Patient-Reported Outcomes with Peripheral Nerve Stimulation for Low Back Pain from Vertebral Plana Deformities: A Case Series
Abstract
1. Introduction
2. Materials and Methods
- Age over 18 years.
- Evaluation at a pain management clinic.
- Radiographically confirmed vertebra plana fracture with >70% vertebral height loss.
- Persistent pain despite standard conservative management.
- Patient’s refusal to participate or to complete a 90-day follow-up.
- Inability to undergo percutaneous PNS due to active infection, severe coagulopathy, or anatomical contraindications.
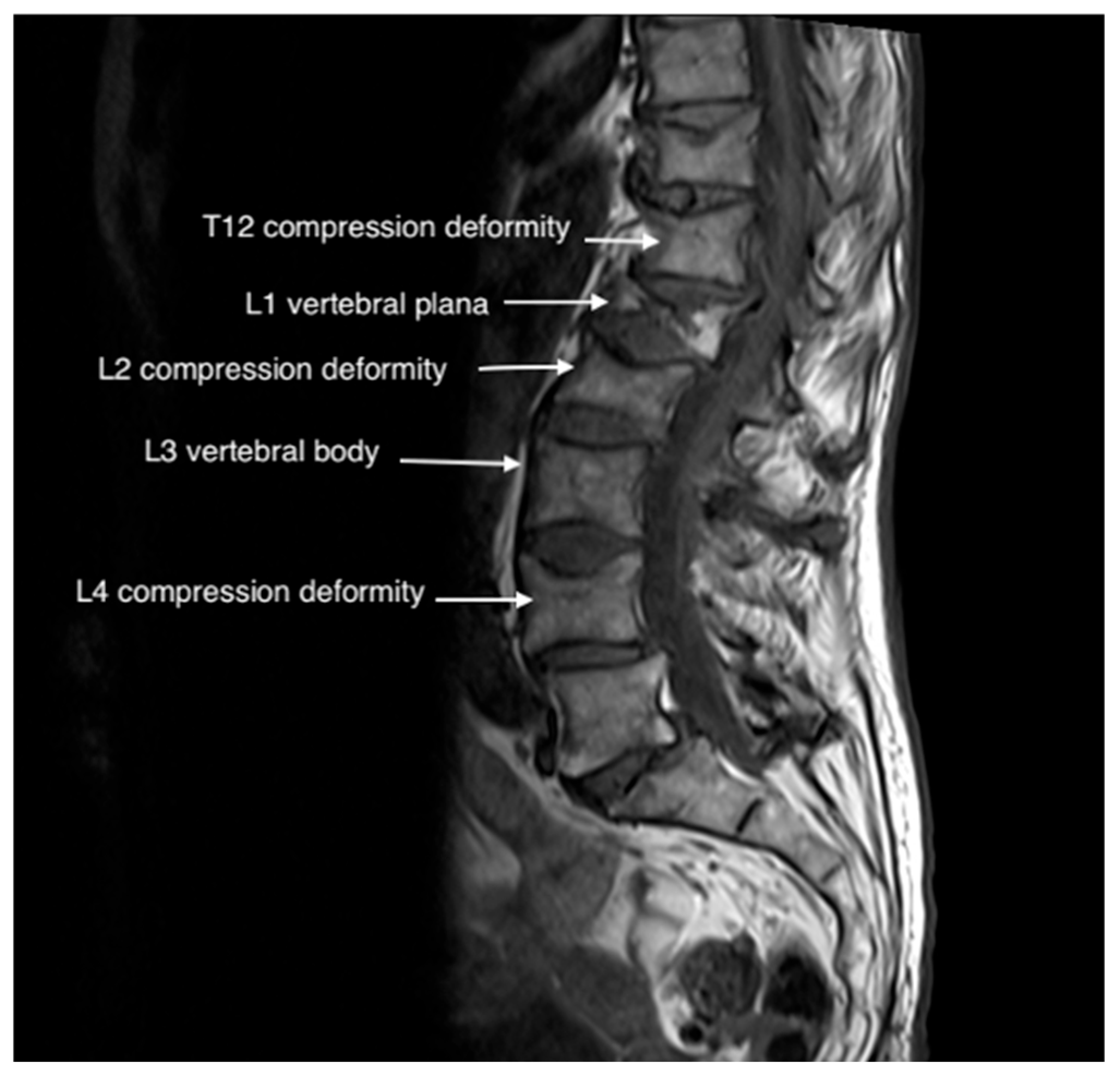
3. Case Presentations
3.1. Case 1
3.2. Case 2
3.3. Case 3
3.4. Case 4
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAD | Coronary artery disease |
| DM | Diabetes mellitus |
| HTN | Hypertension |
| MM | Multiple myeloma |
| MRI | Magnetic resonance imaging |
| OA | Osteoarthritis |
| PNS | Peripheral nerve stimulation |
| PROMIS | Patient-Reported Outcomes Measurement Information System |
| PRO | Patient-reported outcome |
| RFA | Radiofrequency ablation |
| VCF | Vertebral compression fracture |
References
- Kim, S.H.; Jang, S.Y.; Nam, K.; Cha, Y. Analysis of Long-Term Medical Expenses in Vertebral Fracture Patients. Clin. Orthop. Surg. 2023, 15, 989–999. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alexandru, D.; So, W. Evaluation and management of vertebral compression fractures. Perm. J. 2012, 16, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A.; Liguori, S.; Paoletta, M.; Toro, G.; Aulicino, M.; Gimigliano, F.; Iolascon, G. Characterization of neuropathic component of back pain in patients with osteoporotic vertebral fractures. NeuroRehabilitation 2022, 51, 325–331. