TAPAS—A Prospective, Multicentre, Long-Term Cohort Study in Children, Adolescents and Adults with Seasonal Allergic Rhinitis—Design and Early Results
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Design
2.2. Endpoints
- Study inclusion at visit 1
- During active AIT treatment (years 1 to 3), 3 visits were made in year 1 for basic treatment and at least 3 visits per year for continued treatment (on average, 10 injections per year). In years 2 and 3, the maintenance dose is continued at intervals of 4–6 weeks. In general, all injections but also unscheduled visits were documented.
- During the follow-up period in years 4 and 5, 2 visits are planned each year (before the start of the respective pollen season and at the peak of the respective pollen season).
2.3. The Patients
2.4. Pollen Data
2.5. Statistical Methods
2.5.1. Sample Size Estimation
2.5.2. Evaluation Times
2.5.3. Primary Endpoint
2.6. Ethical and Regulatory Framework
3. Results
Safety and Tolerability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACT | Asthma Control Test |
| ADR | Adverse Drug Reaction |
| AE | Adverse Event |
| AIT | Allergen-specific immunotherapy |
| AR | Allergic Rhinitis |
| CSMS | Combined Symptom and Medication Score |
| DBPC | Double-blind, placebo-controlled |
| dMS | Daily Medication Score |
| dSS | Daily Symptom Score |
| DWD | German weather service |
| EAACI | European Academy of Allergy and Clinical Immunology |
| EMA | European Medicines Agency |
| GCP | Good Clinical Practice |
| HTTPS | Hypertext Transfer Protocol Secure |
| MATA | Microcrystalline Tyrosine-associated Allergoid |
| MCT | Microcrystalline Tyrosine |
| NIS | Non-Interventional Study |
| PIP | Paediatric Investigation Plan |
| RCT | Randomised Controlled Trial |
| RCAT | Rhinitis Control Test |
| RQLQ | Rhinoconjunctivitis Quality of Life Questionnaire |
| SAE | Serious Adverse Event |
| SCIT | Subcutaneous allergen-specific immunotherapy |
| SLIT | Sublingual allergen-specific immunotherapy |
| SmPCs | Summary of Product Characteristics |
| VAS | Visual Analogue Scale |
References
- Dhami, S.; Nurmatov, U.; Arasi, S.; Khan, T.; Asaria, M.; Zaman, H.; Agarwal, A.; Netuveli, G.; Roberts, G.; Pfaar, O.; et al. Allergen immunotherapy for allergic rhinoconjunctivitis: A systematic review and meta-analysis. Allergy 2017, 72, 1597–1631. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.; Pfaar, O.; Akdis, C.A.; Ansotegui, I.J.; Durham, S.R.; van Wijk, R.G.; Halken, S.; Larenas-Linnemann, D.; Pawankar, R.; Pitsios, C.; et al. EAACI Guidelines on Allergen Immunotherapy: Allergic rhinoconjunctivitis. Allergy 2018, 73, 765–798. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, L.; Niggemann, B.; Dreborg, S.; Ferdousi, H.A.; Halken, S.; Høst, A.; Koivikko, A.; Norberg, L.A.; Valovirta, E.; Wahn, U.; et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy 2007, 62, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Shah-Hosseini, K.; Mioc, K.; Hadler, M.; Karagiannis, E.; Mösges, R. Optimum treatment strategies for polyallergic patients—Analysis of a large observational trial. Curr. Med. Res. Opin. 2015, 31, 2249–2259. [Google Scholar] [CrossRef] [PubMed]
- Mahler, V.; Mentzer, D.; Bonertz, A.; Muraro, A.; Eigenmann, P.; Bousquet, J.; Halken, S.; Pfaar, O.; Jutel, M.; Wahn, U.; et al. Allergen Immunotherapy (AIT) in children: A vulnerable population with its own rights and legislation—Summary of EMA-initiated multi-stakeholder meeting on Allergen Immunotherapy (AIT) for children, held at Paul-Ehrlich-Institut, Langen, Germany, 16.1.2019. Clin. Transl. Allergy 2020, 10, 28. [Google Scholar] [CrossRef]
- Tang, X.; Rabin, R.L.; Yan, L.K. A three-stage design for allergen immunotherapy trials. Allergy 2022, 77, 1835–1842. [Google Scholar] [CrossRef]
- Tang, X.; Rabin, R.L.; Yan, L.K. An enhanced three-stage design with trend analysis for allergen immunotherapy trials. PLoS ONE 2023, 18, e0291533. [Google Scholar] [CrossRef]
- Valovirta, E.; Piotrowska, T.; Laursen, M.K.; Andersen, J.S.; Sørensen, H.F.; Klink, R.; Varga, E.-M.; Huttegger, I.; Agertoft, L.; Halken, S.; et al. Results from the 5-year SQ grass sublingual immunotherapy tablet asthma prevention (GAP) trial in children with grass pollen allergy. J. Allergy Clin. Immunol. 2018, 141, 529–538.e13. [Google Scholar] [CrossRef]
