Metabolic Syndrome and Liver Disease: Re-Appraisal of Screening, Diagnosis, and Treatment Through the Paradigm Shift from NAFLD to MASLD
Abstract
1. Introduction
2. The Old and the New: From NAFLD to MASLD Definitions
3. Conditions Associated with NAFLD
3.1. Definitions of MetS and Its Components Intertwined with NAFLD
3.1.1. Overweight/Obesity and Their Association with NAFLD
3.1.2. Diabetes and Its Association with NAFLD
3.1.3. HTN and Its Association with NAFLD
3.1.4. Dyslipidemia and Its Association with NAFLD
3.2. Cardiovascular Disease and Its Association with NAFLD
3.3. Other Clinical Conditions and Their Association with NAFLD
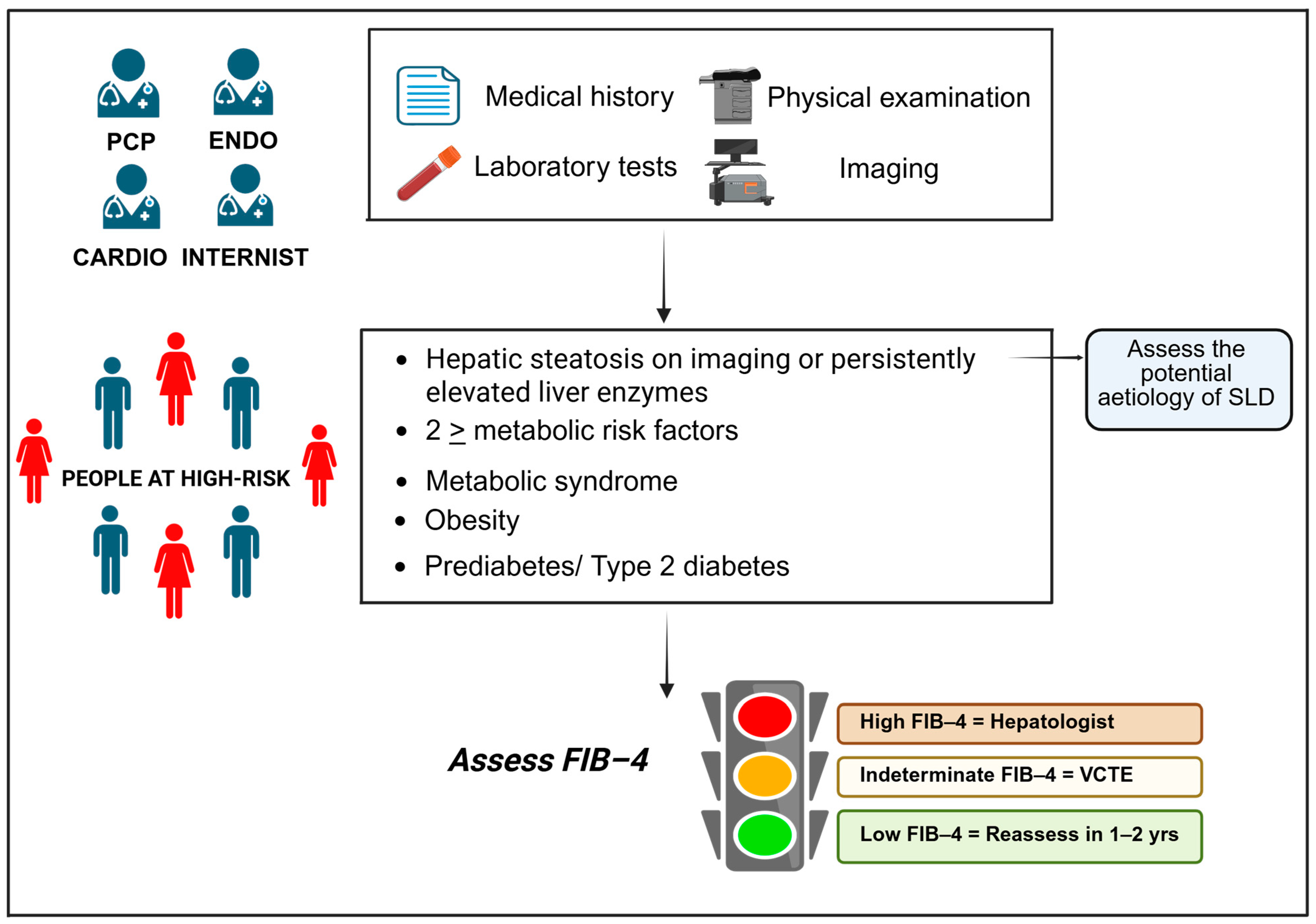
4. Diagnosis and Screening of MASLD
5. Management of MASLD
5.1. Primary Prevention
5.2. Treatment of MASLD
5.3. Secondary Prevention of CVD
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 16 September 2023).
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Le, P.; Tatar, M.; Dasarathy, S.; Alkhouri, N.; Herman, W.H.; Taksler, G.B.; Deshpande, A.; Ye, W.; Adekunle, O.A.; McCullough, A.; et al. Estimated Burden of Metabolic Dysfunction-Associated Steatotic Liver Disease in US Adults, 2020 to 2050. JAMA Netw. Open 2025, 8, e2454707. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Barale, C.; Russo, I. Influence of Cardiometabolic Risk Factors on Platelet Function. Int. J. Mol. Sci. 2020, 21, 623. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, I.; Després, J.P. Metabolic Syndrome: Past, Present and Future. Nutrients 2020, 12, 3501. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Kim, C.H.; Younossi, Z.M. Nonalcoholic fatty liver disease: A manifestation of the metabolic syndrome Clevel. Clin. J. Med. 2008, 75, 721–728. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Loomba, R.; Anstee, Q.M.; Rinella, M.E.; Bugianesi, E.; Marchesini, G.; Neuschwander-Tetri, B.A.; Serfaty, L.; Negro, F.; Caldwell, S.H.; et al. Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatology 2018, 68, 349–360. [Google Scholar] [CrossRef]
- Wong, T.; Wong, R.J.; Gish, R.G. Diagnostic and treatment implications of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Gastroenterol. Hepatol. 2019, 15, 83–89. [Google Scholar]
- Ludwig, J.; Viggiano, T.R.; McGill, D.B.; Oh, B.J. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin. Proc. 1980, 55, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, F.; Thaler, H. Nonalcoholic fatty liver disease. Prog. Liver Dis. 1986, 8, 283–298. [Google Scholar]
- Pais, R.; Maurel, T. Natural History of NAFLD. J. Clin. Med. 2021, 10, 1161. [Google Scholar] [CrossRef]
- Grander, C.; Grabherr, F.; Moschen, A.R.; Tilg, H. Non-Alcoholic Fatty Liver Disease: Cause or Effect of Metabolic Syndrome. Visc. Med. 2016, 32, 329–334. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J.; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastro-Enterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- American Association for the Study of Liver Diseases; Latin American Association for the Study of the Liver; European Association for the Study of the Liver. A call for unity: The path towards a more precise and patient-centric nomenclature for NAFLD. Hepatology 2023, 78, 3–5. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung and Blood Institute scientific statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Balkau, B.; Charles, M.A. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet. Med. 1999, 16, 442–443. [Google Scholar] [PubMed]
- Einhorn, D.; Reaven, G.M.; Cobin, R.H.; Ford, E.; Ganda, O.P.; Handelsman, Y.; Hellman, R.; Jellinger, P.S.; Kendall, D.; Krauss, R.M.; et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr. Pract. 2003, 9, 237–252. [Google Scholar] [CrossRef]
- Dobrowolski, P.; Prejbisz, A.; Kuryłowicz, A.; Baska, A.; Burchardt, P.; Chlebus, K.; Dzida, G.; Jankowski, P.; Jaroszewicz, J.; Jaworski, P.; et al. Metabolic syndrome—A new definition and management guidelines: A joint position paper by the Polish Society of Hypertension, Polish Society for the Treatment of Obesity, Polish Lipid Association, Polish Association for Study of Liver, Polish Society of Family Medicine, Polish Society of Lifestyle Medicine, Division of Prevention and Epidemiology Polish Cardiac Society, “Club 30” Polish Cardiac Society, and Division of Metabolic and Bariatric Surgery Society of Polish Surgeons. Arch. Med. Sci. 2022, 18, 1133–1156. [Google Scholar]
- Suwała, S.; Junik, R. Assessment of the Liver Steatosis and Fibrosis Risk in Metabolic Syndrome and Its Individual Components, Considering the Varying Definitions Used in Clinical Practice throughout Time: A Retrospective Cross-Sectional Study. Biomedicines 2024, 12, 1739. [Google Scholar] [CrossRef]
- Wainwright, P.; Byrne, C.D. Bidirectional Relationships Disconnects between NAFLD and Features of the Metabolic Syndrome. Int. J. Mol. Sci. 2016, 17, 367. [Google Scholar] [CrossRef]
- Meex, R.C.R.; Watt, M.J. Hepatokines: Linking nonalcoholic fatty liver disease and insulin resistance. Nat. Rev. Endocrinol. 2017, 13, 509–520. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Jinjuvadia, R.; Antaki, F.; Lohia, P.; Liangpunsakul, S. The Association Between Nonalcoholic Fatty Liver Disease Metabolic Abnormalities in The United States Population. J. Clin. Gastroenterol. 2017, 51, 160–166. [Google Scholar] [CrossRef]
- WHO WHO European Regional Obesity Report 2022; WHO Regional Office for Europe: Copenhagen, Denmark, 2022; ISBN 978-92-890-5773-8.