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nijs, J.; Kosek, E.; Chiarotto, A.; Cook, C.; Danneels, L.A.; Fernández-de-Las-Peñas, C.; Hodges, P.W.; Koes, B.; Louw, A.; Ostelo, R.; et al. Nociceptive, neuropathic, or nociplastic low back pain? The low back pain phenotyping (BACPAP) consortium’s international and multidisciplinary consensus recommendations. Lancet Rheumatol. 2024, 6, e178–e188. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; McGirt, M.J. Vertebral compression fractures: A review of current management and multimodal therapy. J. Multidiscip. Healthc. 2013, 6, 205–214. [Google Scholar]
- Nevitt, M.C.; Ettinger, B.; Black, D.M.; Stone, K.; Jamal, S.A.; Ensrud, K.; Segal, M.; Genant, H.K.; Cummings, S.R. The association of radiographically detected vertebral fractures with back pain and function: A prospective study. Ann. Intern. Med. 1998, 128, 793–800. [Google Scholar] [CrossRef]
- Barr, J.D.; Jensen, M.E.; Hirsch, J.A.; McGraw, J.K.; Barr, R.M.; Brook, A.L.; Meyers, P.M.; Munk, P.L.; Murphy, K.J.; O’toole, J.E.; et al. Position statement on percutaneous vertebral augmentation: A consensus statement developed by the Society of Interventional Radiology (SIR), American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS), American College of Radiology (ACR), American Society of Neuroradiology (ASNR), American Society of Spine Radiology (ASSR), Canadian Interventional Radiology Association (CIRA), and the Society of NeuroInterventional Surgery (SNIS). J. Vasc. Interv. Radiol. 2014, 25, 171–181. [Google Scholar]
- Donnally, C.J., III; DiPompeo, C.M.; Varacallo, M.A. Vertebral Compression Fractures [Updated 4 August 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025; Available online: https://www.ncbi.nlm.nih.gov/books/NBK448171 (accessed on 15 November 2024).
