Pushing the Boundaries of Ampullectomy for Benign Ampullary Tumors: 25-Year Outcomes of Surgical Ampullary Resection Associated with Duodenectomy or Biliary Resection
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Population, and Setting
2.2. Eligibility Criteria
2.3. Study Procedures
2.3.1. Diagnostic Work-Up and Therapeutic Approach
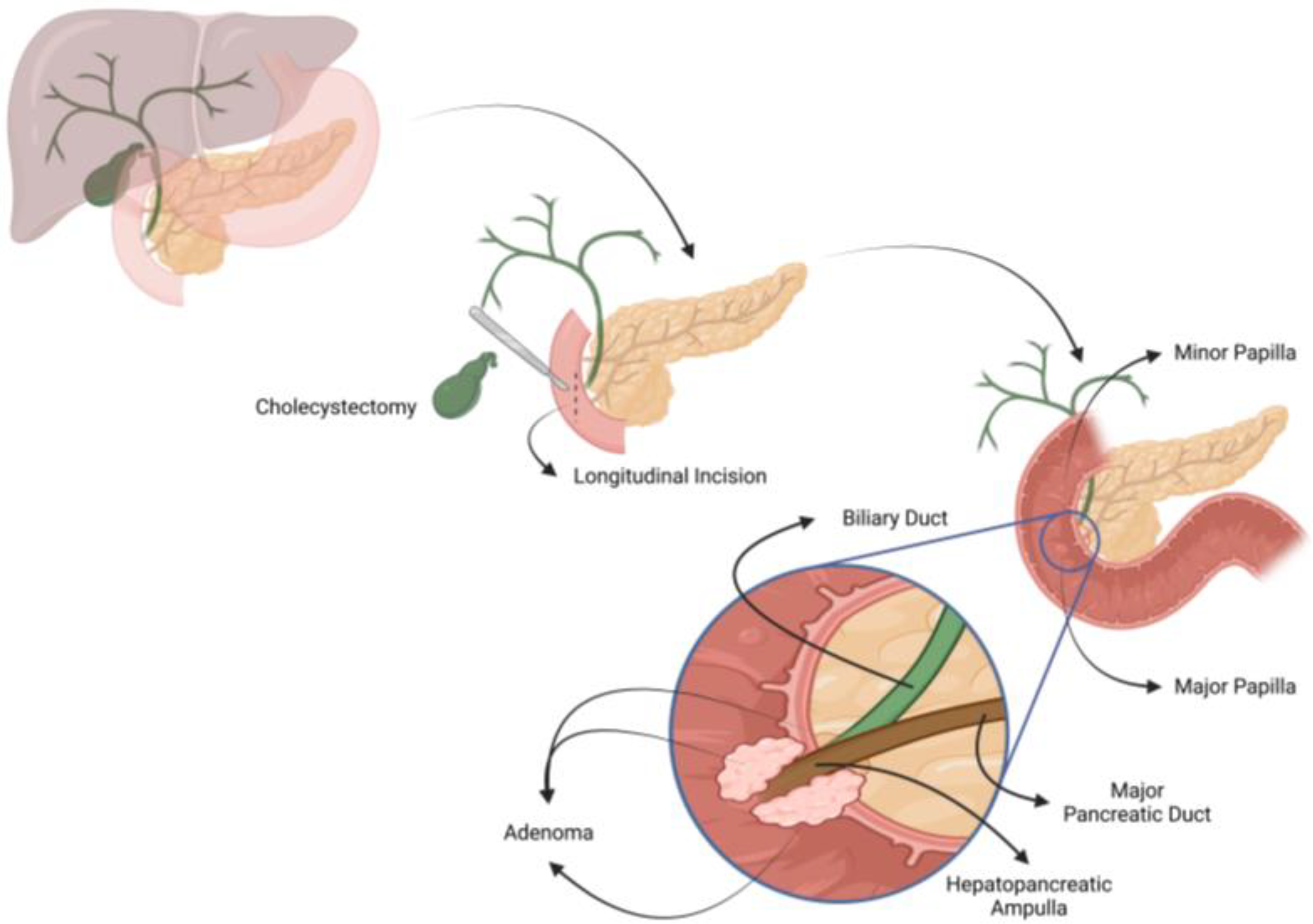
2.3.2. Surgical Technique
2.3.3. Frozen Section Evaluation
2.3.4. Intestinal Reconstruction
2.4. Outcomes
2.5. Data Sources
2.6. Sample Size and Statistical Analysis
3. Results
3.1. Baseline Characteristics and Short-Term Results
3.2. Long-Term Results
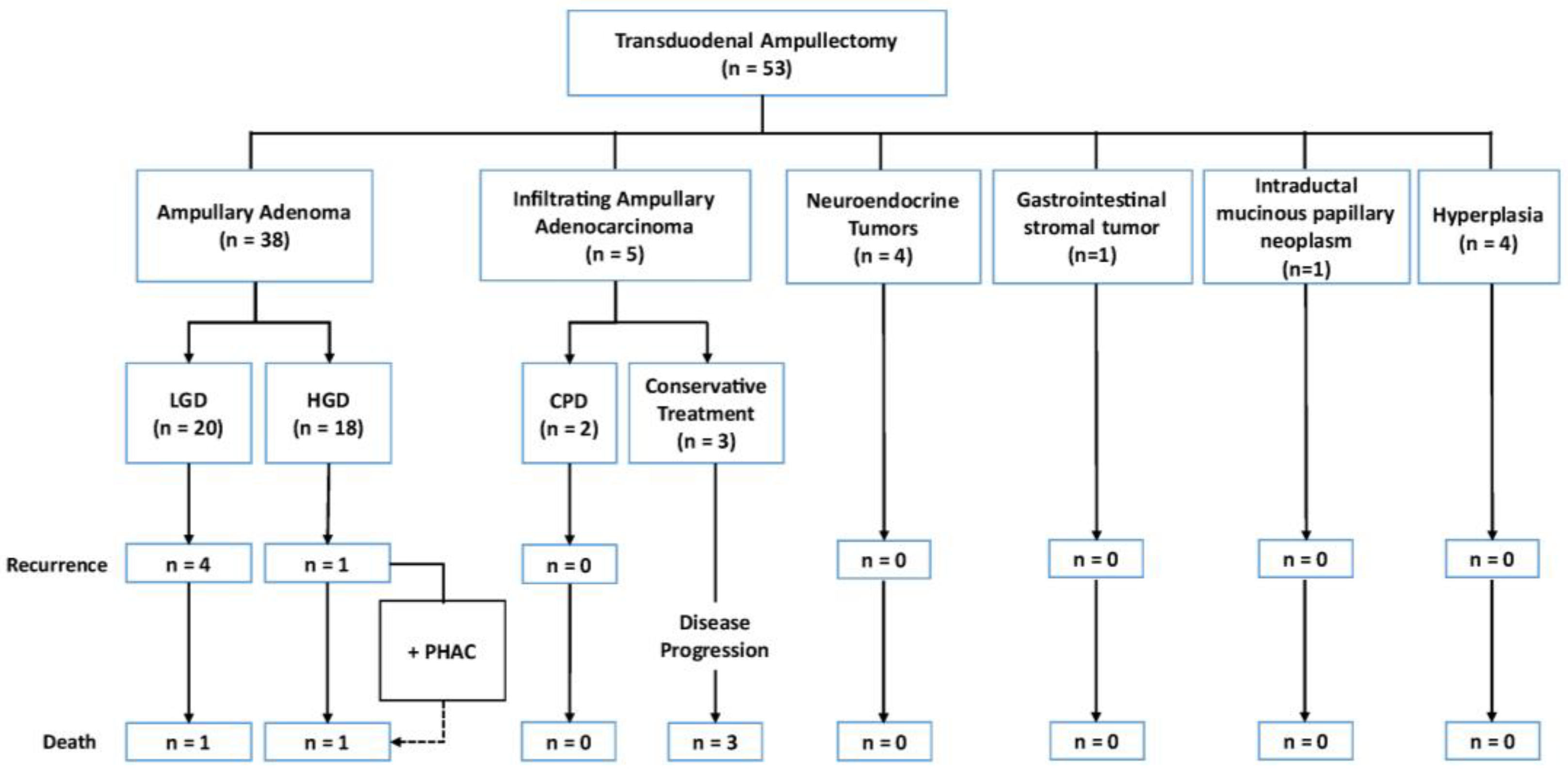
3.3. Expanded Indications for Transduodenal Ampullectomy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenberg, J.; Welch, J.P.; Pyrtek, L.J.; Walker, M.; Trowbridge, P. Benign villous adenomas of the ampulla of Vater. Cancer 1986, 58, 1563–1568. [Google Scholar] [CrossRef] [PubMed]
- Chini, P.; Draganov, P.V. Diagnosis and management of ampullary adenoma: The expanding role of endoscopy. World J. Gastrointest. Endosc. 2011, 3, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Lee, H.S.; Kim, J.; Choe, J.W.; Lee, J.M.; Hyun, J.J.; Yoon, J.H.; Kim, H.J.; Kim, J.S.; Choi, H.S. Clinical outcomes of endoscopic papillectomy of ampullary adenoma: A multi-center study. World J. Gastroenterol. 2022, 28, 1845–1859. [Google Scholar] [CrossRef] [PubMed]
- Irani, S.; Arai, A.; Ayub, K.; Biehl, T.; Brandabur, J.J.; Dorer, R.; Gluck, M.; Jiranek, G.; Patterson, D.; Schembre, D.; et al. Papillectomy for ampullary neoplasm: Results of a single referral center over a 10-year period. Gastrointest. Endosc. 2009, 70, 923–932. [Google Scholar] [CrossRef]
- Pavlovic-Markovic, A.; Dragasevic, S.; Krstic, M.; Stojkovic Lalosevic, M.; Milosavljevic, T. Assessment of Duodenal Adenomas and Strategies for Curative Therapy. Dig. Dis. 2019, 37, 374–380. [Google Scholar] [CrossRef]
- Hoyuela, C.; Cugat, E.; Veloso, E.; Marco, C. Treatment options for villous adenoma of the ampulla of Vater. HPB Surg. 2000, 11, 325–331; discussion 328–329. [Google Scholar] [CrossRef]
- Shapiro, P.F.; Lifvendahl, R.A. Tumors of the Extrahepatic Bile-ducts. Ann. Surg. 1931, 94, 61–79. [Google Scholar] [CrossRef]