- Marchesini, G.; Moscatiello, S.; Di Domizio, S.; Forlani, G. Obesity-associated liver disease. J. Clin. Endocrinol. Metab. 2008, 93 (Suppl. S1), S74–S80. [Google Scholar] [CrossRef]
- Quek, J.; Chan, K.E.; Wong, Z.Y.; Tan, C.; Tan, B.; Lim, W.H.; Tan, D.J.H.; Tang, A.S.P.; Tay, P.; Xiao, J.; et al. Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, D.W.; Yan, H.Y.; Wang, Z.Y.; Zhao, S.H.; Wang, B. Obesity is an independent risk factor for non-alcoholic fatty liver disease: Evidence from a meta-analysis of 21 cohort studies. Obes. Rev. 2016, 17, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yan, C.; Wen, J.; Wang, F.; Wu, H.; Xu, F. Association between different obesity patterns and the risk of NAFLD detected by transient elastography: A cross-sectional study. BMC Gastroenterol. 2024, 24, 221. [Google Scholar] [CrossRef] [PubMed]
- Kotani, K.; Tokunaga, K.; Fujioka, S.; Kobatake, T.; Keno, Y.; Yoshida, S.; Shimomura, I.; Tarui, S.; Matsuzawa, Y. Sexual dimorphism of age-related changes in whole-body fat distribution in the obese. Int. J. Obes. Relat. Metab. Disord. 1994, 18, 207–212. [Google Scholar]
- Wajchenberg, B.L. Subcutaneous and visceral adipose tissue: Their relation to the metabolic syndrome. Endocr. Rev. 2000, 21, 697–738. [Google Scholar] [CrossRef]
- Manolopoulos, K.N.; Karpe, F.; Frayn, K.N. Gluteofemoral body fat as a determinant of metabolic health. Int. J. Obes. 2010, 34, 949–959. [Google Scholar] [CrossRef]
- Karastergiou, K.; Smith, S.R.; Greenberg, A.S.; Fried, S.K. Sex differences in human adipose tissues—The biology of pear shape. Biol. Sex Differ. 2012, 3, 13. [Google Scholar] [CrossRef]
- Neeland, I.J.; Ross, R.; Després, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Tchernof, A.; Després, J.P. Pathophysiology of human visceral obesity: An update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, H.; Liu, Z.; Xu, C. Association of the android to gynoid fat ratio with nonalcoholic fatty liver disease: A cross-sectional study. Front. Nutr. 2023, 10, 1162079. [Google Scholar] [CrossRef]
- Koskinas, K.C.; Van Craenenbroeck, E.M.; Antoniades, C.; Blüher, M.; Gorter, T.M.; Hanssen, H.; Marx, N.; McDonagh, T.A.; Mingrone, G.; Rosengren, A.; et al. Obesity cardiovascular disease: An ESC clinical consensus statement. Eur. J. Prev. Cardiol. 2025, 32, 184–220. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, M.; Yu, H.; Huang, Y.; Yang, B.; Wu, B.; Yang, Y. Body mass index and waist-to-height ratio effect on mortality in non-alcoholic fatty liver: Revisiting the obesity paradox. Front. Endocrinol. 2024, 15, 1419715. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47 (Suppl. S1), S20–S42. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Price, J.K.; Owrangi, S.; Gundu-Rao, N.; Satchi, R.; Paik, J.M. The Global Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis Among Patients with Type 2 Diabetes. Clin. Gastroenterol. Hepatol. 2024, 22, 1999–2010.e8. [Google Scholar] [CrossRef]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Tilg, H.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: An updated meta-analysis of 501 022 adult individuals. Gut 2021, 70, 962–969. [Google Scholar] [CrossRef]
- Targher, G.; Corey, K.E.; Byrne, C.D.; Roden, M. The complex link between NAFLD and type 2 diabetes mellitus—Mechanisms and treatments. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 599–612. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, Y.; He, Y.; Zhang, L.; Liu, J.; Zheng, S.; Bai, Y. The bidirectional relationship between NAFLD and type 2 diabetes: A prospective population-based cohort study. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1521–1528. [Google Scholar] [CrossRef]
- Lomonaco, R.; Bril, F.; Portillo-Sanchez, P.; Ortiz-Lopez, C.; Orsak, B.; Biernacki, D.; Lo, M.; Suman, A.; Weber, M.H.; Cusi, K. Metabolic Impact of Nonalcoholic Steatohepatitis in Obese Patients with Type 2 Diabetes. Diabetes Care 2016, 39, 632–638. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [PubMed]
- World Health Organization (WHO). Hypertension. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 20 March 2023).
- Oikonomou, D.; Georgiopoulos, G.; Katsi, V.; Kourek, C.; Tsioufis, C.; Alexopoulou, A.; Koutli, E.; Tousoulis, D. Non-alcoholic fatty liver disease and hypertension: Coprevalent or correlated? Eur. J. Gastroenterol. Hepatol. 2018, 30, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Aneni, E.C.; Oni, E.T.; Martin, S.S.; Blaha, M.J.; Agatston, A.S.; Feldman, T.; Veledar, E.; Conçeicao, R.D.; Carvalho, J.A.; Santos, R.D.; et al. Blood pressure is associated with the presence severity of nonalcoholic fatty liver disease across the spectrum of cardiometabolic risk. J. Hypertens. 2015, 33, 1207–1214. [Google Scholar] [CrossRef]
- Singh, S.; Allen, A.M.; Wang, Z.; Prokop, L.J.; Murad, M.H.; Loomba, R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: A systematic review and meta-analysis of paired-biopsy studies. Clin. Gastroenterol. Hepatol. 2015, 13, 643–654. [Google Scholar] [CrossRef]
- Li, G.; Peng, Y.; Chen, Z.; Li, H.; Liu, D.; Ye, X. Bidirectional Association between Hypertension and NAFLD: A Systematic Review and Meta-Analysis of Observational Studies. Int. J. Endocrinol. 2022, 2022, 8463640. [Google Scholar] [CrossRef]
- Watt, M.J.; Miotto, P.M.; De Nardo, W.; Montgomery, M.K. The liver as an endocrine organ-linking NAFLD and insulin resistance. Endocr. Rev. 2019, 40, 1367–1393. [Google Scholar] [CrossRef]
- Nakagami, H. Mechanisms underlying the bidirectional association between nonalcoholic fatty liver disease and hypertension. Hypertens. Res. 2023, 46, 539–541. [Google Scholar] [CrossRef]
- López-Suárez, A.; Guerrero, J.M.; Elvira-González, J.; Beltrán-Robles, M.; Cañas-Hormigo, F.; Bascuñana-Quirell, A. Nonalcoholic fatty liver disease is associated with blood pressure in hypertensive and nonhypertensive individuals from the general population with normal levels of alanine aminotransferase. Eur. J. Gastroenterol. Hepatol. 2011, 23, 1011–1017. [Google Scholar] [CrossRef]
- Ng, C.H.; Wong, Z.Y.; Chew, N.W.S.; Chan, K.E.; Xiao, J.; Sayed, N.; Lim, W.H.; Tan, D.J.H.; Loke, R.W.K.; Tay, P.W.L.; et al. Hypertension is prevalent in non-alcoholic fatty liver disease and increases all-cause and cardiovascular mortality. Front. Cardiovasc. Med. 2022, 9, 942753. [Google Scholar] [CrossRef]
- Pirillo, A.; Casula, M.; Olmastroni, E.; Norata, G.D.; Catapano, A.L. Global epidemiology of dyslipidaemias. Nat. Rev. Cardiol. 2021, 18, 689–700. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Raised Cholesterol: Situation and trends. Available online: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/3236 (accessed on 20 September 2023).
