Alignment Between Treatment Decision and Treatment Administration for Squamous Cell Carcinoma of the Upper Aerodigestive Tract Before, During, and After the COVID-19 Pandemic: A Retrospective Analysis
Abstract
1. Introduction
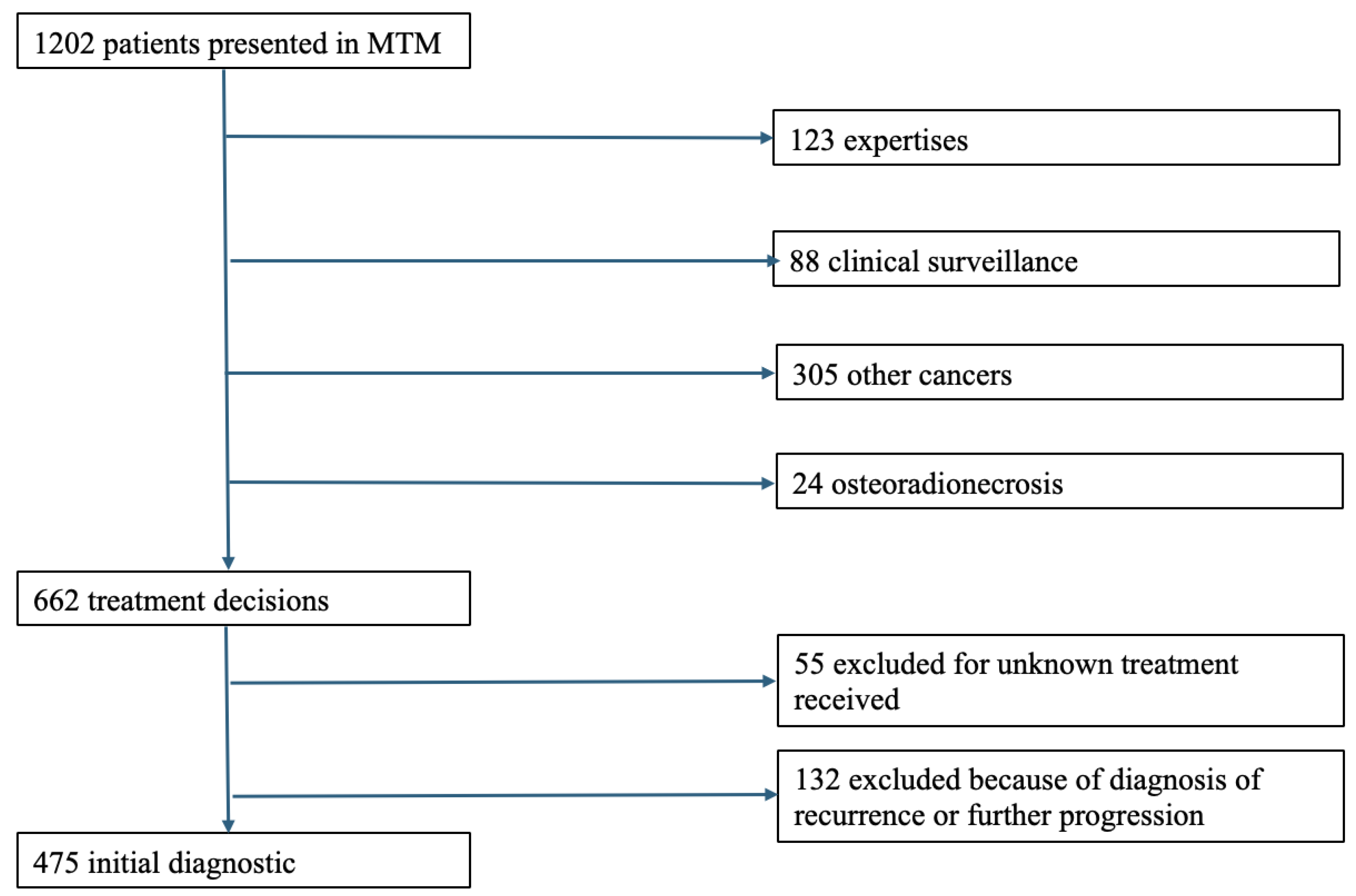
2. Materials and Methods
2.1. Study Design
- -
- Patients whose files were registered and discussed in MTMs at the university hospital center between 1 January 2019 and 31 December 2021.
