Positive Lymph Nodes Independently Affect Long-Term Survival After Pancreaticoduodenectomy for Non-Ampullary Duodenal Adenocarcinoma: A Single-Center, Retrospective Analysis
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Overall Survival Analysis
3.2. Recurrence-Free Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Locher, C.; Batumona, B.; Afchain, P.; Carrère, N.; Samalin, E.; Cellier, C.; Aparicio, T.; Becouarn, Y.; Bedenne, L.; Michel, P.; et al. Small bowel adenocarcinoma: French intergroup clinical practice guidelines for diagnosis, treatments, and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Dig. Liver Dis. 2018, 50, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Hirashita, T.; Ohta, M.; Tada, K.; Saga, K.; Takayama, H.; Endo, Y.; Uchida, H.; Iwashita, Y.; Inomata, M. Prognostic factors of non-ampullary duodenal adenocarcinoma. Jpn. J. Clin. Oncol. 2018, 48, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Struck, A.; Howard, T.; Chiorean, E.G.; Clarke, J.M.; Riffenburgh, R.; Cardenes, H.R. Non-ampullary duodenal adenocarcinoma: Factors important for relapse and survival. J. Surg. Oncol. 2009, 100, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Thiessen, M.; Lee-Ying, R.M.; Monzon, J.G.; Tang, P.A. An Examination of Lymph Node Sampling as a Predictor of Survival in Resected Node-Negative Small Bowel Adenocarcinoma: A SEER Database Analysis. J. Gastrointest. Cancer 2020, 51, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Ecker, B.L.; McMillan, M.T.; Datta, J.; Dempsey, D.T.; Karakousis, G.C.; Fraker, D.L.; Drebin, J.A.; Mamtani, R.; Giantonio, B.J.; Roses, R.E. Lymph node evaluation and survival after curative-intent resection of duodenal adenocarcinoma: A matched cohort study. Eur. J. Cancer Oxf. Engl. 2016, 69, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Yoshimizu, S.; Kawachi, H.; Yamamoto, Y.; Nakano, K.; Horiuchi, Y.; Ishiyama, A.; Tsuchida, T.; Yoshio, T.; Hirasawa, T.; Ito, H.; et al. Clinicopathological features and risk factors for lymph node metastasis in early-stage non-ampullary duodenal adenocarcinoma. J. Gastroenterol. 2020, 55, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.B.; Qadan, M.; Dua, M.M.; Norton, J.A.; Poultsides, G.A.; Visser, B.C. Prognostic relevance of lymph node ratio and total lymph node count for small bowel adenocarcinoma. Surgery 2015, 158, 486–493. [Google Scholar] [PubMed]
- Zheng, L. Negative Lymph Node Count is an Independent Impact Factor for Predicting the Specific Survival of Primary Duodenal Neoplasms under Surgical Procedures. Clin. Lab. 2020, 66, 1113. [Google Scholar]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.A.; Cooper, H.S.; Deming, D.A.; Garrido-Laguna, I.; et al. Small Bowel Adenocarcinoma, Version 1.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2019, 17, 1109–1133. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: Cham, Switzerland, 2017; Available online: https://www.springer.com/gp/book/9783319406176 (accessed on 20 May 2021).