- Deprince, A.; Haas, J.T.; Staels, B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol. Metab. 2020, 42, 101092. [Google Scholar] [CrossRef] [PubMed]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell. Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F.; Mora, S.; Stroes, E.S.G.; Ference, B.A.; Arsenault, B.J.; Berglund, L.; Dweck, M.R.; Koschinsky, M.; Lambert, G.; Mach, F.; et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: A European Atherosclerosis Society consensus statement. Eur. Heart J. 2022, 43, 3925–3946. [Google Scholar] [CrossRef]
- Meroni, M.; Longo, M.; Lombardi, R.; Paolini, E.; Macchi, C.; Corsini, A.; Sirtori, C.R.; Fracanzani, A.L.; Ruscica, M.; Dongiovanni, P. Low Lipoprotein(a) Levels Predict Hepatic Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Hepatol. Commun. 2022, 6, 535–549. [Google Scholar] [CrossRef]
- Simon, T.G.; Roelstraete, B.; Hagström, H.; Sundström, J.; Ludvigsson, J.F. Non-alcoholic fatty liver disease and incident major adverse cardiovascular events: Results from a nation-wide histology cohort. Gut 2022, 71, 1867–1875. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Zhou, X.D.; Wu, S.J.; Fan, D.H.; Van Poucke, S.; Chen, Y.P.; Fu, S.W.; Zheng, M.H. Non-alcoholic fatty liver disease contributes to subclinical atherosclerosis: A systematic review and meta-analysis. Hepatol. Commun. 2018, 2, 376–392. [Google Scholar] [CrossRef]
- Wong, M.Y.Z.; Yap, J.J.L.; Sultana, R.; Cheah, M.; Goh, G.B.B.; Yeo, K.K. Association between non-alcoholic fatty liver disease and subclinical atherosclerosis in Western and Asian cohorts: An updated meta-analysis. Open Heart 2021, 8, e001850. [Google Scholar] [CrossRef]
- Jamalinia, M.; Zare, F.; Lankarani, K.B. Systematic review and meta-analysis: Association between liver fibrosis and subclinical atherosclerosis in nonalcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2023, 58, 384–394. [Google Scholar] [CrossRef]
- Sinn, D.H.; Kang, D.; Chang, Y.; Ryu, S.; Cho, S.J.; Paik, S.W.; Song, Y.B.; Pastor-Barriuso, R.; Guallar, E.; Cho, J.; et al. Non-alcoholic fatty liver disease the incidence of myocardial infarction: A cohort study. J. Gastroenterol. Hepatol. 2020, 35, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Petracca, G.; Csermely, A.; Beatrice, G.; Bonapace, S.; Rossi, A.; Tilg, H.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and risk of new-onset heart failure: An updated meta-analysis of about 11 million individuals. Gut 2022, 72, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Csermely, A.; Petracca, G.; Beatrice, G.; Corey, K.E.; Simon, T.G.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: An updated systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 903–913. [Google Scholar] [CrossRef]
- Xiao, J.; Ng, C.H.; Chan, K.E.; Fu, C.; Tay, P.; Yong, J.N.; Lim, W.H.; Tan, D.J.H.; Syn, N.; Wong, Z.Y.; et al. Hepatic, Extra-hepatic Outcomes and Causes of Mortality in NAFLD—An Umbrella Overview of Systematic Review of Meta-Analysis. J. Clin. Exp. Hepatol. 2023, 13, 656–665. [Google Scholar] [CrossRef]
- Alon, L.; Corica, B.; Raparelli, V.; Cangemi, R.; Basili, S.; Proietti, M.; Romiti, G.F. Risk of cardiovascular events in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2022, 29, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.E.; Ng, C.H.; Yong, J.N.; Chan, K.E.; Xiao, J.; Nah, B.; Bong, S.H.S.; Win, K.M.; Bwa, A.H.; Lim, W.H.; et al. A Meta-analysis on Associated Risk of Mortality in Nonalcoholic Fatty Liver Disease. Endocr. Pract. 2023, 29, 33–39. [Google Scholar] [CrossRef]
- Stahl, E.P.; Dhindsa, D.S.; Lee, S.K.; Sandesara, P.B.; Chalasani, N.P.; Sperling, L.S. Nonalcoholic Fatty Liver Disease and the Heart: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 948–963. [Google Scholar] [CrossRef] [PubMed]
- Duell, P.B.; Welty, F.K.; Miller, M.; Chait, A.; Hammond, G.; Ahmad, Z.; Cohen, D.E.; Horton, J.D.; Pressman, G.S.; Toth, P.P.; et al. Nonalcoholic Fatty Liver Disease and Cardiovascular Risk: A Scientific Statement From the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2022, 42, e168–e185. [Google Scholar] [CrossRef]
- Stefan, N.; Yki-Järvinen, H.; Neuschwander-Tetri, B.A. Metabolic dysfunction-associated steatosis liver disease: Heterogeneous pathomechanisms and effectiveness of metabolism-based treatment. Lancet Diabetes Endocrinol. 2025, 13, 134–148. [Google Scholar] [CrossRef]
- Golabi, P.; Otgonsuren, M.; de Avila, L.; Sayiner, M.; Rafiq, N.; Younossi, Z.M. Components of metabolic syndrome increase the risk of mortality in nonalcoholic fatty liver disease (NAFLD). Medicine 2018, 97, e0214. [Google Scholar] [CrossRef]
- Jin, S.; Jiang, S.; Hu, A. Association between obstructive sleep apnea and non-alcoholic fatty liver disease: A systematic review and meta-analysis. Sleep Breath. 2018, 22, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Mesarwi, O.A.; Loomba, R.; Malhotra, A. Obstructive Sleep Apnea, Hypoxia, and Nonalcoholic Fatty Liver Disease. Am. J. Respir. Crit. Care Med. 2019, 199, 830–841. [Google Scholar] [CrossRef]
- Hany, M.; Abouelnasr, A.A.; Abdelkhalek, M.H.; Ibrahim, M.; Aboelsoud, M.R.; Hozien, A.I.; Torensma, B. Effects of obstructive sleep apnea on non-alcoholic fatty liver disease in patients with obesity: A systematic review. Int. J. Obes. 2023, 47, 1200–1213. [Google Scholar] [CrossRef] [PubMed]
- Kumarendran, B.; O’Reilly, M.W.; Manolopoulos, K.N.; Toulis, K.A.; Gokhale, K.M.; Sitch, A.J.; Wijeyaratne, C.N.; Coomarasamy, A.; Arlt, W.; Nirantharakumar, K. Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: A longitudinal study based on a United Kingdom primary care database. PLoS Med. 2018, 15, e1002542. [Google Scholar] [CrossRef]
- Manzano-Nunez, R.; Santana-Dominguez, M.; Rivera-Esteban, J.; Sabiote, C.; Sena, E.; Bañares, J.; Tacke, F.; Pericàs, J.M. Non-Alcoholic Fatty Liver Disease in Patients with Polycystic Ovary Syndrome: A Systematic Review, Meta-Analysis, and Meta-Regression. J. Clin. Med. 2023, 12, 856. [Google Scholar] [CrossRef]
- Mantovani, A.; Nascimbeni, F.; Lonardo, A.; Zoppini, G.; Bonora, E.; Mantzoros, C.S.; Targher, G. Association Between Primary Hypothyroidism and Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Thyroid 2018, 28, 1270–1284. [Google Scholar] [CrossRef] [PubMed]
- Hatziagelaki, E.; Paschou, S.A.; Schön, M.; Psaltopoulou, T.; Roden, M. NAFLD and thyroid function: Pathophysiological and therapeutic considerations. Trends Endocrinol. Metab. 2022, 33, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Csermely, A.; Bilson, J.; Borella, N.; Enrico, S.; Pecoraro, B.; Shtembari, E.; Morandin, R.; Polyzos, S.A.; Valenti, L.; et al. Association between primary hypothyroidism and metabolic dysfunction-associated steatotic liver disease: An updated meta-analysis. Gut 2024, 73, 1554–1561. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Mousiolis, A.C.; Mintziori, G.; Tarenidou, C.; Polyzos, S.A.; Goulis, D.G. Hypogonadism and nonalcoholic fatty liver disease. Endocrine 2024, 86, 28–47. [Google Scholar] [CrossRef]
- Lu, M.; Flanagan, J.U.; Langley, R.J.; Hay, M.P.; Perry, J.K. Targeting growth hormone function: Strategies and therapeutic applications. Signal Transduct. Target. Ther. 2019, 4, 3. [Google Scholar] [CrossRef]
- Doycheva, I.; Erickson, D.; Watt, K.D. Growth hormone deficiency and NAFLD: An overlooked and underrecognized link. Hepatol. Commun. 2022, 6, 2227–2237. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.A.; Lee, H.W.; Ahn, S.H.; Lee, E.J.; Ku, C.R.; Kim, S.U. Positive association between nonalcoholic fatty liver disease and growth hormone deficiency in patients with nonfunctioning pituitary adenoma. Front. Endocrinol. 2023, 13, 1057769. [Google Scholar] [CrossRef]