- -
- Patients diagnosed initially with squamous cell carcinoma of the oral cavity, oropharynx, or hypopharynx or with cervical lymphadenopathy without a primary or larynx.
- -
- Patients for whom both the decided therapeutic plan and the executed therapeutic plan were recorded.
- -
- Patients in first-line treatment (initial diagnosis).
- -
- Patients undergoing progressive disease or tumor recurrence.
- -
- Patients with tumor localization in the nasal cavities, sinuses, or salivary glands.
- -
- Patients with cancers that were categorized as cutaneous tumors.
- -
- Patients with anatomopathological types other than squamous cell carcinoma.
- -
- Patients with unknown treatment plans.
- -
- Patients whose records were presented in MTMs for expert consultation purposes.
2.2. Data Collected
- -
- Patients’ characteristics (age, gender, World Health Organization Performance Status (PS) index [21]) and medical history (cirrhosis, diabetes, cardiovascular disease, chronic obstructive pulmonary disease (COPD)). Malnutrition status, history of cancer (current or remission), and alcohol and tobacco use were also recorded.
- -
- Squamous cell carcinoma characteristics: site of origin (oral cavity, oropharynx, larynx, hypopharynx, or cervical lymphadenopathy without primary), tumor node metastasis (TNM) status, according to the eighth edition of the UICC (Union for International Cancer Control) 2017 head and neck tumors TNM classification [22], and human papillomavirus (HPV) status.
- -
- Extension assessment, conducted in accordance with the recommendations of the French Society of Oto-Rhinolaryngology (SFORL), included panendoscopy of the UADT with an operative report and a summary diagram. Additionally, it involved a cervical–thoracic CT scan, a Positron Emission Tomography (PET-CT) scan, an Ear, Nose, and Throat (ENT) MRI, or an alternative ultrasound/scan-guided biopsy.
- -
- Multidisciplinary team meeting (MTM): MTMs were held once a week, jointly between the affiliated cancer center and the tertiary referral center. At least three physicians from the following specialties were involved: ENT, maxillofacial or reconstructive surgery, medical oncology, radiation oncology, pathology, and radiology. Before each meeting, a standardized form was completed and verified by the patient’s referring physician. The patient’s case was presented, and all aspects of the extension assessment were reviewed during the meeting. The form included the date, the attending physicians, and relevant prior data. After the meeting, the treatment protocol was collectively determined and validated by the MTM coordinator. The original form was stored in the Cancer Communication Folder (CCF), and a copy of the validated treatment protocol was added to the patient’s electronic medical record for traceability. Patients did not attend the MTM. Instead, the collective treatment decision was communicated to them during a disclosure consultation with their referring ENT physician, where they could either accept or decline the proposed treatment.
- -
- From the patient’s medical record, two time intervals were calculated: the time to care (time between the first symptom and specialist consultation), the time to treatment initiation (time from the MTM decision to the start of treatment).
- -
- Finally, the treatment center was documented (reference center or other center), as well as the 3-year survival.
2.3. Statistical Analysis
3. Results
3.1. Patients Characteristics
3.2. Extension Assessment
3.3. Treatment Performed
3.4. Processing Time
3.5. Inadequacy Between Treatment Decided in MTM and Treatment Delivered
3.6. Factors Associated with Inadequacy Between Treatment Decided at MTM and Treatment Delivered
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MTMs | Multidisciplinary Team Meetings |
| UATD | Upper Aero-Digestive Tract |
| CNIL | Commission National Informatique et Liberté |
| PS | Performance Status |
| TNM | Tumor Node Metastasis |
| COPD | Chronic Obstructive Pulmonary Disease |
| UICC | Union International for Cancer Control |
| HPV | Human Papilloma Virus |
| SFORL | French Society of Oto-Rhino-Laryngology |
| CT | Cervical and Thoracic |
| ENT | Ear, Nose, and Throat |
| MRI | Magnetic Resonance Imaging |
| PET | Positron Emission Tomography |
| CCF | Cancer Communication Folder |
| CDS | Cancer Decision Support |
References
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Khuri, F. Update in the Management of Head and Neck Cancer. Update Cancer Ther [Internet]. 2006. Available online: https://www.academia.edu/62263762/Update_in_the_management_of_head_and_neck_cancer (accessed on 23 July 2023).