- Savage, J.W.; Schroeder, G.D.; Anderson, P.A. Vertebroplasty and kyphoplasty for the treatment of osteoporotic vertebral compression fractures. J. Am. Acad. Orthop. Surg. 2014, 22, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.A.; Froyshteter, A.B.; Tontz, W.L., Jr. Meta-analysis of vertebral augmentation compared with conservative treatment for osteoporotic spinal fractures. J. Bone Miner. Res. 2013, 28, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Perry, K.; Mach, S.; Huh, B. Case report: Use of peripheral nerve stimulation for treatment of pain from vertebral plana fracture. Front. Pain Res. 2023, 3, 1088097. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sudek, E.W.; Mach, S.; Huh, B.; Javed, S. Use of Temporary Percutaneous Peripheral Nerve Stimulation in an Oncologic Population: A Retrospective Review. Neuromodulation 2024, 27, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Ilfeld, B.M.; Gilmore, C.A.; Grant, S.A.; Bolognesi, M.P.; Del Gaizo, D.J.; Wongsarnpigoon, A.; Boggs, J.W. Ultrasound-guided percutaneous peripheral nerve stimulation for analgesia following total knee arthroplasty: A prospective feasibility study. J. Orthop. Surg. Res. 2017, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Hussain, N.; Abd-Elsayed, A.; Boulos, R.; Hakim, M.; Gupta, M.; Weaver, T. Peripheral Nerve Stimulation for Treatment of Headaches: An Evidence-Based Review. Biomedicines 2021, 9, 1588. [Google Scholar] [CrossRef] [PubMed]
- Chmiela, M.A.; Hendrickson, M.; Hale, J.; Liang, C.; Telefus, P.; Sagir, A.; Stanton-Hicks, M. Direct Peripheral Nerve Stimulation for the Treatment of Complex Regional Pain Syndrome: A 30-Year Review. Neuromodulation 2021, 24, 971–982. [Google Scholar] [CrossRef]