- Malik, A.; Javaid, S.; Malik, M.I.; Qureshi, S. Relationship between sarcopenia and metabolic dysfunction-associated steatotic liver disease (MASLD): A systematic review and meta-analysis. Ann. Hepatol. 2024, 29, 101544. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Zaza, G.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Bonora, E.; Targher, G. Nonalcoholic fatty liver disease increases risk of incident chronic kidney disease: A systematic review and meta-analysis. Metabolism 2018, 79, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Ciardullo, S.; Ballabeni, C.; Trevisan, R.; Perseghin, G. Liver Stiffness, Albuminuria and Chronic Kidney Disease in Patients with NAFLD: A Systematic Review and Meta-Analysis. Biomolecules 2022, 12, 105. [Google Scholar] [CrossRef]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Csermely, A.; Lonardo, A.; Schattenberg, J.M.; Tilg, H.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and risk of incident chronic kidney disease: An updated meta analysis. Gut 2022, 71, 156–162. [Google Scholar] [CrossRef]
- Mayén, A.L.; Sabra, M.; Aglago, E.K.; Perlemuter, G.; Voican, C.; Ramos, I.; Debras, C.; Blanco, J.; Viallon, V.; Ferrari, P.; et al. Hepatic steatosis, metabolic dysfunction and risk of mortality: Findings from a multinational prospective cohort study. BMC Med. 2024, 22, 221. [Google Scholar] [CrossRef]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Csermely, A.; Tilg, H.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: A meta-analysis of observational cohort studies. Gut 2022, 71, 778–788. [Google Scholar] [CrossRef]
- Thomas, J.A.; Kendall, B.J.; Dalais, C.; Macdonald, G.A.; Thrift, A.P. Hepatocellular and extrahepatic cancers in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Eur. J. Cancer 2022, 173, 250–262. [Google Scholar] [CrossRef]
- Esposito, K.; Chiodini, P.; Colao, A.; Lenzi, A.; Giugliano, D. Metabolic syndrome and risk of cancer: A systematic review and meta-analysis. Diabetes Care 2012, 35, 2402–2411. [Google Scholar] [CrossRef]
- Uzunlulu, M.; Telci Caklili, O.; Oguz, A. Association between Metabolic Syndrome and Cancer. Ann. Nutr. Metab. 2016, 68, 173–179. [Google Scholar] [CrossRef] [PubMed]
- López-Jiménez, T.; Duarte-Salles, T.; Plana-Ripoll, O.; Recalde, M.; Xavier-Cos, F.; Puente, D. Association between metabolic syndrome and 13 types of cancer in Catalonia: A matched case-control study. PLoS ONE 2022, 17, e0264634. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liu, T.; Liu, C.A.; Zhang, Q.; Song, M.M.; Lin, S.Q.; Wang, Y.M.; Zhang, Q.S.; Shi, H.P. The association of metabolic syndrome score trajectory patterns with risk of all cancer types. Cancer 2024, 130, 2150–2159. [Google Scholar] [CrossRef]
- Souza, M.; Diaz, I.; Barchetta, I.; Mantovani, A. Gastrointestinal cancers in lean individuals with non-alcoholic fatty liver disease: A systematic review and meta-analysis. Liver Int. 2024, 44, 6–14. [Google Scholar] [CrossRef]
- Ng, C.H.; Lim, W.H.; Hui Lim, G.E.; Hao Tan, D.J.; Syn, N.; Muthiah, M.D.; Huang, D.Q.; Loomba, R. Mortality Outcomes by Fibrosis Stage in Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 931–939.e5. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N. Epidemiology and risk-stratification of NAFLD-associated HCC. J. Hepatol. 2021, 75, 1476–1484. [Google Scholar] [CrossRef]
- Tovo, C.V.; de Mattos, A.Z.; Coral, G.P.; Sartori, G.D.P.; Nogueira, L.V.; Both, G.T.; Villela-Nogueira, C.A.; de Mattos, A.A. Hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis. World J. Gastroenterol. 2023, 29, 343–356. [Google Scholar] [CrossRef]
- Grgurevic, I.; Bozin, T.; Mikus, M.; Kukla, M.; O’Beirne, J. Hepatocellular Carcinoma in Non-Alcoholic Fatty Liver Disease: From Epidemiology to Diagnostic Approach. Cancers 2021, 13, 5844. [Google Scholar] [CrossRef]
- Huang, D.Q.; Noureddin, N.; Ajmera, V.; Amangurbanova, M.; Bettencourt, R.; Truong, E.; Gidener, T.; Siddiqi, H.; Majzoub, A.M.; Nayfeh, T.; et al. Type 2 diabetes, hepatic decompensation, and hepatocellular carcinoma in patients with non-alcoholic fatty liver disease: An individual participant-level data meta-analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 829–836. [Google Scholar] [CrossRef]
- Ratziu, V.; Boursier, J.; AFEF Group for the Study of Liver Fibrosis. Confirmatory biomarker diagnostic studies are not needed when transitioning from NAFLD to MASLD. J. Hepatol. 2024, 80, e51–e52. [Google Scholar] [CrossRef]
- Song, S.J.; Lai, J.C.; Wong, G.L.; Wong, V.W.; Yip, T.C. Can we use old NAFLD data under the new MASLD definition? J. Hepatol. 2024, 80, e54–e56. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Paik, J.M.; Stepanova, M.; Ong, J.; Alqahtani, S.; Henry, L. Clinical profiles and mortality rates are similar for metabolic dysfunction-associated steatotic liver disease and non-alcoholic fatty liver disease. J. Hepatol. 2024, 80, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Hagström, H.; Vessby, J.; Ekstedt, M.; Shang, Y. 99% of patients with NAFLD meet MASLD criteria and natural history is therefore identical. J. Hepatol. 2024, 80, e76–e77. [Google Scholar] [CrossRef]
- Kanwal, F.; Neuschwander-Tetri, B.A.; Loomba, R.; Rinella, M.E. Metabolic dysfunction-associated steatotic liver disease: Update and impact of new nomenclature on the American Association for the Study of Liver Diseases practice guidance on nonalcoholic fatty liver disease. Hepatology 2024, 79, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef]
- Marušić, M.; Paić, M.; Knobloch, M.; Liberati Pršo, A.M. NAFLD, Insulin Resistance Diabetes Mellitus Type 2. Can. J. Gastroenterol. Hepatol. 2021, 2021, 6613827. [Google Scholar] [CrossRef]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef]
- Li, J.; Lu, X.; Zhu, Z.; Kalutkiewicz, K.J.; Mounajjed, T.; Therneau, T.M.; Venkatesh, S.K.; Sui, Y.; Glaser, K.J.; Hoodeshenas, S.; et al. Head-to-head comparison of magnetic resonance elastography-based liver stiffness fat fraction T1 relaxation time in identifying at-risk, N.A.S.H. Hepatology 2023, 78, 1200–1208. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M.; Pagano, G. Meta-analysis: Natural history of nonalcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann. Med. 2011, 43, 617–649. [Google Scholar] [CrossRef]
- Loomba, R.; Adams, L.A. The 20% Rule of NASH Progression: The Natural History of Advanced Fibrosis Cirrhosis Caused by, N.A.S.H. Hepatology 2019, 70, 1885–1888. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Eltelbany, A.; Mohammed, A.; Alsabbagh Alchirazi, K.; Trakroo, S.; Asaad, I. The epidemiology of non-alcoholic steatohepatitis (NASH) in the United States between 2010-2020: A population-based study. Ann. Hepatol. 2022, 27, 100727. [Google Scholar] [CrossRef]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Sebastiani, G.; Ekstedt, M.; Hagstrom, H.; Nasr, P.; et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Van Natta, M.L.; Clark, J.; Neuschwander-Tetri, B.A.; Diehl, A.; Dasarathy, S.; Loomba, R.; Chalasani, N.; Kowdley, K.; Hameed, B.; et al. Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N. Engl. J. Med. 2021, 385, 1559–1569. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Mangla, K.K.; Berentzen, T.L.; Grau, K.; Kjær, M.S.; Ladelund, S.; Nitze, L.M.; Coolbaugh, C.; Hsu, C.Y.; Hagström, H. Liver histology is associated with long-term clinical outcomes in patients with metabolic dysfunction-associated steatohepatitis. Hepatol. Commun. 2024, 8, e0423. [Google Scholar] [CrossRef]