- Peng, J.; Raverdy, N.; Ganry, O.; de La Roche-Saint-André, G.; Dubreuil, A.; Lorriaux, A. Descriptive epidemiology of upper aerodigestive tract cancers in the department of Somme. Bull. Cancer 2000, 87, 201–206. [Google Scholar]
- Hill, C. Epidemiology of cancer of the upper aerodigestive tract. Bull. Cancer 2000, 5, 5–8. [Google Scholar]
- Haute Autorité de Santé. DEVELOPPEMENT PROFESSIONNEL CONTRINU (DPC)—Réunion de Concertation Pluridisciplinaire. Available online: https://www.has-sante.fr/jcms/c_2806878/fr/reunion-de-concertation-pluridisciplinaire (accessed on 1 November 2024).
- E.Cancer—Institut Nationnal du Cancer. Les Plans Cancer—Stratégie de Lutte Contre les Cancers en France. Available online: https://www.e-cancer.fr/Institut-national-du-cancer/Strategie-de-lutte-contre-les-cancers-en-France/Les-Plans-cancer (accessed on 30 November 2024).
- Orgerie, M.B.; Duchange, N.; Pélicier, N.; Chapet, S.; Dorval, É.; Rosset, P.; Lemarié, É.; Hervé, C.; Moutel, G. La réunion de concertation pluridisciplinaire: Quelle place dans la décision médicale en cancérologie ? Bull Cancer 2010, 97, 255–264. [Google Scholar] [CrossRef]
- Chbihi, D.; Corda, M.; Thibault, T.; Baude, J.; Guigou, C.; Folia, M. Assessment of Professional Practices in the Care Pathway of Patients with Upper Aerodigestive Tract Cancer in a University Hospital. J. Clin. Med. 2024, 13, 6623. [Google Scholar] [CrossRef]
- Bortot, L.; Targato, G.; Noto, C.; Giavarra, M.; Palmero, L.; Zara, D.; Bertoli, E.; Dri, A.; Andreetta, C.; Pascoletti, G.; et al. Multidisciplinary Team Meeting Proposal and Final Therapeutic Choice in Early Breast Cancer: Is There an Agreement? Front. Oncol. 2022, 12, 885992. [Google Scholar] [CrossRef]
- Pons-Tostivint, E.; Daubisse-Marliac, L.; Grosclaude, P.; Oum Sack, E.; Goddard, J.; Morel, C.; Dunet, C.; Sibrac, L.; Lagadic, C.; Bauvin, E.; et al. Multidisciplinary team meeting and EUSOMA quality indicators in breast cancer care: A French regional multicenter study. Breast 2019, 46, 170–177. [Google Scholar] [CrossRef]
- Vinod, S.K.; Wellege, N.T.; Kim, S.; Duggan, K.J.; Ibrahim, M.; Shafiq, J. Translation of oncology multidisciplinary team meeting (MDM) recommendations into clinical practice. BMC Health Serv. Res. 2021, 21, 461. [Google Scholar] [CrossRef]
- Kaye, A.D.; Okeagu, C.N.; Pham, A.D.; Silva, R.A.; Hurley, J.J.; Arron, B.L.; Sarfraz, N.; Lee, H.N.; Ghali, G.E.; Gamble, J.W.; et al. Economic impact of COVID-19 pandemic on healthcare facilities and systems: International perspectives. Best Pract. Res. Clin. Anaesthesiol. 2021, 35, 293–306. [Google Scholar] [CrossRef]
- Moynihan, R.; Sanders, S.; Michaleff, Z.A.; Scott, A.M.; Clark, J.; To, E.J.; Jones, M.; Kitchener, E.; Fox, M.; Johansson, M.; et al. Impact of COVID-19 pandemic on utilisation of healthcare services: A systematic review. BMJ Open 2021, 11, e045343. [Google Scholar] [CrossRef]
- Vallée, A. Geoepidemiological perspective on COVID-19 pandemic review, an insight into the global impact. Front. Public Health 2023, 11, 1242891. [Google Scholar] [CrossRef]