- Scroggie, D.L.; Mavroeidis, V.K. Surgical ampullectomy: A comprehensive review. World J. Gastrointest. Surg. 2021, 13, 1338–1350. [Google Scholar] [CrossRef]
- Stolte, M.; Pscherer, C. Adenoma-carcinoma sequence in the papilla of Vater. Scand. J. Gastroenterol. 1996, 31, 376–382. [Google Scholar] [CrossRef]
- Askew, J.; Connor, S. Review of the investigation and surgical management of resectable ampullary adenocarcinoma. HPB 2013, 15, 829–838. [Google Scholar] [CrossRef]
- Hernandez, L.V.; Catalano, M.F. Endoscopic papillectomy. Curr. Opin. Gastroenterol. 2008, 24, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Nappo, G.; Gentile, D.; Galvanin, J.; Capretti, G.; Ridolfi, C.; Petitti, T.; Spaggiari, P.; Carrara, S.; Gavazzi, F.; Repici, A.; et al. Trans-duodenal ampullectomy for ampullary neoplasms: Early and long-term outcomes in 36 consecutive patients. Surg. Endosc. 2020, 34, 4358–4368. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.M.; Cameron, J.L.; Olino, K.; Herman, J.M.; de Jong, M.C.; Hruban, R.H.; Wolfgang, C.L.; Eckhauser, F.; Edil, B.H.; Choti, M.A.; et al. Clinicopathologic analysis of ampullary neoplasms in 450 patients: Implications for surgical strategy and long-term prognosis. J. Gastrointest. Surg. 2010, 14, 379–387. [Google Scholar] [CrossRef] [PubMed]
- di Mola, F.F.; Panaccio, P.; Grottola, T.; De Bonis, A.; Sapia, G.; Farrukh, M.; di Sebastiano, P. Transduodenal surgical ampullectomy: A procedure that requires a multidisciplinary approach. Updates Surg. 2021, 73, 2215–2223. [Google Scholar] [CrossRef]
- Kandler, J.; Neuhaus, H. How to Approach a Patient With Ampullary Lesion. Gastroenterology 2018, 155, 1670–1676. [Google Scholar] [CrossRef]
- Min, E.K.; Hong, S.S.; Kim, J.S.; Choi, M.; Hwang, H.S.; Kang, C.M.; Lee, W.J.; Yoon, D.S.; Hwang, H.K. Surgical Outcomes and Comparative Analysis of Transduodenal Ampullectomy and Pancreaticoduodenectomy: A Single-Center Study. Ann. Surg. Oncol. 2022, 29, 2429–2440. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef]
- Ceppa, E.P.; Burbridge, R.A.; Rialon, K.L.; Omotosho, P.A.; Emick, D.; Jowell, P.S.; Branch, M.S.; Pappas, T.N. Endoscopic versus surgical ampullectomy: An algorithm to treat disease of the ampulla of Vater. Ann. Surg. 2013, 257, 315–322. [Google Scholar] [CrossRef]
