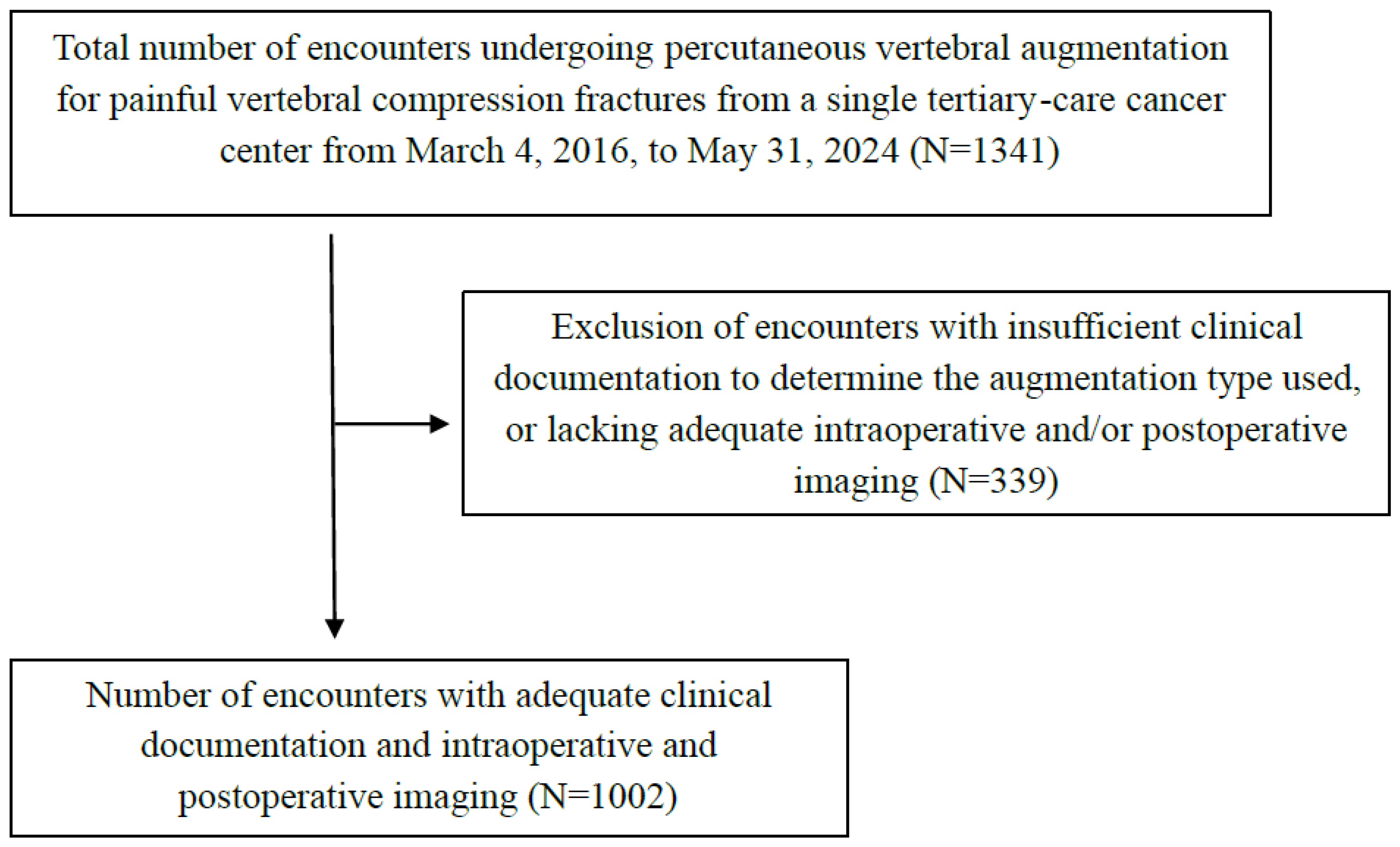
2. Methods
2.1. Study Design
2.2. Study Participants
2.3. Outcomes
2.4. Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvi, M.A.; Zreik, J.; Yolcu, Y.U.; Goyal, A.; Kim, D.K.; Kallmes, D.F.; Freedman, B.A.; Bydon, M. Comparison of Costs and Postoperative Outcomes between Vertebroplasty and Kyphoplasty for Osteoporotic Vertebral Compression Fractures: Analysis from a State-Level Outpatient Database. World Neurosurg. 2020, 141, E801–E814. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Preston, G.; Whaley, J.; Khalil, J.G. Vertebral Augmentation in Spine Surgery. J. Am. Acad. Orthop. Surg. 2023, 31, 477–489. [Google Scholar] [CrossRef]
- Lau, E.; Ong, K.; Kurtz, S.; Schmier, J.; Edidin, A. Mortality Following the Diagnosis of a Vertebral Compression Fracture in the Medicare Population. J. Bone Jt. Surg. 2008, 90, 1479–1486. [Google Scholar] [CrossRef]
- Hinde, K.; Maingard, J.; Hirsch, J.A.; Phan, K.; Asadi, H.; Chandra, R.V. Mortality Outcomes of Vertebral Augmentation (Vertebroplasty and/or Balloon Kyphoplasty) for Osteoporotic Vertebral Compression Fractures: A Systematic Review and Meta-Analysis. Radiology 2020, 295, 96–103. [Google Scholar] [CrossRef]
- Papanastassiou, I.D.; Phillips, F.M.; Meirhaeghe, J.; Berenson, J.R.; Andersson, G.B.J.; Chung, G.; Small, B.J.; Aghayev, K.; Vrionis, F.D. Comparing effects of kyphoplasty, vertebroplasty, and non-surgical management in a systematic review of randomized and non-randomized controlled studies. Eur. Spine J. 2012, 21, 1826–1843. [Google Scholar] [CrossRef]
- Beall, D.; Lorio, M.P.; Yun, B.M.; Runa, M.J.; Ong, K.L.; Warner, C.B. Review of Vertebral Augmentation: An Updated Meta-analysis of the Effectiveness. Int. J. Spine Surg. 2018, 12, 295–321. [Google Scholar] [CrossRef]
- Anderson, P.A.; Froyshteter, A.B.; Tontz, W.L. Meta-analysis of vertebral augmentation compared with conservative treatment for osteoporotic spinal fractures. J. Bone Miner. Res. 2012, 28, 372–382. [Google Scholar] [CrossRef]