- Tuczyńska, M.; Staszewski, R.; Matthews-Kozanecka, M.; Żok, A.; Baum, E. Quality of the Healthcare Services During COVID-19 Pandemic in Selected European Countries. Front. Public Health 2022, 10, 870314. [Google Scholar] [CrossRef]
- Amdaoud, M.; Arcuri, G.; Levratto, N. Healthcare system and social trust in the fight against COVID-19: The case of France. Eur. J. Public Health 2021, 31, 895–900. [Google Scholar] [CrossRef]
- Fotopoulou, C.; Khan, T.; Bracinik, J.; Glasbey, J.; Abu-Rustum, N.; Chiva, L.; Fagotti, A.; Fujiwara, K.; Ghebre, R.; Gutelkin, M.; et al. Outcomes of gynecologic cancer surgery during the COVID-19 pandemic: An international, multicenter, prospective CovidSurg-Gynecologic Oncology Cancer study. Am. J. Obstet. Gynecol. 2022, 227, 735.e1. [Google Scholar] [CrossRef]
- Fagan, M.; Janes, W.C.I.; Andrews, M.; Harvey, D.R.; Warden, G.M.; Organ, M.K.; Johnston, P. Restricted access and advanced disease in post-pandemic testicular cancer. Can. Urol. Assoc. J. 2024, 18, 262–267. [Google Scholar] [CrossRef]
- McKay, S.C.; Pathak, S.; Wilkin, R.J.; Kamarajah, S.K.; Wigmore, S.J.; Rees, J.; Dunne, D.F.; Garcea, G.; Ahmad, J.; de Liguori Carino, N.; et al. Impact of SARS-CoV-2 pandemic on pancreatic cancer services and treatment pathways: United Kingdom experience. HPB 2021, 23, 1656–1665. [Google Scholar] [CrossRef]
- Vidal—Premier Vaccin Contre la COVID19 Disponible en France: Comirnaty, en Pratique. Available online: https://www.vidal.fr/actualites/26442-premier-vaccin-contre-la-covid-19-disponible-en-france-comirnaty-en-pratique.html (accessed on 21 January 2025).
- Chow, R.; Chiu, N.; Bruera, E.; Krishnan, M.; Chiu, L.; Lam, H.; DeAngelis, C.; Pulenzas, N.; Vuong, S.; Chow, E. Inter-rater reliability in performance status assessment among health care professionals: A systematic review. Ann. Palliat. Med. 2016, 5, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; O’Sullivan, B. Overview of the 8th Edition TNM Classification for Head and Neck Cancer. Curr. Treat. Options Oncol. 2017, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Mickey, R.M.; Greenland, S. The Impact of Confounder Selection Criteria on Effect Estimation. Am. J. Epidemiol. 1989, 129, 125–137. [Google Scholar] [CrossRef]
- May, M. Regression Modelling Strategies with Applications to Linear Models, Logistic Regression, and Survival Analysis. Frank E Harrell Jr, New York: Springer 2001, pp. 568, $79.95. ISBN 0-387-95232-2. Int. J. Epidemiol. 2002, 31, 699–700. [Google Scholar] [CrossRef][Green Version]
- Chaillou, D.; Mortuaire, G.; Deken-Delannoy, V.; Rysman, B.; Chevalier, D.; Mouawad, F. Presence in head and neck cancer multidisciplinary team meeting: The patient’s experience and satisfaction. Eur. Ann. Otorhinolaryngol. Head. Neck Dis. 2019, 136, 75–82. [Google Scholar] [CrossRef]
- Massoubre, J.; Lapeyre, M.; Pastourel, R.; Dupuch, V.; Biau, J.; Dillies, A.F.; Mom, T.; Pereira, B.; Gilain, L.; Saroul, N. Will the presence of the patient at multidisciplinary meetings influence the decision in head and neck oncology management? Acta Otolaryngol. 2018, 138, 185–189. [Google Scholar] [CrossRef]