- Gilmore, C.A.; Kapural, L.; McGee, M.J.; Boggs, J.W. Percutaneous Peripheral Nerve Stimulation for Chronic Low Back Pain: Prospective Case Series With 1 Year of Sustained Relief Following Short-Term Implant. Pain. Pract. 2020, 20, 310–320. [Google Scholar] [CrossRef]
- Gilligan, C.; Volschenk, W.; Russo, M.; Green, M.; Gilmore, C.; Mehta, V.; Deckers, K.; De Smedt, K.; Latif, U.; Sayed, D.; et al. Five-Year Longitudinal Follow-Up of Restorative Neurostimulation Shows Durability of Effectiveness in Patients with Refractory Chronic Low Back Pain Associated With Multifidus Muscle Dysfunction. Neuromodulation 2024, 27, 930–943. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.; Do, H.; Alamin, T.; Cheng, I. Facet pain in thoracic compression fractures. Pain. Med. 2010, 11, 1674–1677. [Google Scholar] [CrossRef] [PubMed]
- Amtmann, D.; Cook, K.F.; Jensen, M.P.; Chen, W.-H.; Choi, S.; Revicki, D.; Cella, D.; Rothrock, N.; Keefe, F.; Callahan, L.; et al. Development of a PROMIS item bank to measure pain interference. Pain 2010, 150, 173–182. [Google Scholar] [CrossRef]
- Cook, K.F.; Dunn, W.; Griffith, J.W.; Morrison, M.T.; Tanquary, J.; Sabata, D.; Victorson, D.; Carey, L.M.; MacDermid, J.C.; Dudgeon, B.J.; et al. Pain assessment using the NIH Toolbox. Neurology 2013, 80 (Suppl. S3), S49–S53. [Google Scholar] [CrossRef]
- Ilfeld, B.M.; Plunkett, A.; Vijjeswarapu, A.M.; Hackworth, R.; Dhanjal, S.; Turan, A.; Cohen, S.P.; Eisenach, J.C.; Griffith, S.; Hanling, S.; et al. Percutaneous Peripheral Nerve Stimulation (Neuromodulation) for Postoperative Pain: A Randomized, Sham-controlled Pilot Study. Anesthesiology 