- Bornemann, R.; Jansen, T.R.; Kabir, K.; Pennekamp, P.H.; Stüwe, B.; Wirtz, D.C.; Pflugmacher, R. Comparison of Radiofrequency-targeted Vertebral Augmentation with Balloon Kyphoplasty for the Treatment of Vertebral Compression Fractures. Clin. Spine Surg. A Spine Publ. 2017, 30, E247–E251. [Google Scholar] [CrossRef]
- Tomasian, A.; Jennings, J.W. Spine metastases: Thermal ablation and augmentation. Skelet. Radiol. 2023, 52, 1921–1928. [Google Scholar] [CrossRef]
- Mattie, R.; Brar, N.; Tram, J.T.; McCormick, Z.L.; Beall, D.P.; Fox, A.; Saltychev, M. Vertebral Augmentation of Cancer-Related Spinal Compression Fractures: A Systematic Review and Meta-analysis. Spine 2021, 46, 1729–1737. [Google Scholar] [CrossRef]
- Hou, J.-G.; Zhang, N.; Chen, G.-D. Factors affecting cement leakage in percutaneous vertebroplasty: A retrospective cohort study of 309 patients. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 3877–3886. [Google Scholar] [CrossRef] [PubMed]
- Dohm, M.; Black, C.; Dacre, A.; Tillman, J.; Fueredi, G. A Randomized Trial Comparing Balloon Kyphoplasty and Vertebroplasty for Vertebral Compression Fractures due to Osteoporosis. Am. J. Neuroradiol. 2014, 35, 2227–2236. [Google Scholar] [CrossRef] [PubMed]
- Semaan, H.; Obri, T.; Bazerbashi, M.; Paull, D.; Liu, X.; Sarrouj, M.; Elgafy, H. Clinical outcome and subsequent sequelae of cement extravasation after percutaneous kyphoplasty and vertebroplasty: A comparative review. Acta Radiol. 2017, 59, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.N.; Brinjikji, W.; Evans, A.J.; Murad, M.H.; Kallmes, D.F. Outcomes of vertebroplasty compared with kyphoplasty: A systematic review and meta-analysis. J. NeuroInterv. Surg. 2015, 8, 636–642. [Google Scholar] [CrossRef]
- Taylor, R.S.; Taylor, R.J.; Fritzell, P. Balloon kyphoplasty and vertebroplasty for vertebral compression fractures: A comparative systematic re-view of efficacy and safety. Spine 2006, 31, 2747–2755. [Google Scholar] [CrossRef]
- Liu, J.T.; Liao, W.J.; Tan, W.C.; Lee, J.K.; Liu, C.H.; Chen, Y.H.; Lin, T.B. Balloon kyphoplasty versus vertebroplasty for treatment of osteoporotic vertebral compression fracture: A prospective, comparative, and randomized clinical study. Osteoporos. Int. 2009, 21, 359–364. [Google Scholar] [CrossRef]