- Saroul, N.; Massoubre, J.; Biau, J.; Pastourel, R.; Mom, T.; Dillies, A.F.; Barthelemy, I.; Lapeyre, M.; Gilain, L. Decision-making in head and neck cancer: Investigation on the mode of presentation of files and composition of the multidisciplinary team meetings. Bull Cancer 2017, 104, 812–814. [Google Scholar] [CrossRef]
- Potier, A.L.; Leroy, M.; Mortuaire, G.; Rysman, B.; Morisse, M.; Mouawad, F. Impact of the 2nd, 3rd and 4th waves of the COVID-19 pandemic on wait times in head and neck cancer: A retrospective study in a French expert center. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2024, 141, 268–274. [Google Scholar] [CrossRef]
- Vanderhaegen, T.; Pierache, A.; Mortuaire, G.; Rysman, B.; Nicot, R.; Chevalier, D.; Mouawad, F. The first wave of COVID-19 did not cause longer wait times in head and neck cancer. Experience of a French expert center. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2022, 139, 261–267. [Google Scholar] [CrossRef]
- Tevetoğlu, F.; Kara, S.; Aliyeva, C.; Yıldırım, R.; Yener, H.M. Delayed presentation of head and neck cancer patients during COVID-19 pandemic. Eur. Arch. Otorhinolaryngol. 2021, 278, 5081–5085. [Google Scholar] [CrossRef]
- Solis, R.N.; Mehrzad, M.; Faiq, S.; Frusciante, R.P.; Sekhon, H.K.; Abouyared, M.; Bewley, A.F.; Farwell, D.G.; Birkeland, A.C. The Impact of COVID-19 on Head and Neck Cancer Treatment: Before and During the Pandemic. OTO Open 2021, 5, 2473974X211068075. [Google Scholar] [CrossRef]
- Balk, M.; Rupp, R.; Craveiro, A.V.; Allner, M.; Grundtner, P.; Eckstein, M.; Hecht, M.; Iro, H.; Gostian, A.O. The COVID-19 pandemic and its consequences for the diagnosis and therapy of head and neck malignancies. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 284–290. [Google Scholar] [CrossRef]
- Grumstrup Simonsen, M.; Fenger Carlander, A.L.; Kronberg Jakobsen, K.; Grønhøj, C.; Von Buchwald, C. The impact of the COVID-19 pandemic on time to treatment in head and neck cancer management: A systematic review. Acta Oncol. 2025, 64, 156–166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lachâtre, M.; Launay, O. Vaccination COVID-19: Technologies vaccinales, efficacité en vie réelle et spécificités. Méd. Mal. Infect. Form. 2022, 1, 129–135. [Google Scholar] [CrossRef]
- Bessis, S. Pandémie de COVID-19. Med. Mal. Infect. 2020, 50, 8S20–8S24. [Google Scholar] [CrossRef]
- Milcent, C. Système de santé français face à la crise sanitaire: De désorganisation à réorganisation. Inf. Psychiatr. 2021, 97, 857–864. [Google Scholar] [CrossRef]
- Thomas, P. Quelles conséquences auront les déprogrammations hospitalières liées au COVID-19? Npg 2021, 21, 135–137. [Google Scholar] [CrossRef]
- Direction Général de l’Offre de Soin—Les Plans Nationaux Cancers—Ministère de la Santé et de l’Accès aux Soins. Available online: https://sante.gouv.fr/soins-et-maladies/maladies/cancer-11425/article/les-plans-nationaux-cancer (accessed on 19 November 2024).