2021, 135, 95–110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Theodorou, D.J.; Theodorou, S.J.; Duncan, T.D.; Garfin, S.R.; Wong, W.H. Percutaneous balloon kyphoplasty for the correction of spinal deformity in painful vertebral body compression fractures. Clin. Imaging 2002, 26, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.; Burge, R.T.; Strauss, D.M. One year outcomes and costs following a vertebral fracture. Osteoporos. Int. 2005, 16, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Zuercher, M.; Ummenhofer, W. Cardiac arrest during anesthesia. Curr. Opin. Crit. Care 2008, 14, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Guven, A.E.; Evangelisti, G.; Burkhard, M.D.; Köhli, P.; Hambrecht, J.; Zhu, J.; Chiapparelli, E.; Kelly, M.; Tsuchiya, K.; Amoroso, K.; et al. Asymmetrical atrophy of the paraspinal muscles in patients undergoing unilateral lumbar medial branch radiofrequency neurotomy. Pain 2024, 165, 2130–2134. [Google Scholar] [CrossRef]
- D’Souza, R.S.; Jin, M.Y.; Abd-Elsayed, A. Peripheral Nerve Stimulation for Low Back Pain: A Systematic Review. Curr. Pain. Headache Rep. 2023, 27, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Gargya, A.; Singh, H.; Sivanesan, E.; Gulati, A. Mechanism of Peripheral Nerve Stimulation in Chronic Pain. Pain. Med. 2020, 21, S6–S12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deer, T.R.; Gilmore, C.A.; Desai, M.J.; Li, S.C.; DePalma, M.J.; Hopkins, T.J.; Burgher, A.H.; Spinner, D.A.; Cohen, S.P.; McGee, M.J.; et al. Percutaneous Peripheral Nerve Stimulation of the Medial Branch Nerves for the Treatment of Chronic Axial Back Pain in Patients After Radiofrequency Ablation. Pain. Med. 2021, 22, 548–560. [Google Scholar] [CrossRef]