- Kanwal, F.; Shubrook, J.H.; Adams, L.A.; Pfotenhauer, K.; Wai-Sun Wong, V.; Wright, E.; Abdelmalek, M.F.; Harrison, S.A.; Loomba, R.; Mantzoros, C.S.; et al. Clinical Care Pathway for the Risk Stratification and Management of Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2021, 161, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- National Guideline Centre (UK). Non-Alcoholic Fatty Liver Disease: Assessment and Management; National Institute for Health and Care Excellence (NICE): London, UK, 2016. [Google Scholar]
- Cusi, K.; Isaacs, S.; Barb, D.; Basu, R.; Caprio, S.; Garvey, W.T.; Kashyap, S.; Mechanick, J.I.; Mouzaki, M.; Nadolsky, K.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pract. 2022, 28, 528–562. [Google Scholar]
- Wattacheril, J.J.; Abdelmalek, M.F.; Lim, J.K.; Sanyal, A.J. AGA Clinical Practice Update on the Role of Noninvasive Biomarkers in the Evaluation and Management of Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2023, 165, 1080–1088. [Google Scholar] [CrossRef]
- Lee, J.; Vali, Y.; Boursier, J.; Spijker, R.; Anstee, Q.M.; Bossuyt, P.M.; Zafarmand, M.H. Prognostic accuracy of FIB-4, NAFLD fibrosis score and APRI for NAFLD-related events: A systematic review. Liver Int. 2021, 41, 261–270. [Google Scholar] [CrossRef]
- Papatheodoridi, M.; Hiriart, J.B.; Lupsor-Platon, M.; Bronte, F.; Boursier, J.; Elshaarawy, O.; Marra, F.; Thiele, M.; Markakis, G.; Payance, A.; et al. Refining the Baveno VI elastography criteria for the definition of compensated advanced chronic liver disease. J. Hepatol. 2021, 74, 1109–1116. [Google Scholar] [CrossRef]
- Mózes, F.E.; Lee, J.A.; Selvaraj, E.A.; Jayaswal, A.N.A.; Trauner, M.; Boursier, J.; Fournier, C.; Staufer, K.; Stauber, R.E.; Bugianesi, E.; et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: An individual patient data meta-analysis. Gut 2022, 71, 1006–1019. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Cusi, K.; Hilliard, M.E.; Isaacs, D.; et al. 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. S1), S49–S67. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47 (Suppl. S1), S52–S76. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Hardy, T.; Dufour, J.F.; Petta, S.; Romero-Gomez, M.; Allison, M.; Oliveira, C.P.; Francque, S.; Van Gaal, L.; Schattenberg, J.M.; et al. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am. J. Gastroenterol. 2017, 112, 740–751. [Google Scholar] [CrossRef]
- Ishiba, H.; Sumida, Y.; Tanaka, S.; Yoneda, M.; Hyogo, H.; Ono, M.; Fujii, H.; Eguchi, Y.; Suzuki, Y.; Yoneda, M.; et al. The novel cutoff points for the FIB4 index categorized by age increase the diagnostic accuracy in NAFLD: A multi-center study. J. Gastroenterol. 2018, 53, 1216–1224. [Google Scholar] [CrossRef]
- Caussy, C.; Soni, M.; Cui, J.; Bettencourt, R.; Schork, N.; Chen, C.H.; Ikhwan, M.A.; Bassirian, S.; Cepin, S.; Gonzalez, M.P.; et al. Nonalcoholic fatty liver disease with cirrhosis increases familial risk for advanced fibrosis. J. Clin. Investig. 2017, 127, 2697–2704. [Google Scholar] [CrossRef]
- Tamaki, N.; Ahlholm, N.; Luukkonen, P.K.; Porthan, K.; Sharpton, S.R.; Ajmera, V.; Kono, Y.; Dave, S.; Ahmed, A.; Sundaram, V.; et al. Risk of advanced fibrosis in first-degree relatives of patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2022, 132, e162513. [Google Scholar] [CrossRef] [PubMed]
- Yeo, Y.H.; Hwang, J.; Jeong, D.; Dang, N.; Kam, L.Y.; Henry, L.; Park, H.; Cheung, R.; Nguyen, M.H. Surveillance of patients with cirrhosis remains suboptimal in the United States. J. Hepatol. 2021, 75, 856–864. [Google Scholar] [CrossRef]
- Tincopa, M.A.; Loomba, R. Non-invasive diagnosis and monitoring of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Lancet Gastroenterol. Hepatol. 2023, 8, 660–670. [Google Scholar] [CrossRef]
- Yilmaz, Y. Letter: Beyond advanced fibrosis-The critical need for assessing NITs performance in identifying F2-F3 fibrosis. Aliment. Pharmacol. Ther. 2024, 60, 974–975. [Google Scholar] [CrossRef]
- Ratziu, V.; Harrison, S.A.; Hajji, Y.; Magnanensi, J.; Petit, S.; Majd, Z.; Delecroix, E.; Rosenquist, C.; Hum, D.; Staels, B.; et al. NIS2+TM as a screening tool to optimize patient selection in metabolic dysfunction-associated steatohepatitis clinical trials. J. Hepatol. 2024, 80, 209–219. [Google Scholar] [CrossRef]
- Kim, B.K.; Tamaki, N.; Imajo, K.; Yoneda, M.; Sutter, N.; Jung, J.; Lin, T.; Tu, X.M.; Bergstrom, J.; Nguyen, K.; et al. Head-to-head comparison between, M.E.F.I.B.; MAST; FAST for detecting stage 2 fibrosis or higher among patients with NAFLD. J. Hepatol. 2022, 77, 1482–1490. [Google Scholar] [CrossRef]
- Ravaioli, F.; Dajti, E.; Mantovani, A.; Newsome, P.N.; Targher, G.; Colecchia, A. Diagnostic accuracy of FibroScan-AST (FAST) score for the non-invasive identification of patients with fibrotic non-alcoholic steatohepatitis: A systematic review and meta-analysis. Gut 2023, 72, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Stefanakis, K.; Mingrone, G.; George, J.; Mantzoros, C.S. Accurate non-invasive detection of MASH with fibrosis F2-F3 using a lightweight machine learning model with minimal clinical and metabolomic variables. Metabolism 2025, 163, 156082. [Google Scholar] [CrossRef] [PubMed]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple non-invasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Vergniol, J.; Guillet, A.; Hiriart, J.B.; Lannes, A.; Le Bail, B.; Michalak, S.; Chermak, F.; Bertrais, S.; Foucher, J.; et al. Diagnostic accuracy prognostic significance of blood fibrosis tests liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J. Hepatol. 2016, 65, 570–578. [Google Scholar] [CrossRef]
- Vali, Y.; Lee, J.; Boursier, J.; Petta, S.; Wonders, K.; Tiniakos, D.; Bedossa, P.; Geier, A.; Francque, S.; Allison, M.; et al. Biomarkers for staging fibrosis and non-alcoholic steatohepatitis in non-alcoholic fatty liver disease (the LITMUS project): A comparative diagnostic accuracy study. Lancet Gastroenterol. Hepatol. 2023, 8, 714–725. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef]
- Hagström, H.; Nasr, P.; Ekstedt, M.; Stål, P.; Hultcrantz, R.; Kechagias, S. Accuracy of Noninvasive Scoring Systems in Assessing Risk of Death and Liver-Related Endpoints in Patients with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2019, 17, 1148–1156.e4. [Google Scholar] [CrossRef]
- Rosenberg, W.M.; Voelker, M.; Thiel, R.; Becka, M.; Burt, A.; Schuppan, D.; Hubscher, S.; Roskams, T.; Pinzani, M.; Arthur, M.J.; et al. Serum markers detect the presence of liver fibrosis: A cohort study. Gastroenterology 2004, 127, 1704–1713. [Google Scholar] [CrossRef]
- Day, J.; Patel, P.; Parkes, J.; Rosenberg, W. Derivation and Performance of Standardized Enhanced Liver Fibrosis (ELF) Test Thresholds for the Detection Prognosis of Liver Fibrosis. J. Appl. Lab. Med. 2019, 3, 815–826. [Google Scholar] [CrossRef]
- Hinkson, A.; Lally, H.; Gibson, H.; Jones, R.; Rowe, I.A.; Shinkins, B.; Parker, R. Meta-analysis: Enhanced liver fibrosis test to identify hepatic fibrosis in chronic liver diseases. Aliment. Pharmacol. Ther. 2023, 57, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Vergniol, J.; Wong, G.L.; Foucher, J.; Chan, H.L.; Le Bail, B.; Choi, P.C.; Kowo, M.; Chan, A.W.; Merrouche, W.; et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010, 51, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Lee, H.W.; Yip, T.C.; Tsochatzis, E.; Petta, S.; Bugianesi, E.; Yoneda, M.; Zheng, M.H.; Hagström, H.; Boursier, J.; et al. Vibration-Controlled Transient Elastography Scores to Predict Liver-Related Events in Steatotic Liver Disease. JAMA 2024, 331, 1287–1297. [Google Scholar] [CrossRef]