- Castel, P.; Merle, I. Quand les normes de pratiques deviennent une ressource pour les médecins. Sociol. Trav. 2002, 44, 337–355. [Google Scholar] [CrossRef]
- Sidhom, M.A.; Poulsen, M. Group decisions in oncology: Doctors’ perceptions of the legal responsibilities arising from multidisciplinary meetings. J. Med. Imaging Radiat. Oncol. 2008, 52, 287–292. [Google Scholar] [CrossRef]
- Sidhom, M.A.; Poulsen, M.G. Multidisciplinary care in oncology: Medicolegal implications of group decisions. Lancet Oncol. 2006, 7, 951–954. [Google Scholar] [CrossRef]
- Ruhstaller, T.; Roe, H.; Thürlimann, B.; Nicoll, J.J. The multidisciplinary meeting: An indispensable aid to communication between different specialities. Eur. J. Cancer 2006, 42, 2459–2462. [Google Scholar] [CrossRef]
- Penel, N.; Valentin, F.; Giscard, S.; Vanseymortier, L.; Beuscart, R. General practitioners assessment of a structured report on medical decision making by a regional multidisciplinary cancer committee. Bull Cancer 2007, 94, E23–E26. [Google Scholar] [PubMed]
- Somashekhar, S.P.; Sepúlveda, M.J.; Puglielli, S.; Norden, A.D.; Shortliffe, E.H.; Rohit Kumar, C.; Rauthan, A.; Arun Kumar, N.; Patil, P.; Rhee, K.; et al. Watson for Oncology and breast cancer treatment recommendations: Agreement with an expert multidisciplinary tumor board. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 418–423. [Google Scholar] [CrossRef]
- Zou, F.W.; Tang, Y.; Liu, C.; Ma, J.; Hu, C. Concordance Study Between IBM Watson for Oncology and Real Clinical Practice for Cervical Cancer Patients in China: A Retrospective Analysis. Front. Genet. 2020, 11, 200. [Google Scholar] [CrossRef]
- Pan, H.; Tao, J.; Qian, M.; Zhou, W.; Qian, Y.; Xie, H.; Jing, S.; Xu, T.; Zhang, X.; Dai, Z.; et al. Concordance assessment of Watson for Oncology in breast cancer chemotherapy: First China experience. Transl. Cancer Res. 2019, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.I.; Chung, J.W.; Kim, K.O.; Kwon, K.A.; Kim, Y.J.; Park, D.K.; Ahn, S.M.; Park, S.H.; Sym, S.J.; Shin, D.B.; et al. Concordance Rate between Clinicians and Watson for Oncology among Patients with Advanced Gastric Cancer: Early, Real-World Experience in Korea. Can. J. Gastroenterol. Hepatol. 2019, 2019, 8072928. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, S.H. Artificial Intelligence-Driven Oncology Clinical Decision Support System for Multidisciplinary Teams. Sensors 2020, 20, 4693. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | 2019 N = 147 1 | 2020 N = 162 1 | 2021 N = 166 1 | p-Value 2 |
|---|---|---|---|---|
| Age | 64 (56.72) | 65 (59.73) | 65 (59.73) | 0.2 |
| Male gender | 117 (80%) | 126 (78%) | 127 (77%) | 0.8 |
| Performance status (≥2) | 12 (8.2%) | 36 (22%) | 46 (28%) | <0.001 |
| COPD | 39 (27%) | 32 (20%) | 26 (16%) | 0.061 |
| Hepatopathy | 11 (7.5%) | 16 (9.9%) | 8 (4.8%) | 0.2 |
| Cardiovascular disease | 84 (57%) | 87 (54%) | 95 (57%) | 0.8 |
| Diabetes | 16 (11%) | 18 (11%) | 30 (18%) | 0.11 |
| Undernutrition | 56 (38%) | 120 (74%) | 124 (75%) | <0.001 |
| Previous cancer history | 26 (18%) | 42 (26%) | 32 (19%) | 0.2 |
| Smoke quantity (PY) | 40 (15.50) | 30 (10.45) | 40 (20.50) | 0.3 |
| Active smoking | 88 (60%) | 89 (55%) | 82 (49%) | 0.2 |