- Demetriades, H.; Zacharakis, E.; Kirou, I.; Pramateftakis, M.G.; Sapidis, N.; Kanellos, I.; Betsis, D. Local excision as a treatment for tumors of ampulla of Vater. World J. Surg. Oncol. 2006, 4, 14. [Google Scholar] [CrossRef][Green Version]
- Jordan, P.H., Jr.; Ayala, G.; Rosenberg, W.R.; Kinner, B.M. Treatment of ampullary villous adenomas that may harbor carcinoma. J. Gastrointest. Surg. 2002, 6, 770–775. [Google Scholar] [CrossRef]
- Lai, J.H.; Shyr, Y.M.; Wang, S.E. Ampullectomy versus pancreaticoduodenectomy for ampullary tumors. J. Chin. Med. Assoc. 2015, 78, 339–344. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Papalampros, A.; Moris, D.; Petrou, A.; Dimitrokallis, N.; Karavokyros, I.; Schizas, D.; Delladetsima, I.; Pappas, T.N.; Felekouras, E. Non-Whipple Operations in the Management of Benign, Premalignant and Early Cancerous Duodenal Lesions. Anticancer Res. 2017, 37, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Ramia Ángel, J.M.; Quiñones Sampedro, J.E.; Veguillas Redondo, P.; Sabater Maroto, C.; Garcia-Parreño Jofré, J. Ampulectomía transduodenal como tratamiento del adenoma de ampolla de Vater. Cirugía Española 2010, 87, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.L.; Choi, Y.I. Safety of duodenal ampullectomy for benign periampullary tumors. Ann. Hepatobiliary Pancreat. Surg. 2017, 21, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Vanbiervliet, G.; Strijker, M.; Arvanitakis, M.; Aelvoet, A.; Arnelo, U.; Beyna, T.; Busch, O.; Deprez, P.H.; Kunovsky, L.; Larghi, A.; et al. Endoscopic management of ampullary tumors: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2021, 53, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.K.; Liu, F.; Wang, Y.; Wang, X.D.; Tang, P.; Li, W. Preliminary experience of hybrid endoscopic submucosal dissection by duodenoscope for recurrent laterally spreading papillary lesions. World J. Gastroenterol. 2020, 26, 5673–5681. [Google Scholar] [CrossRef]