- Newsome, P.N.; Sasso, M.; Deeks, J.J.; Paredes, A.; Boursier, J.; Chan, W.K.; Yilmaz, Y.; Czernichow, S.; Zheng, M.H.; Wong, V.W.; et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: A prospective derivation and global validation study. Lancet Gastroenterol. Hepatol. 2020, 5, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Woreta, T.A.; Van Natta, M.L.; Lazo, M.; Krishnan, A.; Neuschwander-Tetri, B.A.; Loomba, R.; Mae Diehl, A.; Abdelmalek, M.F.; Chalasani, N.; Gawrieh, S.; et al. Validation of the accuracy of the FAST™ score for detecting patients with at-risk nonalcoholic steatohepatitis (NASH) in a North American cohort and comparison to other non-invasive algorithms. PLoS ONE 2022, 17, e0266859. [Google Scholar] [CrossRef]
- Loomba, R.; Wolfson, T.; Ang, B.; Hooker, J.; Behling, C.; Peterson, M.; Valasek, M.; Lin, G.; Brenner, D.; Gamst, A.; et al. Magnetic resonance elastography predicts advanced fibrosis in patients with non-alcoholic fatty liver disease: A prospective study. Hepatology 2014, 60, 1920–1928. [Google Scholar] [CrossRef]
- Lai, J.C.; Liang, L.Y.; Wong, G.L. Noninvasive tests for liver fibrosis in 2024: Are there different scales for different diseases? Gastroenterol. Rep. 2024, 12, goae024. [Google Scholar] [CrossRef]
- Gu, J.; Liu, S.; Du, S.; Zhang, Q.; Xiao, J.; Dong, Q.; Xin, Y. Diagnostic value of MRI-PDFF for hepatic steatosis in patients with non-alcoholic fatty liver dis-ease: A meta-analysis. Eur. Radiol. 2019, 29, 3564–3573. [Google Scholar] [CrossRef]
- Jung, J.; Loomba, R.R.; Imajo, K.; Madamba, E.; Gandhi, S.; Bettencourt, R.; Singh, S.; Hernandez, C.; Valasek, M.A.; Behling, C.; et al. MRE combined with FIB-4 (MEFIB) index in detection of candidates for pharmacological treatment of NASH-related fibrosis. Gut 2021, 70, 1946–1953. [Google Scholar] [CrossRef]
- Ajmera, V.; Kim, B.K.; Yang, K.; Majzoub, A.M.; Nayfeh, T.; Tamaki, N.; Izumi, N.; Nakajima, A.; Idilman, R.; Gumussoy, M.; et al. Liver Stiffness on Magnetic Resonance Elastography and the MEFIB Index and Liver-Related Outcomes in Nonalcoholic Fatty Liv-er Disease: A Systematic Review and Meta-Analysis of Individual Participants. Gastroenterology 2022, 163, 1079–1089.e5. [Google Scholar] [CrossRef]
- Andersson, A.; Kelly, M.; Imajo, K.; Nakajima, A.; Fallowfield, J.A.; Hirschfield, G.; Pavlides, M.; Sanyal, A.J.; Noureddin, M.; Banerjee, R.; et al. Clinical Utility of Magnetic Resonance Imaging Biomarkers for Identifying Nonalcoholic Steatohepatitis Patients at High Risk of Progression: A Multicenter Pooled Data and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 2451–2461.e3. [Google Scholar] [CrossRef]
- Plauth, M.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Bischoff, S.C. ESPEN guideline on clinical nutrition in liver disease. Clin. Nutr. 2019, 38, 485–521. [Google Scholar] [CrossRef]
- Long, M.T.; Noureddin, M.; Lim, J.K. AGA Clinical Practice Update: Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Lean Individuals: Expert Review. Gastroenterology 2022, 163, 764–774.e1. [Google Scholar] [CrossRef]
- Gepner, Y.; Shelef, I.; Komy, O.; Cohen, N.; Schwarzfuchs, D.; Bril, N.; Rein, M.; Serfaty, D.; Kenigsbuch, S.; Zelicha, H.; et al. The beneficial effects of Mediterranean diet over low-fat diet may be mediated by decreasing hepatic fat content. J. Hepatol. 2019, 71, 379–388. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 3. Prevention or Delay of Diabetes and Associated Comorbidities: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48 (Suppl. S1), S50–S58. [Google Scholar] [CrossRef]
- Henney, A.E.; Gillespie, C.S.; Alam, U.; Hydes, T.J.; Cuthbertson, D.J. Ultra-Processed Food Intake Is Associated with Non-Alcoholic Fatty Liver Disease in Adults: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 2266. [Google Scholar] [CrossRef]
- Ivancovsky-Wajcman, D.; Fliss-Isakov, N.; Webb, M.; Bentov, I.; Shibolet, O.; Kariv, R.; Zelber-Sagi, S. Ultra-processed food is associated with features of metabolic syndrome and non-alcoholic fatty liver disease. Liver Int. 2021, 41, 2635–2645. [Google Scholar] [CrossRef]
- Yki-Järvinen, H.; Luukkonen, P.K.; Hodson, L.; Moore, J.B. Dietary carbohydrates, and fats in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 770–786. [Google Scholar] [CrossRef]
- Noureddin, M.; Zelber-Sagi, S.; Wilkens, L.R.; Porcel, J.; Boushey, C.J.; Le Marchand, L.; Rosen, H.R.; Setiawan, V.W. Diet Associations with Nonalcoholic Fatty Liver Disease in an Ethnically Diverse Population: The Multiethnic Cohort. Hepatology 2020, 71, 1940–1952. [Google Scholar] [CrossRef]
- Salomone, F.; Ivancovsky-Wajcman, D.; Fliss-Isakov, N.; Webb, M.; Grosso, G.; Godos, J.; Galvano, F.; Shibolet, O.; Kariv, R.; Zelber-Sagi, S. Higher phenolic acid intake independently associates with lower prevalence of insulin resistance and non-alcoholic fatty liver disease. JHEP Rep. 2020, 2, 100069. [Google Scholar] [CrossRef]
- Bell, W.; Jennings, A.; Thompson, A.S.; Bondonno, N.P.; Tresserra-Rimbau, A.; Kühn, T.; Cassidy, A. A flavonoid-rich diet is associated with lower risk and improved imaging biomarkers of nonalcoholic fatty liver disease: A prospective cohort study. Am. J. Clin. Nutr. 2024, 120, 1325–1334. [Google Scholar] [CrossRef]
- Marti-Aguado, D.; Calleja, J.L.; Vilar-Gomez, E.; Iruzubieta, P.; Rodríguez-Duque, J.C.; Del Barrio, M.; Puchades, L.; Rivera-Esteban, J.; Perelló, C.; Puente, A.; et al. Low-to-moderate alcohol consumption is associated with increased fibrosis in individuals with metabolic dysfunction-associated steatotic liver disease. J. Hepatol. 2024, 81, 930–940. [Google Scholar] [CrossRef]
- Jung, H.S.; Chang, Y.; Kwon, M.J.; Sung, E.; Yun, K.E.; Cho, Y.K.; Shin, H.; Ryu, S. Smoking and the Risk of Non-Alcoholic Fatty Liver Disease: A Cohort Study. Am. J. Gastroenterol. 2019, 114, 453–463. [Google Scholar] [CrossRef]
- Abdel-Rahman, O.; Helbling, D.; Schöb, O.; Eltobgy, M.; Mohamed, H.; Schmidt, J.; Giryes, A.; Mehrabi, A.; Iype, S.; John, H.; et al. Cigarette smoking as a risk factor for the development of mortality from hepatocellular carcinoma: An updated systematic review of 81 epidemiological studies. J. Evid. Based Med. 2017, 10, 245–254. [Google Scholar] [CrossRef]
- Charatcharoenwitthaya, P.; Karaketklang, K.; Aekplakorn, W. Cigarette Smoking Increased Risk of Overall Mortality in Patients with Non-alcoholic Fatty Liver Disease: A Nationwide Population-Based Cohort Study. Front. Med. 2020, 7, 604919. [Google Scholar] [CrossRef]
- Kim, D.; Vazquez-Montesino, L.M.; Li, A.A.; Cholankeril, G.; Ahmed, A. Inadequate Physical Activity and Sedentary Behavior Are Independent Predictors of Nonalcoholic Fatty Liver Disease. Hepatology 2020, 72, 1556–1568. [Google Scholar] [CrossRef]
- Hashida, R.; Kawaguchi, T.; Bekki, M.; Omoto, M.; Matsuse, H.; Nago, T.; Takano, Y.; Ueno, T.; Koga, H.; George, J.; et al. Aerobic vs resistance exercise in non-alcoholic fatty liver disease: A systematic review. J. Hepatol. 2017, 66, 142–152. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef]
- Keam, S.J. Resmetirom: First Approval. Drugs 2024, 84, 729–735. [Google Scholar] [CrossRef]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.S.; Harrison, S.A.; NN9931-4296 Investigators. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef]