- Adsay, N.V.; Basturk, O.; Altinel, D.; Khanani, F.; Coban, I.; Weaver, D.W.; Kooby, D.A.; Sarmiento, J.M.; Staley, C. The number of lymph nodes identified in a simple pancreatoduodenectomy specimen: Comparison of conventional vs orange-peeling approach in pathologic assessment. Mod. Pathol. 2009, 22, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Vuarnesson, H.; Lupinacci, R.M.; Semoun, O.; Svrcek, M.; Julié, C.; Balladur, P.; Penna, C.; Bachet, J.B.; Resche-Rigon, M.; Paye, F. Number of examined lymph nodes and nodal status assessment in pancreaticoduodenectomy for pancreatic adenocarcinoma. Eur. J. Surg. Oncol. 2013, 39, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Malleo, G.; Maggino, L.; Ferrone, C.R.; Marchegiani, G.; Mino-Kenudson, M.; Capelli, P.; Rusev, B.; Lillemoe, K.D.; Bassi, C.; Fernàndez-del Castillo, C.; et al. Number of Examined Lymph Nodes and Nodal Status Assessment in Distal Pancreatectomy for Body/Tail Ductal Adenocarcinoma. Ann. Surg. 2019, 270, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Saiura, A.; Ono, Y.; Mise, Y.; Inoue, Y.; Ishizawa, T.; Takahashi, Y.; Ito, H. Optimal Lymphadenectomy for Duodenal Adenocarcinoma: Does the Number Alone Matter? Ann. Surg. Oncol. 2017, 24, 3368–3375. [Google Scholar] [CrossRef] [PubMed]
- Sarela, A.I.; Brennan, M.F.; Karpeh, M.S.; Klimstra, D.; Conlon, K.C.P. Adenocarcinoma of the duodenum: Importance of accurate lymph node staging and similarity in outcome to gastric cancer. Ann. Surg. Oncol. 2004, 11, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Nishio, K.; Kimura, K.; Eguchi, S.; Shirai, D.; Tauchi, J.; Kinoshita, M.; Murata, A.; Ohira, G.; Shinkawa, H.; Shintaro, K.; et al. Prognostic Factors and Lymph Node Metastasis Patterns of Primary Duodenal Cancer. World J. Surg. 2022, 46, 163–171. [Google Scholar] [PubMed]


| Node Negative (n = 34, 49%) | Node Positive (n = 36, 51%) | p-Value | |
|---|---|---|---|
| Baseline Parameters | |||
| Female, n (%) | 17 (50%) | 10 (28%) | 0.048 |
| Age > 65 y, n (%) | 13 (38%) | 13 (36%) | 0.525 |
| BMI > 25 kg/m2, n (%) | 11 (32%) | 14 (39%) | 0.375 |
| ASA ≥ 3, n (%) | 4 (12%) | 7 (19%) | 0.291 |
| Diabetes, n (%) | 0 (0%) | 4 (11%) | 0.064 |
| Preoperative Jaundice, n (%) | 5 (15%) | 14 (39%) | 0.021 |
| History of Malignancy, n (%) | 9 (27%) | 5 (14%) | 0.155 |
| Neoadjuvant Therapy, n (%) | 4 (12%) | 0 (0%) | 0.051 |