| Alcoholism | 54 (37%) | 69 (43%) | 77 (46%) | 0.2 |
| HPV | 22 (15%) | 23 (14%) | 25 (15%) | >0.9 |
| T at diagnosis (3 or 4) | 84 (57%) | 99 (61%) | 99 (60%) | 0.8 |
| N + at diagnosis | 88 (60%) | 87 (54% | 80(48%) | 0.12 |
| M + at diagnosis | 7 (4.8%) | 20 (12%) | 7 (4.2%) | 0.011 |
| Discovery of synchronous cancer | 14 (9.5%) | 16 (9.9%) | 19 (11%) | 0.9 |
| Complete file | 113 (77%) | 75 (46%) | 102 (61%) | <0.001 |
| Adequacy MTM–treatment performed | 119 (81%) | 121 (75%) | 127 (77%) | 0.4 |
| Place of treatment (university hospital or another center) | 112 (81%) | 134 (83%) | 135 (81%) | 0.5 |
| Unknown | 10 | 0 | 0 | |
| MTM reconducted | 17 (12%) | 59 (37%) | 51 (31%) | <0.001 |
| Unknown | 0 | 1 | 0 | |
| Gross survival at 3 years | 71 (48%) | 89 (55%) | 100 (60%) | 0.11 |
| Diagnoses | 2019 N = 147 (100%) | 2020 N = 162 (100%) | 2021 N = 166 (100%) | |
|---|---|---|---|---|
| Number of incompletes (%) | 34 (23) | 87 (54) | 64 (39) | |
| Missing element in extension assessment | Imaging | 26 (18) | 67 (41) | 47 (28) |
| Anathomopathology | 3 (2) | 1 (1) | ||
| Imaging + anathomopathology | 2 (1) | 2 (1) | 4 (2) | |
| Pan endoscopy | 6 (4) | 8 (6) | 7 (4) | |
| Pan endoscopy + imaging | 7 (4) | 5 (3) | ||
| 2019 N = 147 (100%) | 2020 N = 162 (100%) | 2021 N = 166 (100%) | |
|---|---|---|---|
| Surgery | 32 (22) | 30 (19) | 23 (14) |
| Exclusive radiotherapy | 20 (14) | 16 (10) | 21 (13) |
| Radio-chemotherapy | 40 (27) | 49 (30) | 49 (30) |
| Surgery and adjuvant radiotherapy | 14 (10) | 12 (7) | 21 (13) |
| Surgery and radio-chemotherapy | 20 (14) | 16 (10) | 26 (16) |
| Exclusive chemotherapy | 6 (4) | 23 (14) | 14 (8) |
| No treatment performed | 15 (10) | 16 (10) | 12 (7) |
| Delay | 2019 N = 147 | 2020 N = 162 | 2021 N = 166 | p-Value 1 |
|---|---|---|---|---|
| Initial care time (first consultation and MTM) in days | 23 (16.31) | 24 (16.40) | 26 (17.38) | 0.5 |
| Unknown | 0 | 2 | 0 | |
| Time between MTM and treatment | 32 (20.42) | 24 (14.38) | 26 (13.37) | <0.001 |
| Unknown | 15 | 18 | 14 |
| Treatment | S. | RT. | S. + Adjuvant RT. | S. + Adjuvant RTCT. | Concomitant RTCT. | Palliative CT. | Supportive Care Treatment | Death Before Treatment | Treatment Refused by the Patient | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MTM Decision | |||||||||||
| (A) | |||||||||||
| S. | - | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| RT. | 0 | - | 0 | 0 | 0 | 0 | 3 | 3 | 0 | 6 | |
| S. + adjuvant RT. | 3 | 0 | - | 0 | 0 | 0 | 0 | 1 | 0 | 4 | |
| S. + adjuvant RTCT. | 1 | 0 | 1 | - | 2 | 0 | 0 | 0 | 0 | 4 | |
| Concomitant RTCT. | 1 | 2 | 0 | 1 | - | 1 | 2 | 2 | 0 | 9 | |
| Palliative CT. | 0 | 0 | 0 | 0 | 1 | - | 0 | 2 | 1 | 4 | |
| Total | 5 | 3 | 1 | 1 | 3 | 1 | 5 | 8 | 1 | 28 | |
| (B) | |||||||||||
| S. | - | 2 | 1 | 2 | 3 | 1 | 0 | 0 | 0 | 9 | |
| RT. | 0 | - | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 3 | |
| S. + adjuvant RT. | 3 | 0 | - | 0 | 0 | 1 | 0 | 0 | 0 | 4 | |
| S. + adjuvant RTCT. | 0 | 0 | 3 | - | 0 | 0 | 0 | 2 | 0 | 5 | |
| Concomitant RTCT. | 1 | 1 | 0 | 0 | - | 4 | 1 | 3 | 1 | 11 | |
| Palliative CT. | 1 | 0 | 0 | 0 | 1 | - | 1 | 6 | 0 | 9 | |
| Total | 5 | 3 | 4 | 2 | 5 | 7 | 3 | 11 | 1 | 41 | |
| (C) | |||||||||||