- Xiao, H.; Yang, J.; Feng, X.; Chen, P.; Li, Y.; Huang, C.; Liang, Y.; Chen, H. Comparing complications of vertebroplasty and kyphoplasty for treating osteoporotic vertebral compression fractures: A meta-analysis of the randomized and non-randomized controlled studies. Eur. J. Orthop. Surg. Traumatol. 2014, 25, 77–85. [Google Scholar] [CrossRef]
- Vogl, T.J.; Pflugmacher, R.; Hierholzer, J.; Stender, G.; Gounis, M.; Wakhloo, A.; Fiebig, C.; Hammerstingl, R. Cement directed kyphoplasty reduces cement leakage as compared with vertebroplasty. Spine 2013, 38, 1730–1736. [Google Scholar] [CrossRef]
- Zhan, Y.; Jiang, J.; Liao, H.; Tan, H.; Yang, K. Risk Factors for Cement Leakage After Vertebroplasty or Kyphoplasty: A Meta-Analysis of Published Evidence. World Neurosurg. 2017, 101, 633–642. [Google Scholar] [CrossRef]
- Wang, C.-H.; Ma, J.-Z.; Zhang, C.-C.; Nie, L. Comparison of high-viscosity cement vertebroplasty and balloon kyphoplasty for the treatment of osteoporotic vertebral compression fractures. Pain Physician 2015, 18, E187–E194. [Google Scholar]
- Chen, W.C.; Tsai, S.H.L.; Goyal, A.; Fu, T.-S.; Lin, T.-Y.; Bydon, M. Comparison between vertebroplasty with high or low viscosity cement augmentation or kyphoplasty in cement leakage rate for patients with vertebral compression fracture: A systematic review and network meta-analysis. Eur. Spine J. 2020, 30, 2680–2690. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.W.; Mendoza, T.; Gebhardt, R.; Hamid, B.; Nouri, K.; Perez-Toro, M.; Ting, J.; Koyyalagunta, D. Vertebral Compression Fracture Treatment with Vertebroplasty and Kyphoplasty: Experience in 407 Patients with 1,156 Fractures in a Tertiary Cancer Center. Pain Med. 2011, 12, 1750–1757. [Google Scholar] [CrossRef]
- Zhang, H.-R.; Xu, M.-Y.; Yang, X.-G.; Qiao, R.-Q.; Li, J.-K.; Hu, Y.-C. Percutaneous vertebral augmentation procedures in the management of spinal metastases. Cancer Lett. 2020, 475, 136–142. [Google Scholar] [CrossRef]