- Loomba, R.; Hartman, M.L.; Lawitz, E.J.; Vuppalanchi, R.; Boursier, J.; Bugianesi, E.; Yoneda, M.; Behling, C.; Cummings, O.W.; Tang, Y.; et al. Tirzepatide for Metabolic Dysfunction-Associated Steatohepatitis with Liver Fibrosis. N. Engl. J. Med. 2024, 391, 299–310. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Bedossa, P.; Fraessdorf, M.; Neff, G.W.; Lawitz, E.; Bugianesi, E.; Anstee, Q.M.; Hussain, S.A.; Newsome, P.N.; Ratziu, V.; et al. A Phase 2 Randomized Trial of Survodutide in MASH and Fibrosis. N. Engl. J. Med. 2024, 391, 311–319. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Kaplan, L.M.; Frias, J.P.; Brouwers, B.; Wu, Q.; Thomas, M.K.; Harris, C.; Schloot, N.C.; Du, Y.; Mather, K.J.; et al. Triple hormone receptor agonist retatrutide for metabolic dysfunction-associated steatotic liver disease: A randomized phase 2a trial. Nat. Med. 2024, 30, 2037–2048. [Google Scholar] [CrossRef]
- Harrison, S.A.; Frias, J.P.; Neff, G.; Abrams, G.A.; Lucas, K.J.; Sanchez, W.; Gogia, S.; Sheikh, M.Y.; Behling, C.; Bedossa, P.; et al. Safety and efficacy of once-weekly efruxifermin versus placebo in non-alcoholic steatohepatitis (HARMONY): A multicentre, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Gastroenterol. Hepatol. 2023, 8, 1080–1093. [Google Scholar] [CrossRef]
- Loomba, R.; Sanyal, A.J.; Kowdley, K.V.; Bhatt, D.L.; Alkhouri, N.; Frias, J.P.; Bedossa, P.; Harrison, S.A.; Lazas, D.; Barish, R.; et al. Randomized, Controlled Trial of the FGF21 Analogue Pegozafermin in NASH. N. Engl. J. Med. 2023, 389, 998–1008. [Google Scholar] [CrossRef]
- Francque, S.M.; Bedossa, P.; Ratziu, V.; Anstee, Q.M.; Bugianesi, E.; Sanyal, A.J.; Loomba, R.; Harrison, S.A.; Balabanska, R.; Mateva, L.; et al. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N. Engl. J. Med. 2021, 385, 1547–1558. [Google Scholar] [CrossRef]
- Bril, F.; Kalavalapalli, S.; Clark, V.C.; Lomonaco, R.; Soldevila-Pico, C.; Liu, I.C.; Orsak, B.; Tio, F.; Cusi, K. Response to Pioglitazone in Patients with Nonalcoholic Steatohepatitis With vs Without Type 2 Diabetes. Clin. Gastroenterol. Hepatol. 2018, 16, 558–566.e2. [Google Scholar] [CrossRef]
- Hsiang, J.C.; Wong, V.W. SGLT2 Inhibitors in Liver Patients. Clin. Gastroenterol. Hepatol. 2020, 18, 2168–2172.e2. [Google Scholar] [CrossRef]
- Kahl, S.; Gancheva, S.; Straßburger, K.; Herder, C.; Machann, J.; Katsuyama, H.; Kabisch, S.; Henkel, E.; Kopf, S.; Lagerpusch, M.; et al. Empagliflozin Effectively Lowers Liver Fat Content in Well-Controlled Type 2 Diabetes: A Randomized, Double-Blind, Phase 4, Placebo-Controlled Trial. Diabetes Care 2020, 43, 298–305. [Google Scholar] [CrossRef]
- Wei, Q.; Xu, X.; Guo, L.; Li, J.; Li, L. Effect of SGLT2 Inhibitors on Type 2 Diabetes Mellitus with Non-Alcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 2021, 12, 635556. [Google Scholar] [CrossRef]
- Liu, W.; You, D.; Lin, J.; Zou, H.; Zhang, L.; Luo, S.; Yuan, Y.; Wang, Z.; Qi, J.; Wang, W.; et al. SGLT2 inhibitor promotes ketogenesis to improve MASH by suppressing CD8+ T cell activation. Cell Metab. 2024, 36, 2245–2261.e6. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef]
- Wen, H.; Deng, H.; Yang, L.; Li, L.; Lin, J.; Zheng, P.; Bjelakovic, M.; Ji, G. Vitamin E for people with non-alcoholic fatty liver disease. Cochrane Database Syst. Rev. 2024, 10, CD015033. [Google Scholar]
- Aminian, A.; Al-Kurd, A.; Wilson, R.; Bena, J.; Fayazzadeh, H.; Singh, T.; Albaugh, V.L.; Shariff, F.U.; Rodriguez, N.A.; Jin, J.; et al. Association of Bariatric Surgery with Major Adverse Liver and Cardiovascular Outcomes in Patients with Biopsy-Proven Nonalcoholic Steatohepatitis. JAMA 2021, 326, 2031–2042. [Google Scholar] [CrossRef]
- Sutanto, A.; Wungu, C.D.K.; Susilo, H.; Sutanto, H. Reduction of Major Adverse Cardiovascular Events (MACE) after Bariatric Surgery in Patients with Obesity and Cardiovascular Diseases: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3568. [Google Scholar] [CrossRef]
- Verrastro, O.; Panunzi, S.; Castagneto-Gissey, L.; De Gaetano, A.; Lembo, E.; Capristo, E.; Guidone, C.; Angelini, G.; Pennestrì, F.; Sessa, L.; et al. Bariatric-metabolic surgery versus lifestyle intervention plus best medical care in non-alcoholic steatohepatitis (BRAVES): A multicentre, open-label, randomized trial. Lancet 2023, 401, 1786–1797. [Google Scholar] [CrossRef]
- Havranek, B.; Loh, R.; Torre, B.; Redfield, R.; Halegoua-DeMarzio, D. Glucagon-like peptide-1 receptor agonists improve metabolic dysfunction-associated steatotic liver disease outcomes. Sci. Rep. 2025, 15, 4947. [Google Scholar] [CrossRef]
- Boutari, C.; Rizos, C.V.; Liamis, G.; Skoumas, I.; Rallidis, L.; Garoufi, A.; Kolovou, G.; Sfikas, G.; Tziomalos, K.; Skalidis, E.; et al. The effect of lipid-lowering treatment on indices of MASLD in familial hypercholesterolemia patients. Clin. Nutr. 2024, 43, 84–91. [Google Scholar] [CrossRef]
- Butera, E.; Termite, F.; Esposto, G.; Galasso, L.; Mignini, I.; Borriello, R.; Ainora, M.E.; Miele, L.; Gasbarrini, A.; Zocco, M.A. Exploring the Role of Bempedoic Acid in Metabolic Dysfunction Associated Steatotic Liver Disease: Actual Evidence and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 6938. [Google Scholar] [CrossRef]
- Barkas, F.; Sener, Y.Z.; Golforoush, P.A.; Kheirkhah, A.; Rodriguez-Sanchez, E.; Novak, J.; Apellaniz-Ruiz, M.; Akyea, R.K.; Bianconi, V.; Ceasovschih, A.; et al. Advancements in risk stratification and management strategies in primary cardiovascular prevention. Atherosclerosis 2024, 395, 117579. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.M.; Younossi, Z.M.; Diehl, A.M.; Charlton, M.R.; Lazarus, J.V. Envisioning how to advance the MASH field. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 726–738. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Pirola, C.J.; Sanyal, A.J. MASLD as a non-communicable disease. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 148–149. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Non-Alcoholic Fatty Liver Disease (NAFLD) | Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) | Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) |
|---|---|---|---|
| Hepatic steatosis > 5% assessed by liver histology or imaging techniques | ✔ | ✔ | ✔ |
| Hepatic steatosis > 5% assessed through blood biomarkers/scores | X | ✔ | X |
| Absence of significant alcohol consumption (≥30 g/day in men, ≥20 g/day in women) | ✔ | X | ✔ |
| Exclusion of other causes of steatosis | ✔ | X | ✔ |
| Presence of a cardiometabolic criteria (metabolic dysfunction) | X | ✔ | ✔ |
| 1. Overweight/Obesity (BMI ≥ 25 kg/m2 in Caucasians [23 kg/m2 in Asians]) 2. T2D (Based on widely recognized international guidelines) 3. Lean/normal weight (BMI < 25 kg/m2 in Caucasians or BMI < 23 kg/m2 in Asians) if present at least two metabolic abnormalities: A—WC ≥ 102 cm (M), ≥88 cm (F) in Caucasians (or ≥90 cm (M), ≥88 cm (F) in Asians B—Plasma HDL–C ≤ 40 mg/dL [1 mmol/L] (M) and ≤50 mg/dL [1.3 mmol/L] (F) OR lipid-lowering treatment C—Plasma TG ≥ 150 mg/dL [1.70 mmol/L] OR lipid-lowering treatment D—BP ≥ 130/85 mmHg OR specific antihypertensive drug treatment E—Prediabetes (Fasting Serum Glucose ≥ 100 to 125 mg/dL [5.6 to 6.9 mmol/L] OR 2 h post-load glucose levels ≥ 140 to 199 mg/dL [7.8 to 11.0 mmol/L] OR HbA1c ≥ 5.7 to 6.4% [39 to 47 mmol/mol]) F—Homeostasis model assessment of insulin resistance score ≥ 2.5 G—Plasma high sensitivity C-reactive protein level > 2 mg/L | BMI ≥ 25 kg/m2 [23 kg/m2 in Asians] OR WC > 94 cm (M) 80 cm (F) OR ethnicity adjusted measurements Fasting Serum Glucose ≥ 100 mg/dL [5.6 mmol/L] OR 2 h post-load glucose levels ≥ 140 mg/dL [7.8 mmol/L] OR HbA1c ≥ 5.7% [39 mmol/mol] OR T2D OR treatment for T2D Plasma HDL–cholesterol ≤ 40 mg/dL [1 mmol/L] (M) and ≤50 mg/dL [1.3 mmol/L] (F) OR lipid-lowering treatment Plasma TG ≥ 150 mg/dL [1.70 mmol/L] OR lipid-lowering treatment BP ≥ 130/85 mmHg OR specific antihypertensive drug treatment | ||