- Nakajima, Y.; Nemoto, D.; Suzuki, K.; Sato, A.; Shibukawa, G.; Lefor, A.K.; Togashi, K. Miniprobe ultrasonography guidance during endoscopic submucosal dissection of an ampullary duodenal lesion. Endoscopy 2023, 55, E92–E93. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Karwat, T.; Dubewar, S.; Mukewar, S.; Mukewar, S. Endoscopic resection of a large ampullary tumor using a hybrid endoscopic submucosal dissection and mucosal resection technique. Endoscopy 2023, 55, E1114–E1115. [Google Scholar] [CrossRef]
- Ridtitid, W.; Schmidt, S.E.; Al-Haddad, M.A.; LeBlanc, J.; DeWitt, J.M.; McHenry, L.; Fogel, E.L.; Watkins, J.L.; Lehman, G.A.; Sherman, S.; et al. Performance characteristics of EUS for locoregional evaluation of ampullary lesions. Gastrointest. Endosc. 2015, 81, 380–388. [Google Scholar] [CrossRef]
- Gluck, N.; Strul, H.; Rozner, G.; Leshno, M.; Santo, E. Endoscopy and EUS are key for effective surveillance and management of duodenal adenomas in familial adenomatous polyposis. Gastrointest. Endosc. 2015, 81, 960–966. [Google Scholar] [CrossRef]
- Labib, P.L.; Goodchild, G.; Turbett, J.P.; Skipworth, J.; Shankar, A.; Johnson, G.; Clark, S.; Latchford, A.; Pereira, S.P. Endoscopic ultrasound in the assessment of advanced duodenal adenomatosis in familial adenomatous polyposis. BMJ Open Gastroenterol. 2019, 6, e000336. [Google Scholar] [CrossRef] [PubMed]
- Committee, A.S.o.P.; Chathadi, K.V.; Khashab, M.A.; Acosta, R.D.; Chandrasekhara, V.; Eloubeidi, M.A.; Faulx, A.L.; Fonkalsrud, L.; Lightdale, J.R.; Salztman, J.R.; et al. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest. Endosc. 2015, 82, 773–781. [Google Scholar] [CrossRef]
- Grobmyer, S.R.; Stasik, C.N.; Draganov, P.; Hemming, A.W.; Dixon, L.R.; Vogel, S.B.; Hochwald, S.N. Contemporary results with ampullectomy for 29 “benign” neoplasms of the ampulla. J. Am. Coll. Surg. 2008, 206, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.S.; Han, S.S.; Kwon, W.; Jang, J.Y.; Kim, H.J.; Cho, C.K.; Ahn, K.S.; Yang, J.D.; Park, Y.; Min, S.K.; et al. Comparison of Oncologic Outcomes between Transduodenal Ampullectomy and Pancreatoduodenectomy in Ampulla of Vater Cancer: Korean Multicenter Study. Cancers 2021, 13, 2038. [Google Scholar] [CrossRef]
- Patel, V.; Jowell, P.; Obando, J.; Guy, C.D.; Burbridge, R.A. Does ampullary adenoma size predict invasion on EUS? Does invasion on EUS predict presence of malignancy? Endosc. Int. Open 2016, 4, E1313–E1318. [Google Scholar] [CrossRef]