- Strand, N.H.; D’Souza, R.; Wie, C.; Covington, S.; Maita, M.; Freeman, J.; Maloney, J. Mechanism of Action of Peripheral Nerve Stimulation. Curr. Pain. Headache Rep. 2021, 25, 47–49. [Google Scholar] [CrossRef]
- Gilmore, C.A.; Desai, M.J.; Hopkins, T.J.; Li, S.; DePalma, M.J.; Deer, T.R.; Grace, W.; Burgher, A.H.; Sayal, P.K.; Amirdelfan, K.; et al. Treatment of chronic axial back pain with 60-day percutaneous medial branch PNS: Primary end point results from a prospective, multicenter study. Pain. Pract. 2021, 21, 877–889. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abd-Elsayed, A.; Keith, M.K.; Cao, N.N.; Fiala, K.J.; Martens, J.M. Temporary Peripheral Nerve Stimulation as Treatment for Chronic Pain. Pain. Ther. 2023, 12, 1415–1426. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gilmore, C.A.; Ilfeld, B.M.; Rosenow, J.M.; Li, S.; Desai, M.J.; Hunter, C.W.; Rauck, R.L.; Nader, A.; Mak, J.; Cohen, S.P.; et al. Percutaneous 60-day peripheral nerve stimulation implant provides sustained relief of chronic pain following amputation: 12-month follow-up of a randomized, double-blind, placebo-controlled trial. Reg. Anesth. Pain. Med. 2019, 17, rapm-2019-100937. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Gilmore, C.A.; Rauck, R.L.; Lester, D.D.; Trainer, R.J.; Phan, T.; Kapural, L.; North, J.M.; Crosby, N.D.; Boggs, J.W. Percutaneous Peripheral Nerve Stimulation for the Treatment of Chronic Pain Following Amputation. Mil. Med. 2019, 184, e267–e274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Albright-Trainer, B.; Phan, T.; Trainer, R.J.; Crosby, N.D.; Murphy, D.P.; Disalvo, P.; Amendola, M.; Lester, D.D. Peripheral nerve stimulation for the management of acute and subacute post-amputation pain: A randomized, controlled feasibility trial. Pain. Manag. 2022, 12, 357–369. [Google Scholar] [CrossRef] [PubMed]