- Yu, Z.; Tian, S.; Wang, W.; Li, Y.; Wang, Y. Biomembrane formation after radiofrequency ablation prevents bone cement extravasation during percutaneous vertebroplasty for treating vertebral metastases with posterior margin destruction: An animal study. J. Cancer Res. Ther. 2020, 16, 1082–1087. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, C.; Liang, H.; Huang, T.; Zhong, W.; Zhao, Z.; Luo, X. Cement leakage in percutaneous vertebroplasty for spinal metastases: A retrospective study of risk factors and clinical outcomes. World J. Surg. Oncol. 2022, 20, 112. [Google Scholar] [CrossRef]
- Corcos, G.; Dbjay, J.; Mastier, C.; Leon, S.; Auperin, A.; De Baere, T.; Deschamps, F. Cement Leakage in Percutaneous Vertebroplasty for Spinal Metastases: A Retrospective Evaluation of Incidence and Risk Factors. Spine 2014, 39, E332–E338. [Google Scholar] [CrossRef]
- Saad, A.; Botchu, R.; James, S. The Rates of Cement Leakage Following Vertebroplasty in Osteoporotic versus Metastatic Disease. Indian J. Radiol. Imaging 2022, 32, 46–50. [Google Scholar] [CrossRef]
- Chew, C.; Craig, L.; Edwards, R.; Moss, J.; O’dwyer, P. Safety and efficacy of percutaneous vertebroplasty in malignancy: A systematic review. Clin. Radiol. 2011, 66, 63–72. [Google Scholar] [CrossRef]
- Shi, X.; Cui, Y.; Pan, Y.; Wang, B.; Lei, M. Epidemiology and detection of cement leakage in patients with spine metastases treated with percutaneous vertebroplasty: A 10-year observational study. J. Bone Oncol. 2021, 28, 100365. [Google Scholar] [CrossRef]
- Nakano, M.; Hirano, N.; Ishihara, H.; Kawaguchi, Y.; Matsuura, K. Calcium phosphate cement leakage after percutaneous vertebroplasty for osteoporotic vertebral fractures: Risk factor analysis for cement leakage. J. Neurosurg. Spine 2005, 2, 27–33. [Google Scholar] [CrossRef]
- Röder, C.; Boszczyk, B.; Perler, G.; Aghayev, E.; Külling, F.; Maestretti, G. Cement volume is the most important modifiable predictor for pain relief in BKP: Results from SWISSspine, a nationwide registry. Eur. Spine J. 2013, 22, 2241–2248. [Google Scholar] [CrossRef] [PubMed]