- Lee, H.; Park, J.Y.; Kwon, W.; Heo, J.S.; Choi, D.W.; Choi, S.H. Transduodenal Ampullectomy for the Treatment of Early-Stage Ampulla of Vater Cancer. World J. Surg. 2016, 40, 967–973. [Google Scholar] [CrossRef]
- Busquets, J.; Lopez-Dominguez, J.; Gonzalez-Castillo, A.; Vila, M.; Pelaez, N.; Secanella, L.; Ramos, E.; Fabregat, J. Pancreas sparing duodenectomy in the treatment of primary duodenal neoplasms and other situations with duodenal involvement. Hepatobiliary Pancreat. Dis. Int. 2021, 20, 485–492. [Google Scholar] [CrossRef]
- Heise, C.; Abou Ali, E.; Hasenclever, D.; Auriemma, F.; Gulla, A.; Regner, S.; Gaujoux, S.; Hollenbach, M. Systematic Review with Meta-Analysis: Endoscopic and Surgical Resection for Ampullary Lesions. J. Clin. Med. 2020, 9, 3622. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, Y.; Huang, X.; Wang, H.; Huang, X.; Yuan, Z. Transduodenal ampullectomy provides a less invasive technique to cure early ampullary cancer. BMC Surg. 2016, 16, 36. [Google Scholar] [CrossRef]
- Sekine, M.; Watanabe, F.; Ishii, T.; Miura, T.; Koito, Y.; Kashima, H.; Matsumoto, K.; Noda, H.; Rikiyama, T.; Mashima, H. Investigation of the Indications for Endoscopic Papillectomy and Transduodenal Ampullectomy for Ampullary Tumors. J. Clin. Med. 2021, 10, 4463. [Google Scholar] [CrossRef]
- Moekotte, A.L.; Lof, S.; Van Roessel, S.; Fontana, M.; Dreyer, S.; Shablak, A.; Casciani, F.; Mavroeidis, V.K.; Robinson, S.; Khalil, K.; et al. Histopathologic Predictors of Survival and Recurrence in Resected Ampullary Adenocarcinoma: International Multicenter Cohort Study. Ann. Surg. 2020, 272, 1086–1093. [Google Scholar] [CrossRef]
| Baseline and Sociodemographic Characteristics | N = 53 |
|---|---|
| Age (years), mean (SD) | 62.5 (14.6) |
| Sex, n (%) | |
| Male | 29 (54.7) |
| Female | 24 (45.3) |
| ASA, n (%) | |
| I | 10 (18.9) |
| II | 23 (43.4) |
| III | 20 (37.7) |
| Postoperative Morbidity | N = 53 |
|---|---|
| Infections | |
| Intra-abdominal Abscess | 9 (17.0) |
| Surgical Site Infection | 9 (17.0) |
| Other Infections | 5 (9.4) |
| Acute Pancreatitis | 5 (9.4) |
| Fistulae | |
| Pancreatic Fistula | 4 (7.6) |