| S. | - | 2 | 0 | 0 | 4 | 0 | 1 | 2 | 1 | 10 | |
| RT. | 0 | - | 0 | 0 | 2 | 2 | 1 | 2 | 0 | 7 | |
| S. + adjuvant RT. | 2 | 0 | - | 1 | 0 | 1 | 0 | 0 | 0 | 4 | |
| S. + adjuvant RTCT. | 0 | 2 | 0 | - | 0 | 0 | 0 | 0 | 0 | 2 | |
| Concomitant RTCT. | 0 | 1 | 0 | 1 | - | 6 | 0 | 2 | 0 | 10 | |
| Palliative CT. | 0 | 0 | 0 | 0 | 1 | - | 0 | 3 | 0 | 4 | |
| Total | 2 | 5 | 0 | 2 | 7 | 9 | 2 | 9 | 1 | 37 | |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variables | Mismatching N = 108 1 | Matching N = 367 1 | p-Value 2 | OR For No-Matching | 95% CI | p-Value |
| PS ≥ 2 | 45 (42%) | 49 (13%) | <0.001 | 3.03 | 1.78, 5.15 | <0.001 |
| COPD | 27 (25%) | 70 (19%) | 0.2 | 1.03 | 0.57, 1.80 | >0.9 |
| Hepatopathy | 14 (13%) | 21 (5.7%) | 0.011 | 1.59 | 0.68, 3.65 | 0.3 |
| Cardiovascular disease | 71 (66%) | 195 (53%) | 0.020 | 1.34 | 0.82, 2.23 | 0.2 |
| Diabetes | 17 (16%) | 47 (13%) | 0.4 | |||
| Undernutrition | 90 (83%) | 210 (57%) | <0.001 | 1.77 | 0.97, 3.36 | 0.07 |
| Smoking (active or withdrawal) | 50 (46%) | 209 (57%) | 0.051 | 0.85 | 0.50, 1.44 | 0.5 |
| Alcoholism | 58 (54%) | 142 (39%) | 0.005 | 1.11 | 0.64, 1.92 | 0.7 |
| Previous cancer history | 27 (25%) | 73 (20%) | 0.3 | |||
| T at diagnostic (3/4) | 87 (81%) | 195 (53%) | <0.001 | 2.43 | 1.40, 4.35 | 0.002 |
| N at diagnostic (1/2/3+) | 69 (64%) | 186 (51%) | 0.016 | 1.20 | 0.72, 1.99 | 0.5 |
| M at diagnostic | 16 (15%) | 18 (4.9%) | <0.001 | 1.77 | 0.79, 3.92 | 0.2 |
| Discovery of synchronous cancer | 12 (11%) | 37 (10%) | 0.8 | |||
| Complete file | 61 (56%) | 229 (62%) | 0.3 | |||
| Study period | 0.4 | |||||
| 28 (26%) | 119 (32%) | ||||
| 41 (38%) | 121 (33%) | ||||
| 39 (36%) | 127 (35%) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reliquet, B.; Thibault, T.; Elhomsy, P.; Chbihi, D.; Folia, M.; Guigou, C. Alignment Between Treatment Decision and Treatment Administration for Squamous Cell Carcinoma of the Upper Aerodigestive Tract Before, During, and After the COVID-19 Pandemic: A Retrospective Analysis. J. Clin. Med. 2025, 14, 2613. https://doi.org/10.3390/jcm14082613
Reliquet B, Thibault T, Elhomsy P, Chbihi D, Folia M, Guigou C. Alignment Between Treatment Decision and Treatment Administration for Squamous Cell Carcinoma of the Upper Aerodigestive Tract Before, During, and After the COVID-19 Pandemic: A Retrospective Analysis. Journal of Clinical Medicine. 2025; 14(8):2613. https://doi.org/10.3390/jcm14082613
Chicago/Turabian StyleReliquet, Benjamin, Thomas Thibault, Paul Elhomsy, Dounia Chbihi, Mireille Folia, and Caroline Guigou. 2025. "Alignment Between Treatment Decision and Treatment Administration for Squamous Cell Carcinoma of the Upper Aerodigestive Tract Before, During, and After the COVID-19 Pandemic: A Retrospective Analysis" Journal of Clinical Medicine 14, no. 8: 2613. https://doi.org/10.3390/jcm14082613
APA StyleReliquet, B., Thibault, T., Elhomsy, P., Chbihi, D., Folia, M., & Guigou, C. (2025). Alignment Between Treatment Decision and Treatment Administration for Squamous Cell Carcinoma of the Upper Aerodigestive Tract Before, During, and After the COVID-19 Pandemic: A Retrospective Analysis. Journal of Clinical Medicine, 14(8), 2613. https://doi.org/10.3390/jcm14082613