| Intraoperative Parameters | |||
| Whipple Resection, n (%) | 17 (50%) | 14 (39%) | 0.244 |
| Multiorgan Resection, n (%) | 1 (3%) | 3 (8%) | 0.329 |
| Vascular Resection, n (%) | 0 (0%) | 1 (3%) | 0.514 |
| EBL > 400 mL, n (%) | 15 (44%) | 19 (53%) | 0.314 |
| OT > 360 min, n (%) | 23 (68%) | 20 (56%) | 0.214 |
| Pathological Parameters | |||
| Intestinal Phenotype | 26 (77) | 23 (64) | 0.188 |
| Tumor Size (mm), median (IQR) | 30 (0–75) | 35 (0–160) | 0.425 |
| Location, n (%) | 0.146 | ||
| Oral | 10 (29%) | 16 (44%) | |
| Anal | 24 (71%) | 20 (56%) | |
| ELN, median (IQR) | 30 (21–43) | 38 (31–46) | 0.032 |
| pT, n° (%) | <0.001 | ||
| 1 | 15 (44%) | 0 (0%) | |
| 2 | 3 (9%) | 3 (8%) | |
| 3 | 9 (27%) | 8 (22%) | |
| 4 | 7 (21%) | 25 (69%) | |
| Grading, n (%) | <0.001 | ||
| Well Differentiated | 15 (44%) | 2 (6%) | |
| Moderately differentiated | 11 (32%) | 22 (61%) | |
| Poorly Differentiated | 8 (24%) | 12(33%) | |
| R1, n (%) | 2 (6%) | 5 (14%) | 0.239 |
| Pancreatic Infiltration, n (%) | 7 (21%) | 25 (69%) | <0.001 |
| Perineural Invasion, n (%) | 9 (27%) | 26 (72%) | <0.001 |
| Adjuvant Therapy | 5 (15%) | 30 (83%) | <0.001 |
| Recurrence | 2 (6%) | 17 (47%) | <0.001 |
| Variables | Univariate Hazard Ratio (95% CI) | p-Value | Multivariate Hazard Ratio (95% CI) | p-Value |
|---|---|---|---|---|
| Baseline Parameters | ||||
| Female | 0.394 (0.145–1.071) | 0.068 | ||
| Age > 65 y | 1.941 (0.839–4.486) | 0.121 | ||
| BMI > 25 kg/m2 | 1.631 (0.702–3.793) | 0.255 | ||
| ASA Score ≥ 3 | 1.983 (0.720–5.461) | 0.185 | ||
| Diabetes | 2.375 (0.549–10.268) | 0.247 | ||
| Preoperative Jaundice | 1.035 (0.404–2.651) | 0.943 | ||
| History of Other Malignancies | 0.880 (0.298–2.601) | 0.817 | ||
| Neoadjuvant Therapy | 0.680 (0.091–5.082) | 0.707 | ||
| Intraoperative Parameters | ||||
| Whipple Resection | 0.626 (0.262–1.496) | 0.292 | ||
| Multiorgan Resection | 0.810 (0.109–6.037) | 0.837 | ||
| Vascular Resection | 8.114 (1.015–64.881) | 0.048 | 12.070 (1.44–101.11) | 0.022 |
| OT > 360 min | 1.115 (0.467–2.662) | 0.806 | ||
| EBL > 400 mL | 1.165 (0.504–2.690) | 0.721 | ||
| Postoperative Parameters | ||||
| Postoperative Complications | 2.172 (0.634–7.438) | 0.217 | ||
| Clavien–Dindo ≥ III | 2.538 (1.094–5.892) | 0.030 | 2.766 (1.171–6.533) | 0.020 |
| Variables | Univariate Hazard Ratio (95% CI) | p-Value | Multivariate Hazard Ratio (95% CI) | p-Value |