| Biliary Fistula | 2 (3.8) |
| Intestinal Fistula | 4 (7.6) |
| Bleeding | |
| Intra-abdominal Bleeding | 2 (3.8) |
| Upper Gastrointestinal Bleeding | 1 (1.9) |
| Blood Transfusion Requirement | |
| ≤48 h | 2 (3.8) |
| >48 h | 7 (13.2) |
| Delayed Gastric Emptying | 3 (5.7) |
| Parenteral Nutrition Requirement | 10 (18.9) |
| Long-Term Mortality | N = 9 |
|---|---|
| Postoperative death (≤90 days) | 1 (11.1) |
| Recurrent disease + de novo pancreatic head adenocarcinoma | 1 (11.1) |
| Non-intervened infiltrating ampullary adenocarcinoma | 3 (33.3) |
| Unrelated with TDA/ampullary lesion | 4 (44.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorribas, M.; Carnaval, T.; Secanella, L.; Peláez, N.; Salord, S.; Gornals, J.B.; Leiva, D.; Serrano, T.; Fabregat, J.; Busquets, J. Pushing the Boundaries of Ampullectomy for Benign Ampullary Tumors: 25-Year Outcomes of Surgical Ampullary Resection Associated with Duodenectomy or Biliary Resection. J. Clin. Med. 2024, 13, 7220. https://doi.org/10.3390/jcm13237220
Sorribas M, Carnaval T, Secanella L, Peláez N, Salord S, Gornals JB, Leiva D, Serrano T, Fabregat J, Busquets J. Pushing the Boundaries of Ampullectomy for Benign Ampullary Tumors: 25-Year Outcomes of Surgical Ampullary Resection Associated with Duodenectomy or Biliary Resection. Journal of Clinical Medicine. 2024; 13(23):7220. https://doi.org/10.3390/jcm13237220
Chicago/Turabian StyleSorribas, Maria, Thiago Carnaval, Luis Secanella, Núria Peláez, Silvia Salord, Joan B. Gornals, David Leiva, Teresa Serrano, Joan Fabregat, and Juli Busquets. 2024. "Pushing the Boundaries of Ampullectomy for Benign Ampullary Tumors: 25-Year Outcomes of Surgical Ampullary Resection Associated with Duodenectomy or Biliary Resection" Journal of Clinical Medicine 13, no. 23: 7220. https://doi.org/10.3390/jcm13237220
APA StyleSorribas, M., Carnaval, T., Secanella, L., Peláez, N., Salord, S., Gornals, J. B., Leiva, D., Serrano, T., Fabregat, J., & Busquets, J. (2024). Pushing the Boundaries of Ampullectomy for Benign Ampullary Tumors: 25-Year Outcomes of Surgical Ampullary Resection Associated with Duodenectomy or Biliary Resection. Journal of Clinical Medicine, 13(23), 7220. https://doi.org/10.3390/jcm13237220