| Embraces the concept of a multifactorial aetiology | X | ✔ | ✔ |
| National Cholesterol Education Program/Adult Treatment Panel III | International Diabetes Federation | Unifying Metabolic Syndrome Statement | |
|---|---|---|---|
| Criteria | At least 3 of the following criteria | Central obesity, plus at least 2 of the following criteria | At least 3 of the following criteria |
| Waist Circumference/Obesity | |||
| Abdominal obesity a Men ≥ 102 cm (40 in) Women ≥ 88 cm (35 in) | Central Obesity * WC—ethnicity specific: a. Europids, Sub-Saharan Africans, Middle East, Eastern Mediterranean ≥94 cm (M), ≥80 cm (F) b. South Asians, Chinese, Ethnic central and South Americans ≥90 cm (M), ≥80 cm (F) c. Japanese ≥85 cm (M), ≥90 cm (F) | Elevated WC ** Population specific and country specific definitions | |
| Fasting plasma glucose | |||
| ≥100 mg/dL (5.6 mmol/L) | ✔ b | ✔ | ✔ |
| Or | |||
| prior T2D diagnosis | X | ✔ | X |
| Or | |||
| pharmacological therapy for high glucose | X | X | ✔ |
| High-density lipoprotein cholesterol | |||
| Men < 40 mg/dL (1.04 mmol/L) Women < 50 mg/dL (1.30 mmol/L) | ✔ | ✔ | ✔ |
| Or | |||
| Targeted therapy for this lipid disorder | X | ✔ | ✔ |
| Triglycerides | |||
| ≥150 mg/dL (1.7 mmol/L) | ✔ | ✔ | ✔ |
| Or | |||
| Targeted therapy for this lipid disorder | X | ✔ | ✔ |
| Blood Pressure | |||
| SBP ≥ 130 mmHg | ✔ | ✔ | ✔ |
| Or | |||
| DBP ≥ 85 mmHg | ✔ | ✔ | ✔ |
| Or | |||
| Treatment for HTN | X | ✔ | ✔ |
| Test | Advantages | Disadvantages |
| Fibrosis index based on 4 factors (FIB-4) score FIB-4 score = age (years) × AST (U/L)/(platelet count (109/L) × √ALT (U/L) | -Easy to calculate -Commonly measured parameters -Widely validated -Cost-effective -Good alternative for initial screening | -Reduced accuracy in specific populations (individuals under 35 years of age, those over 65, people with significant alcohol consumption, and patients with other underlying liver conditions). -No information on aetiology -Not perfect for early-stage fibrosis -Accuracy influenced by coexisting conditions |
| NAFLD fibrosis score (NFS) NFS = −1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × impaired fasting glucose/diabetes (yes = 1, no = 0) + 0.99 × (AST/ALT ratio) − 0.013 × platelet count (×109/L) − 0.66 × albumin (g/dL) | -Easy to calculate -Commonly measured parameters -Widely validated -Cost-effective -Good alternative for initial screening | -Reduced accuracy in specific populations (individuals with very high or very low BMI, significant alcohol consumption, or other underlying liver diseases). -No information on aetiology -Not perfect for early-stage fibrosis -Accuracy can be influenced by coexisting conditions -Requires multiple variables |
| Enhanced liver fibrosis (ELF) score ELF score = 2.278 + 0.851 ln (hyaluronic acid) + 0.751 ln (PIIINP) + 0.394 ln (TIMP-1) | -Widely validated -High accuracy for detecting fibrosis | -Cost -No information on aetiology -Not perfect for early-stage fibrosis -Accuracy affected by coexisting conditions (obesity, diabetes, kidney disease) -Limited availability |
| Liver Stiffness Measurement-Vibration Controlled Transient Elastography Liver (LSM- VCTE) [FibroScan] It assesses liver stiffness using ultrasound-based elastography (e.g., FibroScan), with values measured in kilopascals (kPa), and liver steatosis using the Controlled Attenuation Parameter (CAP), with values measured in decibels per meter (dB/m). | -Widely validated -High accuracy for detecting fibrosis -Quick and easy with real-time results | -Cost -No information on aetiology -Not perfect for early-stage fibrosis -Accuracy can be impacted by coexisting conditions, such as obesity, ascites, liver congestion, and others. -Possible operator dependence -Limited availability |
| FibroScan-AST (FAST) score FAST = VCTE [Liver Stiffness Measurement (LSM), Controlled Attenuation Parameter (CAP)] + AST FAST score = e−1.65 + 1.07 × ln (LSM) + 2.66*10−8 × CAP3 − 63.3 × AST−1/1 + e−1.65 + 1.07 × ln (LSM) + 2.66*10−8 × CAP3 − 63.3 × AST−1 | -Widely validated -Enhanced accuracy for detecting fibrosis -Quick and easy | -Cost -No information on aetiology -Not perfect for early-stage fibrosis -Accuracy can be impacted by coexisting conditions, such as obesity, ascites, liver congestion, and others. -Possible operator dependence -Limited availability |
| Magnetic resonance imaging (MRI), MRI proton density fat fraction (MRI-PDFF) measures liver steatosis Magnetic resonance elastography (MRE) (Standard MRI machines utilizing a phase contrast technique, along with specialized software, to evaluate liver stiffness by analysing the propagation of mechanical waves through the liver tissue. MRI-iron-corrected T1 mapping (cT1) | -Widely validated -Highly accurate for detecting fibrosis -Better for heterogeneous liver disease -Provides detailed imaging of the entire liver -Can be combined with other parameters (MAST = MRE + MRI-PDFF + AST; MEFIB = MRE + FIB-4) | -Cost -No information on aetiology -Requires specialized equipment and expertise -Limited availability |
| Area | Past | Present | Future |
|---|---|---|---|
| Treatment | Lifestyle Modifications Management of MetS components, comorbidities, and cardiovascular disease. | Lifestyle Modifications Stronger emphasis on managing MetS components with preferred pharmacologic treatments, addressing comorbidities, and implementing an integrated approach to cardiovascular risk. | Lifestyle Modifications Stronger emphasis on managing MetS components with personalized pharmacologic treatments, addressing comorbidities, and implementing an integrated approach to cardiovascular risk. |
| No specific drug approved | Resmetirom (Only in US) | Resmetiron (global use) Potential approval of new drugs, including Semaglutide, Tirzepatide, Survodutide, Retatrutide, Efruxifermin, Pegozafermin, and Lanifibranor. Combination therapy Specific treatments for individuals with MASH- related cirrhosis | |
| Bariatric surgery Liver transplantation | Bariatric surgery Liver transplantation | Bariatric surgery Liver transplantation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecani, M.; Andreozzi, P.; Cangemi, R.; Corica, B.; Miglionico, M.; Romiti, G.F.; Stefanini, L.; Raparelli, V.; Basili, S. Metabolic Syndrome and Liver Disease: Re-Appraisal of Screening, Diagnosis, and Treatment Through the Paradigm Shift from NAFLD to MASLD. J. Clin. Med. 2025, 14, 2750. https://doi.org/10.3390/jcm14082750
Pecani M, Andreozzi P, Cangemi R, Corica B, Miglionico M, Romiti GF, Stefanini L, Raparelli V, Basili S. Metabolic Syndrome and Liver Disease: Re-Appraisal of Screening, Diagnosis, and Treatment Through the Paradigm Shift from NAFLD to MASLD. Journal of Clinical Medicine. 2025; 14(8):2750. https://doi.org/10.3390/jcm14082750
Chicago/Turabian StylePecani, Marin, Paola Andreozzi, Roberto Cangemi, Bernadette Corica, Marzia Miglionico, Giulio Francesco Romiti, Lucia Stefanini, Valeria Raparelli, and Stefania Basili. 2025. "Metabolic Syndrome and Liver Disease: Re-Appraisal of Screening, Diagnosis, and Treatment Through the Paradigm Shift from NAFLD to MASLD" Journal of Clinical Medicine 14, no. 8: 2750. https://doi.org/10.3390/jcm14082750
APA StylePecani, M., Andreozzi, P., Cangemi, R., Corica, B., Miglionico, M., Romiti, G. F., Stefanini, L., Raparelli, V., & Basili, S. (2025). Metabolic Syndrome and Liver Disease: Re-Appraisal of Screening, Diagnosis, and Treatment Through the Paradigm Shift from NAFLD to MASLD. Journal of Clinical Medicine, 14(8), 2750. https://doi.org/10.3390/jcm14082750