|---|---|---|---|---|
| Tumor Size | 0.999 (0.983–1.014) | 0.855 | ||
| pT3–4 | 3.395 (1.003–11.493) | 0.050 | - | 0.546 |
| pN+ | 4.359 (1.597–11.900) | 0.004 | - | 0.281 |
| ELN > 25 | 2.695 (0.908–7.998) | 0.074 | ||
| 0.2 ≤ LNR < 0.4 | 4.648 (1.821–11.867) | 0.001 | - | 0.157 |
| LNR ≥ 0.4 | 6.863 (1.520–30.994) | 0.012 | - | 0.424 |
| Grading (G2–G3) | 4.278 (0.992–18.450) | 0.002 | - | 0.455 |
| Location Anal | 0.639 (0.275–1.480) | 0.296 | ||
| Non-Intestinal Phenotype | 2.120 (0.901–4.987) | 0.085 | ||
| R-Status | 7.241 (2.724–19.246) | <0.001 | 5.994 (1.906–18.847) | 0.002 |
| Lymph-vascular invasion | 4.543 (1.341–15.386) | 0.015 | - | 0.718 |
| Perineural Invasion | 2.614 (1.063–6.426) | 0.036 | - | 0.941 |
| Pancreatic Infiltration | 2.795 (1.161–6.730) | 0.022 | - | 0.910 |
| Adjuvant Therapy | 2.547 (1.037–6.254) | 0.041 | - | 0.535 |
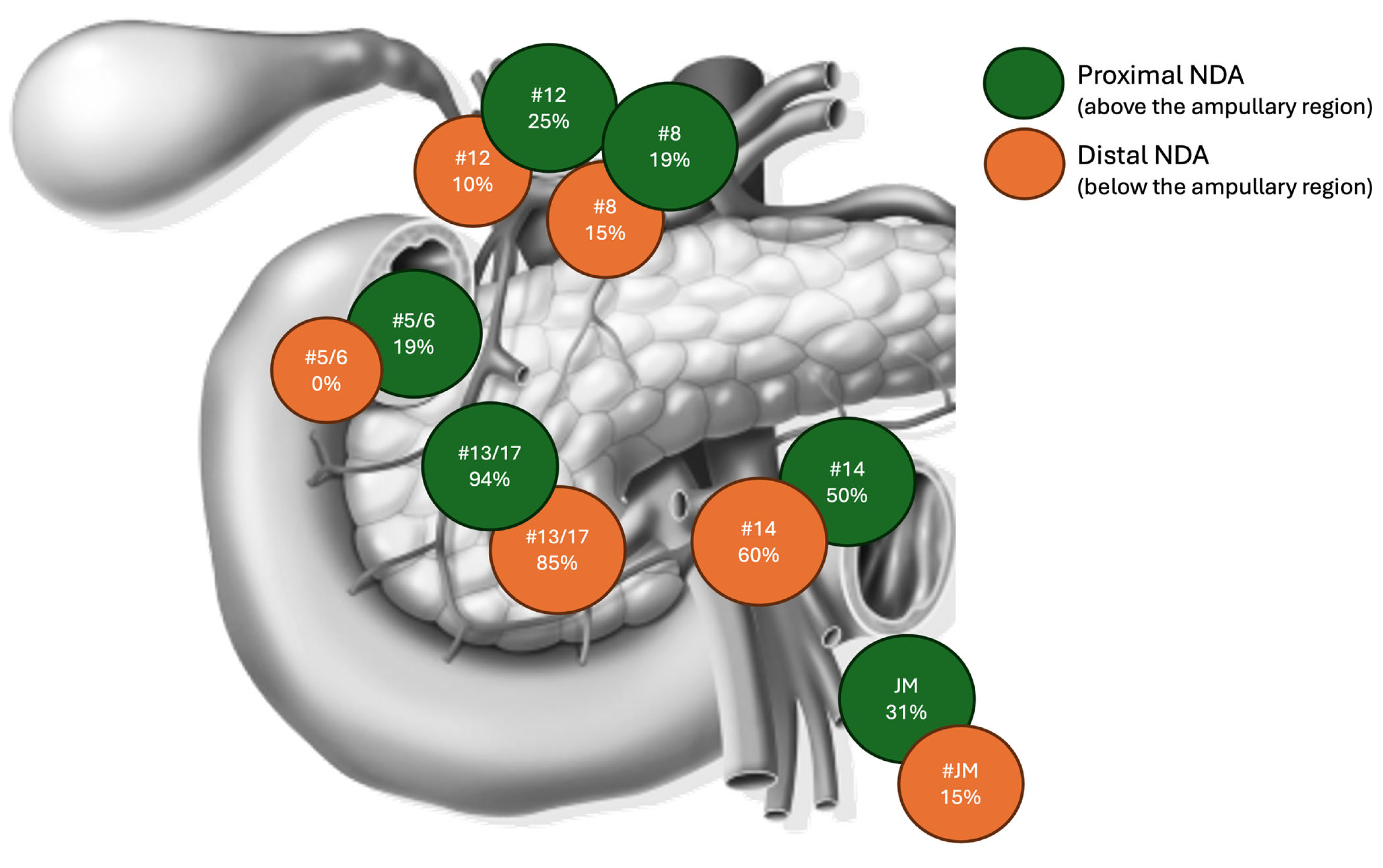
| Lymph Nodes Station Sub-Analysis | ||||
| Positive Station 14 | 4.677 (1.870–11.700) | <0.001 | - | 0.514 |
| Positive Stations 13–17 | 5.085 (1.853–13.957) | 0.002 | - | 0.563 |
| Positive Station 12 a, b, c | 7.783 (2.756–21.975) | <0.001 | - | 0.307 |
| Positive Stations 5–6 | 3.776 (1.194–11.944) | 0.024 | - | 0.507 |
| Positive Stations 7–9 | 4.763 (0.766–29.619) | 0.094 | ||
| Positive Station 8 a, p | 4.367 (1.406–13.564) | 0.011 | 8.076 (1.015–64.25) | 0.048 |
| Positive Station 16 | - | - | ||
| Positive Station Jejunal Mesentery | 10.005 (3.710–26.981) | <0.001 | - | - |
| Variables | Univariate Hazard Ratio (95% CI) | p-Value | Multivariate Hazard Ratio (95% CI) | p-Value |
|---|---|---|---|---|
| Tumor Size | 0.966 (0.979–1.014) | 0.688 | ||
| pT3–4 | 4.117 (0.946–17.915) | 0.059 | ||
| pN+ | 9.550 (2.192–41.603) | 0.003 | - | 0.451 |
| ELN > 25 | 4.580 (1.050–19.973) | 0.043 | - | 0.065 |
| 0.2 ≤ LNR < 0.4 | 8.090 (3.003–21.795) | <0.001 | - | 0.105 |
| LNR ≥ 0.4 | 23.891 (4.652–122.691) | <0.001 | 53.537 (4.563–623.886) | 0.002 |
| Grading (G2–3) | 6.707 (0.892–50.455) | 0.065 | ||
| Location Anal | 0.678 (0.267–1.719) | 0.412 | ||
| Non-Intestinal Phenotype | 2.934 (1.158–7.433) | 0.023 | - | 0.923 |
| R-Status | 8.127 (2.810–23.502) | <0.001 | 5.946 (1.315–26.874) | 0.021 |
| Lymph-vascular invasion | 11.781 (1.567–88.589) | 0.017 | - | 0.374 |
| Perineural Invasion | 3.042 (1.083–8.545) | 0.035 | - | 0.937 |