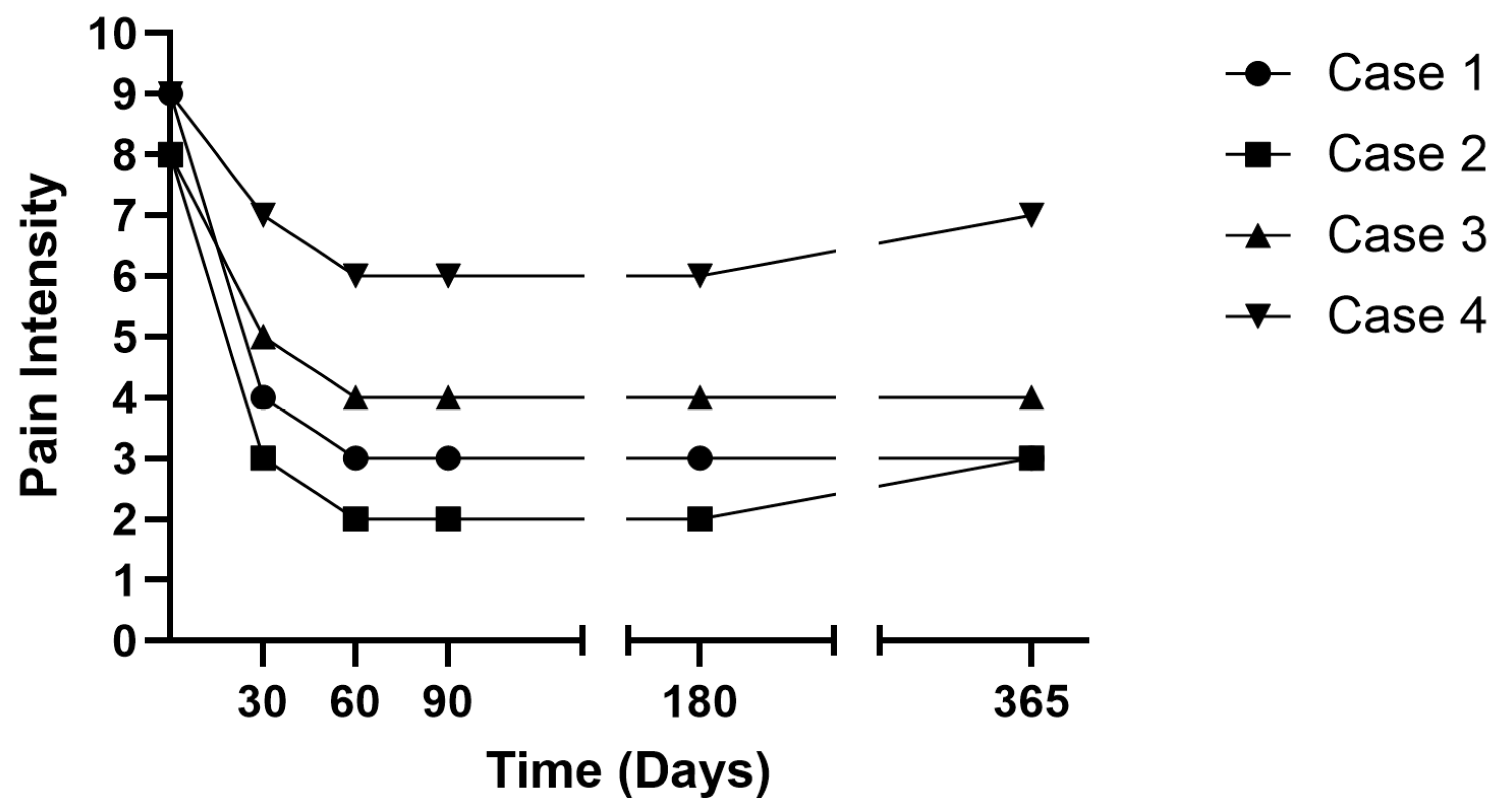



| Patient | Age at PNS Procedure (y) | Sex | Level of Plana Compression | Pathologic vs. Osteoporotic Fracture | PNS Medial Nerve Target | Relevant Comorbidities | Percent Pain Relief |
|---|---|---|---|---|---|---|---|
| 1 | 80 | Female | L1 | Osteoporotic | T12 bilateral | Coronary Artery Disease, Prior Cardiac Arrest | 66% |
| 2 | 71 | Male | L4 | Pathologic | L3 bilateral | Diabetes Mellitus, Rheumatoid Arthritis, Degenerative Disk Disease, Multiple Myeloma | 75% |
| 3 | 85 | Female | L3 | Osteoporotic | L2 bilateral | Coronary Artery Disease, Hypertension, DM, Osteoarthritis | 60% |
| 4 | 85 | Female | L3 | Pathologic | L2 bilateral | Hypertension, Diabetes Mellitus, Osteoarthritis, Multiple Myeloma | 33% |
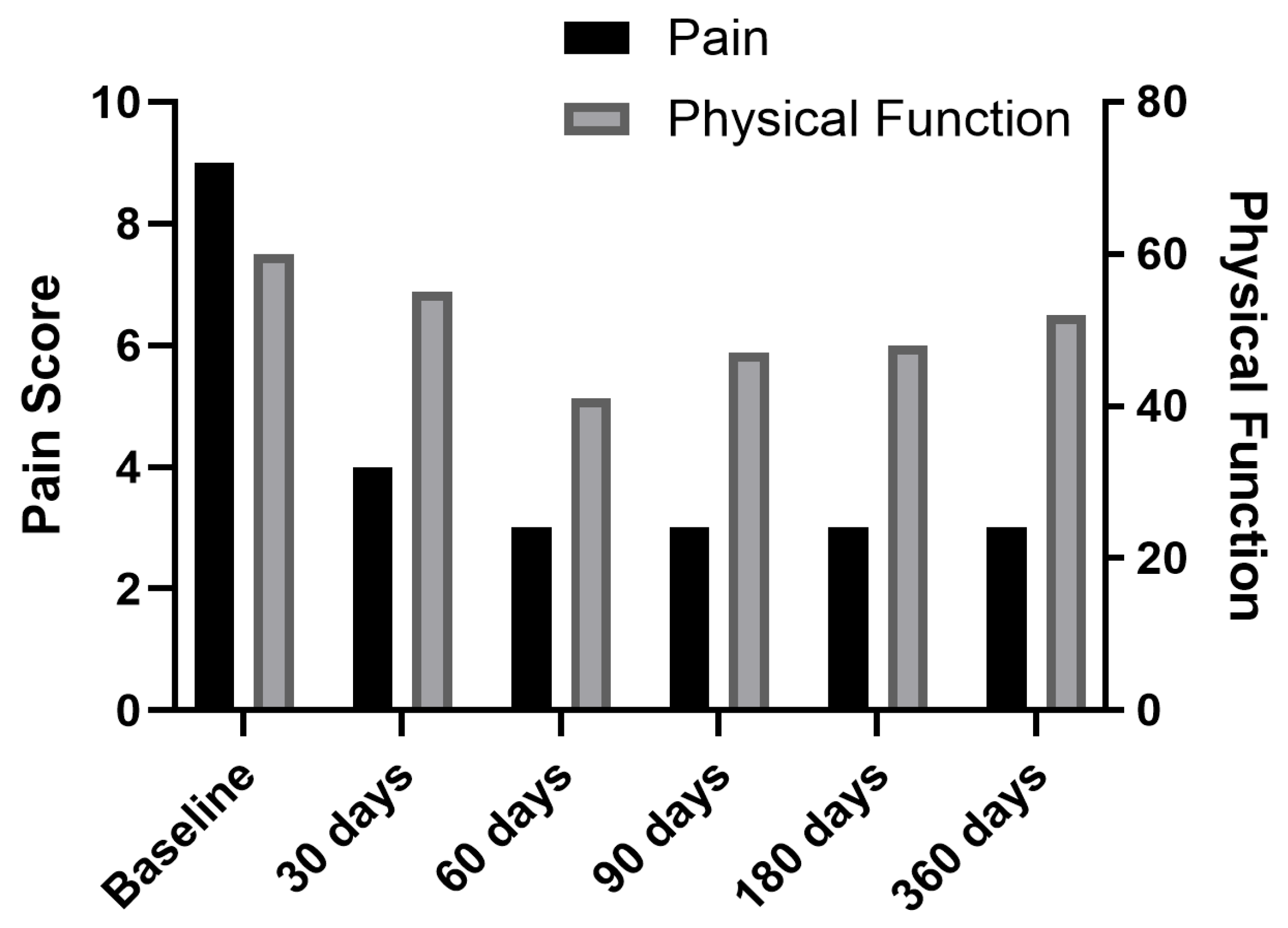
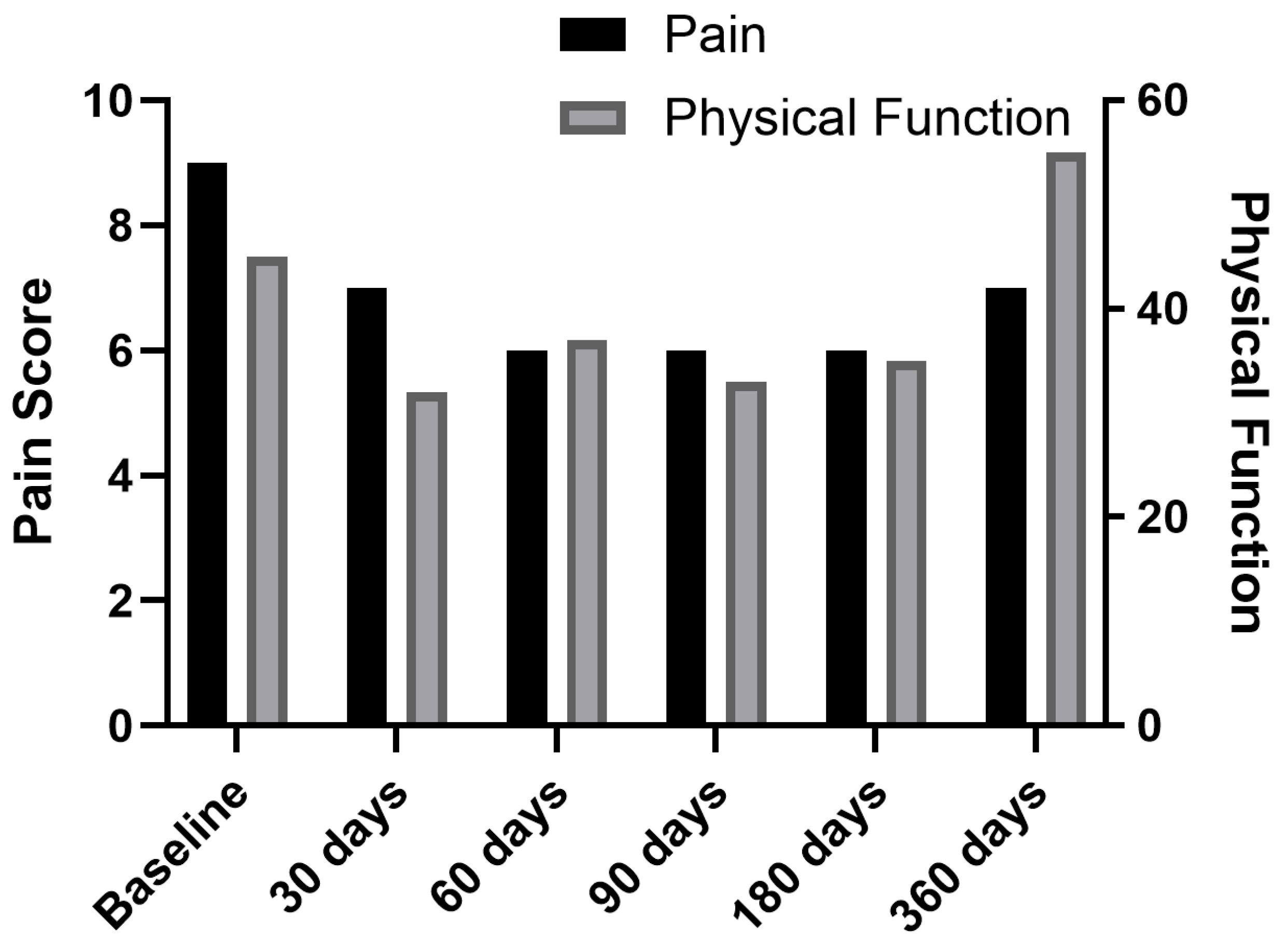
| PROMIS-29 Domains | Baseline | 30 Days | 60 Days | 3 Months | 6 Months | 1 Year |
|---|---|---|---|---|---|---|
| Pain intensity (0–10) | 8.5 (0.6) | 4.75 (1.7) | 3.75 (1.7) | 3.75 (1.7) | 3.75 (1.7) | 4.25 (1.9) |
| Physical function | 50.5 (6.9) | 44.75 (9.5) | 40.25 (2.1) | 38.5 (6.0) | 40.25 (5.5) | 46.75 (7.9) |
| Anxiety | 71 (4.2) | 71.5 (4.4) | 69.75 (4.3) | 69.5 (4.2) | 70 (4.5) | 69.25 (3.8) |
| Depression | 69.25 (2.1) | 65 (1.8) | 64 (1.8) | 69.25 (2.2) | 65.5 (3.9) | 68 (2.3) |
| Fatigue | 68.5 (4.1) | 70.25 (4.3) | 67 (2.3) | 67 (2.3) | 65.25 (2.1) | 65 (2.3) |