| Variable | Overall Cohort (n = 888) |
|---|---|
| Age | |
| Mean (SD), years | 64.3 (12.4) |
| Sex | |
| Female, n (%) | 434 (48.9%) |
| Male | 454 (51.1%) |
| BMI | |
| Mean (SD), kg/m2 | 27.4 (6.2) |
| Race | |
| American Indian or Alaska Native | 7 (0.8%) |
| Asian | 39 (4.4%) |
| Black or African American | 82 (9.2%) |
| Caucasian or White | 702 (79.1%) |
| Native Hawaiian or Other Pacific Islander | 2 (0.2%) |
| Other | 51 (5.7%) |
| Unknown | 5 (0.6%) |
| Primary Cancer Diagnosis | |
| Bladder Cancer | 26 (2.9%) |
| Breast Cancer | 114 (12.8%) |
| Colorectal Cancer | 41 (4.6%) |
| Esophageal/Gastric Cancer | 25 (2.8%) |
| Gynecologic Cancer | 25 (2.8%) |
| Head and Neck Cancer | 23 (2.6%) |
| Kidney Cancer | 95 (10.7%) |
| Leukemia | 18 (2.0%) |
| Liver/Gallbladder Cancer | 26 (2.9%) |
| Lung Cancer | 106 (11.9%) |
| Lymphoma | 24 (2.7%) |
| Multiple Myeloma | 122 (13.7%) |
| Myelofibrosis/ Myelodysplastic Syndrome | 14 (1.6%) |
| Pancreatic Cancer | 26 (2.9%) |
| Prostate Cancer | 112 (12.6%) |
| Sarcoma | 25 (2.8%) |
| Skin Cancer | 39 (4.4%) |
| Thyroid Cancer | 10 (1.1%) |
| Other | 12 (1.4%) |
| No Cancer History | 5 (0.6%) |
| Overall Cohort (n = 1002) | Cement Extravasation (n = 573) | No Cement Extravasation (n = 429) | OR | 95% CI | p-Value | |
|---|---|---|---|---|---|---|
| Age | ||||||
| Mean (SD), years | 64.6 (12.3) | 63.4 (12.5) | 66.2 (11.9) | 0.98 | 0.97–0.99 | 0.0005 |
| Age groups | ||||||
| <40 a, n (%) | 42 (4.2%) | 30 (5.2%) | 12 (2.8%) | |||
| 40–49 | 69 (6.9%) | 45 (7.9%) | 24 (5.6%) | 0.75 | 0.33–1.73 | 0.50 |
| 50–59 | 188 (18.8%) | 122 (21.3%) | 66 (15.4%) | 0.74 | 0.35–1.54 | 0.42 |
| 60–69 | 326 (32.5%) | 178 (31.1%) | 148 (34.5%) | 0.48 | 0.24–0.97 | 0.04 |
| 70–79 | 289 (28.8%) | 155 (27.1%) | 134 (31.2%) | 0.46 | 0.23–0.94 | 0.03 |
| 80 or older | 88 (8.8%) | 43 (7.5%) | 45 (10.5%) | 0.28 | 0.17–0.02 | 0.02 |
| Sex | ||||||
| Female a, n (%) | 490 (48.9%) | 305 (53.2%) | 185 (43.1%) | |||
| Male | 512 (51.1%) | 268 (46.8%) | 244 (56.9%) | 0.66 | 0.51–0.86 | 0.002 |
| BMI | ||||||