| Pancreatic Infiltration | 3.808 (1.354–10.706) | 0.011 | - | 0.796 |
| Adjuvant Therapy | 2.231 (0.837–5.946) | 0.109 | ||
| Lymph Nodes Station Sub-Analysis | ||||
| Positive Station 14 | 10.494 (3.352–32.855) | <0.001 | - | 0.059 |
| Positive Stations 13–17 | 11.245 (2.566–49.271) | 0.001 | - | 0.130 |
| Positive Station 12 a, b, c | 9.216 (3.123–27.197) | <0.001 | - | 0.905 |
| Positive Stations 5–6 | 2.136 (0.466–9.788) | 0.328 | ||
| Positive Stations 7–9 | 4.763 (0.766–29.619) | 0.094 | ||
| Positive Station 8 a, p | 7.908 (2.694–23.831) | <0.001 | - | 0.102 |
| Positive Station 16 | 5.660 (0.513–62.450) | 0.157 | ||
| Positive Station 15 | 9.606 (3.499–26.369) | <0.001 | - | 0.680 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Pastena, M.; Zingaretti, C.C.; Paiella, S.; Lionetto, G.; Guerriero, M.; De Santis, N.; Luchini, C.; Malleo, G.; Salvia, R. Positive Lymph Nodes Independently Affect Long-Term Survival After Pancreaticoduodenectomy for Non-Ampullary Duodenal Adenocarcinoma: A Single-Center, Retrospective Analysis. J. Clin. Med. 2025, 14, 2616. https://doi.org/10.3390/jcm14082616
De Pastena M, Zingaretti CC, Paiella S, Lionetto G, Guerriero M, De Santis N, Luchini C, Malleo G, Salvia R. Positive Lymph Nodes Independently Affect Long-Term Survival After Pancreaticoduodenectomy for Non-Ampullary Duodenal Adenocarcinoma: A Single-Center, Retrospective Analysis. Journal of Clinical Medicine. 2025; 14(8):2616. https://doi.org/10.3390/jcm14082616
Chicago/Turabian StyleDe Pastena, Matteo, Caterina Costanza Zingaretti, Salvatore Paiella, Gabriella Lionetto, Massimo Guerriero, Nicoletta De Santis, Claudio Luchini, Giuseppe Malleo, and Roberto Salvia. 2025. "Positive Lymph Nodes Independently Affect Long-Term Survival After Pancreaticoduodenectomy for Non-Ampullary Duodenal Adenocarcinoma: A Single-Center, Retrospective Analysis" Journal of Clinical Medicine 14, no. 8: 2616. https://doi.org/10.3390/jcm14082616
APA StyleDe Pastena, M., Zingaretti, C. C., Paiella, S., Lionetto, G., Guerriero, M., De Santis, N., Luchini, C., Malleo, G., & Salvia, R. (2025). Positive Lymph Nodes Independently Affect Long-Term Survival After Pancreaticoduodenectomy for Non-Ampullary Duodenal Adenocarcinoma: A Single-Center, Retrospective Analysis. Journal of Clinical Medicine, 14(8), 2616. https://doi.org/10.3390/jcm14082616