| Ability to participate in social roles and activities | 50.75 (1.5) | 48.25 (2.6) | 48 (2.3) | 49 (2.3) | 48.75 (3.2) | 48 (2.3) |
| Pain interference | 61.75 (3.5) | 56.25 (1.0) | 55.5 (1.3) | 55.5 (1.3) | 54.75 (1.7) | 54.75 (1.7) |
| PROMIS-29 Domains | Baseline Score | 1-Year Score | Change Score | MCID | Meets MCID? |
|---|---|---|---|---|---|
| Pain intensity (0–10) | 8.5 | 4.25 | 4.25 | 2.0 | Yes |
| Physical function | 50.5 | 46.75 | 3.75 | 3.0 | Yes |
| Anxiety | 71.0 | 69.25 | 1.75 | 3.0 | No |
| Depression | 69.25 | 68.0 | 1.25 | 3.0 | No |
| Fatigue | 68.5 | 65.0 | 3.5 | 3.0 | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javed, S.; Lam, L.; Nwankwo, A.; Komachkov, Z. Patient-Reported Outcomes with Peripheral Nerve Stimulation for Low Back Pain from Vertebral Plana Deformities: A Case Series. J. Clin. Med. 2025, 14, 3964. https://doi.org/10.3390/jcm14113964
Javed S, Lam L, Nwankwo A, Komachkov Z. Patient-Reported Outcomes with Peripheral Nerve Stimulation for Low Back Pain from Vertebral Plana Deformities: A Case Series. Journal of Clinical Medicine. 2025; 14(11):3964. https://doi.org/10.3390/jcm14113964
Chicago/Turabian StyleJaved, Saba, Loc Lam, Angela Nwankwo, and Zaur Komachkov. 2025. "Patient-Reported Outcomes with Peripheral Nerve Stimulation for Low Back Pain from Vertebral Plana Deformities: A Case Series" Journal of Clinical Medicine 14, no. 11: 3964. https://doi.org/10.3390/jcm14113964
APA StyleJaved, S., Lam, L., Nwankwo, A., & Komachkov, Z. (2025). Patient-Reported Outcomes with Peripheral Nerve Stimulation for Low Back Pain from Vertebral Plana Deformities: A Case Series. Journal of Clinical Medicine, 14(11), 3964. https://doi.org/10.3390/jcm14113964