| Mean (SD), kg/m2 | 27.4 (6.2) | 27.4 (6.4) | 27.4 (5.9) | 1.00 | 0.98–1.02 | 0.93 |
| Fracture type | ||||||
| Non-pathologic a, n (%) | 226 (22.6%) | 108 (18.8%) | 118 (27.5%) | |||
| Pathologic | 776 (77.4%) | 465 (81.2%) | 311 (72.5%) | 1.64 | 1.21–2.21 | 0.001 |
| Levels | ||||||
| Single a, n (%) | 587 (58.6%) | 307 (53.6%) | 280 (65.3%) | |||
| Multilevel | 415 (41.4%) | 266 (46.4%) | 149 (34.7%) | 1.63 | 1.26–2.13 | 0.0003 |
| Bone Density b | ||||||
| Normal a, n (%) | 33 (3.3%) | 20 (3.5%) | 13 (3.0%) | |||
| Osteopenia | 90 (9.0%) | 55 (9.6%) | 35 (8.2%) | 1.02 | 0.45–2.32 | 0.96 |
| Osteoporosis | 111 (11.1%) | 54 (9.4%) | 57 (13.3%) | 0.62 | 0.28–1.36 | 0.23 |
| Unknown | 768 (76.6%) | 444 (77.5%) | 324 (75.5%) | 0.89 | 0.44–1.82 | 0.75 |
| Overall Cohort (n = 1002) | Cement Extravasation (n = 573) | No cement Extravasation (n = 429) | OR | 95% CI | p-Value b | |
|---|---|---|---|---|---|---|
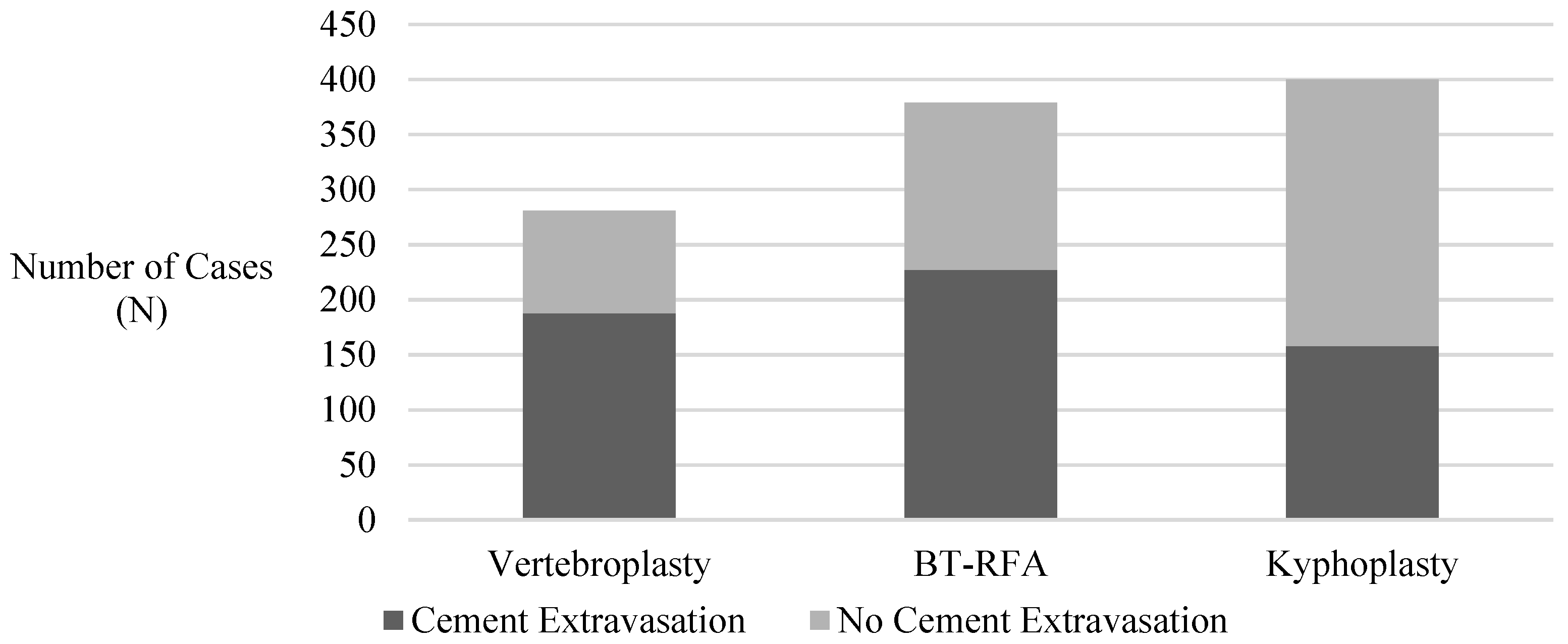
| Kyphoplasty vs. BT-RFA | ||||||
| BT-RFA a, n (%) | 379 (37.8%) | 227 (39.6%) | 152 (35.4%) | |||
| Kyphoplasty | 342 (34.1%) | 158 (27.6%) | 184 (42.9%) | 0.57 | 0.42–0.77 | 0.0009 |
| Kyphoplasty vs. Vertebroplasty | ||||||
| Vertebroplasty a, n (%) | 281 (28.0%) | 188 (32.8%) | 93 (21.7%) | |||
| Kyphoplasty | 342 (34.1%) | 158 (27.6%) | 184 (42.9%) | 0.42 | 0.30–0.58 | <0.0001 |
| Vertebroplasty vs. BT-RFA | ||||||
| BT-RFA a, n (%) | 379 (37.8%) | 227 (39.6%) | 152 (35.4%) | |||
| Vertebroplasty | 281 (28.0%) | 188 (32.8%) | 93 (21.7%) | 1.36 | 0.98–1.89 | 0.1577 |
| Overall Cohort (n = 1002) | Cement Extravasation (n = 573) | No Cement Extravasation (n = 429) | OR | 95% CI | p-Value b | |
|---|---|---|---|---|---|---|
| CT vs. Combined | ||||||
| Combined a, n (%) | 23 (2.3%) | 12 (2.1%) | 11 (2.6%) | |||
| CT | 521 (52.0%) | 315 (55.0%) | 206 (48.0%) | 1.4 | 0.60–3.25 | 0.86 |
| CT vs. Fluoroscopy | ||||||
| Fluoroscopy a, n (%) | 457 (45.6%) | 246 (42.9%) | 211 (49.2%) | |||
| CT | 521 (52.0%) | 315 (55.0%) | 206 (48.0%) | 1.32 | 1.02–1.70 | 0.15 |
| Combined vs. Fluoroscopy | ||||||
| Fluoroscopy a, n (%) | 457 (45.6%) | 246 (42.9%) | 211 (49.2%) | |||
| Combined | 23 (2.3%) | 12 (2.1%) | 11 (2.6%) | 0.94 | 0.40–2.18 | 0.99 |
| Procedure a | Extravasation Location b | p-Value |
|---|---|---|
| Kyphoplasty vs. BT-RFA | Intervertebral disc space vs. Posterior | 0.30 |
| Vertebroplasty vs. BT-RFA | Intervertebral disc space vs. Posterior | 0.81 |
| Kyphoplasty vs. BT-RFA | Paravertebral vs. Posterior | 0.83 |
| Vertebroplasty vs. BT-RFA | Paravertebral vs. Posterior | 0.65 |
| Kyphoplasty vs. BT-RFA | Multidirectional vs. Posterior | 0.53 |
| Vertebroplasty vs. BT-RFA | Multidirectional vs. Posterior | 0.74 |
| Procedure | Pre-Procedure NRS, Mean (SD) | Post-Procedure NRS, Mean (SD) | p-Value |
|---|---|---|---|
| Overall | 3.15 (3.08) | 3.03 (3.22) | 0.39 |
| BT-RFA | 3.24 (3.15) | 3.20 (3.15) | 0.99 |
| Kyphoplasty | 2.89 (2.91) | 3.07 (3.26) | 0.34 |
| Vertebroplasty | 3.40 (3.16) | 2.75 (3.25) | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheen, S.; Hasan, P.; Sun, X.; Wang, J.; Tatsui, C.; Nouri, K.; Javed, S. Retrospective Analysis of Cement Extravasation Rates in Vertebroplasty, Kyphoplasty, and Bone Tumor Radiofrequency Ablation. J. Clin. Med. 2025, 14, 2908. https://doi.org/10.3390/jcm14092908
Sheen S, Hasan P, Sun X, Wang J, Tatsui C, Nouri K, Javed S. Retrospective Analysis of Cement Extravasation Rates in Vertebroplasty, Kyphoplasty, and Bone Tumor Radiofrequency Ablation. Journal of Clinical Medicine. 2025; 14(9):2908. https://doi.org/10.3390/jcm14092908
Chicago/Turabian StyleSheen, Soun, Prit Hasan, Xiaowen Sun, Jian Wang, Claudio Tatsui, Kent Nouri, and Saba Javed. 2025. "Retrospective Analysis of Cement Extravasation Rates in Vertebroplasty, Kyphoplasty, and Bone Tumor Radiofrequency Ablation" Journal of Clinical Medicine 14, no. 9: 2908. https://doi.org/10.3390/jcm14092908
APA StyleSheen, S., Hasan, P., Sun, X., Wang, J., Tatsui, C., Nouri, K., & Javed, S. (2025). Retrospective Analysis of Cement Extravasation Rates in Vertebroplasty, Kyphoplasty, and Bone Tumor Radiofrequency Ablation. Journal of Clinical Medicine, 14(9), 2908. https://doi.org/10.3390/jcm14092908