- Pfaar, O.; Ankermann, T.; Augustin, M.; Bubel, P.; Böing, S.; Brehler, R.; Eng, P.A.; Fischer, P.J.; Gerstlauer, M.; Hamelmann, E.; et al. Guideline on allergen immunotherapy in IgE-mediated allergic diseases: S2K Guideline of the German Society of Allergology and Clinical Immunology (DGAKI), Society of Pediatric Allergology and Environmental Medicine (GPA), Medical Association of German Allergologists (AeDA), Austrian Society of Allergology and Immunology (ÖGAI), Swiss Society for Allergology and Immunology (SSAI), German Dermatological Society (DDG), German So-ciety of Oto-Rhino-Laryngology, Head and Neck Surgery (DGHNO-KHC), German Society of Pediatrics and Ado-lescent Medicine (DGKJ), Society of Pediatric Pulmonology (GPP), German Respiratory Society (DGP), German Pro-fessional Association of Otolaryngologists (BVHNO), German Association of Paediatric and Adolescent Care Spe-cialists (BVKJ), Federal Association of Pneumologists, Sleep and Respiratory Physicians (BdP), Professional Asso-ciation of German Dermatologists (BVDD). Allergol. Sel. 2022, 6, 167–232. [Google Scholar] [CrossRef]
- Al Saleh, H.; Mösges, R. MicroCrystalline Tyrosine-adsorbed immunotherapy. Curr. Opin. Allergy Clin. Immunol. 2022, 22, 413–420. [Google Scholar] [CrossRef]
- Becker, S.; Zieglmayer, P.; Canto, G.; Fassio, F.; Yong, P.; Acikel, C.; Raskopf, E.; Steveling-Klein, E.H.; Allekotte, S.; Mösges, R. A meta-analysis on allergen-specific immunotherapy using MCT® (MicroCrystalline Tyrosine)-adsorbed allergoids in pollen allergic patients suffering from allergic rhinoconjunctivitis. Clin. Transl. Allergy 2021, 11, e12037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vogelberg, C.; Gerstlauer, M. AIT in pediatric allergology: Opportunities and difficulties on the home stretch of the Therapy Allergen Ordinance. Allergol. Sel. 2023, 7, 236–241. [Google Scholar] [CrossRef]
- Pfaar, O.; Demoly, P.; Gerth van Wijk, R.; Bonini, S.; Bousquet, J.; Canonica, G.W.; Durham, S.R.; Jacobsen, L.; Malling, H.J.; Mösges, R.; et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: An EAACI Position Paper. Allergy 2014, 69, 854–867. [Google Scholar] [CrossRef]
- Mösges, R.; Raskopf, E.; Klimek, L.; Pfaar, O.; Zielen, S.; Xenofontos, E.; Decker, L.; Neuhof, C.; Rybachuk, A.; Acikel, C.; et al. Short-course subcutaneous treatment with birch pollen allergoids greatly improves symptom and medication scores in birch allergy. Allergy 2024, 80, 817–826. [Google Scholar] [CrossRef]
- Juniper, E.F.; Guyatt, G.H.; Dolovich, J. Assessment of quality of life in adolescents with allergic rhinoconjunctivitis: Development and testing of a questionnaire for clinical trials. J. Allergy Clin. Immunol. 1994, 93, 413–423. [Google Scholar] [CrossRef]
- Juniper, E.F.; Howland, W.C.; Roberts, N.B.; Thompson, A.K.; King, D.R. Measuring quality of life in children with rhinoconjunctivitis. J. Allergy Clin. Immunol. 1998, 101, 163–170. [Google Scholar] [CrossRef]
- Juniper, E.F.; Thompson, A.K.; Ferrie, P.J.; Roberts, J.N. Validation of the standardised version of the Rhinoconjunctivitis Quality of Life Questionnaire. J. Allergy Clin. Immunol. 1999, 104 Pt 1, 364–369. [Google Scholar] [CrossRef]
- Nathan, R.A.; Sorkness, C.A.; Kosinski, M.; Schatz, M.; Li, J.T.; Marcus, P.; Murray, J.J.; Pendergraft, T.B. Development of the Asthma Control Test: A survey for assessing asthma control. J. Allergy Clin. Immunol. 2004, 113, 59–65. [Google Scholar] [CrossRef]
- Liu, A.H.; Zeiger, R.; Sorkness, C.; Mahr, T.; Ostrom, N.; Burgess, S.; Rosenzweig, J.C.; Manjunath, R. Development and cross-sectional validation of the Childhood Asthma Control Test. J. Allergy Clin. Immunol. 2007, 119, 817–825. [Google Scholar] [CrossRef]
- Nathan, R.A.; Dalal, A.A.; Stanford, R.H.; Meltzer, E.O.; Schatz, M.; Derebery, J.; Mintz, M.; Thompson, M.A.; DiBenedetti, D.B. Qualitative Development of the Rhinitis Control Assessment Test (RCAT), an Instrument for Evaluating Rhinitis Symptom Control. Patient 2010, 3, 91–99. [Google Scholar] [CrossRef]
- Liedtke, J.; Mandl, A.; Köther, J.; Chwieralski, J.; Shah-Hosseini, K.; Raskopf, E.; Pieper-Fürst, U.; Allekotte, S.; Mösges, R. RCAT reflects symptom control and quality of life in allergic rhinoconjunctivitis patients. Allergy 2017, 73, 1101–1109. [Google Scholar] [CrossRef]
- Sieber, J.; Shah-Hosseini, K.; Mösges, R. Specific immunotherapy for allergic rhinitis to grass and tree pollens in daily medical practice—Symptom load with sublingual immunotherapy compared to subcutaneous immunotherapy. Ann. Med. 2011, 43, 418–424. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on the Choice of the Non-Inferiority Margin; EMA/CHMP/EWP/2158/99; European Medicines Agency: London, UK, 2005. [Google Scholar]
- Food and Drug Administration (US). Non-Inferiority Clinical Trials to Establish Effectiveness [Draft Guidance for Industry]; Food and Drug Administration: Silver Spring, MD, USA, 2016. [Google Scholar]
- Pfaar, O.; Richter, H.; Sager, A.; Miller, C.; Müller, T.; Jutel, M. Persistence in allergen immunotherapy: A longitudinal, prescription data-based real-world analysis. Clin. Transl. Allergy 2023, 13, e12245. [Google Scholar] [CrossRef]
- Licari, A.; Magri, P.; De Silvestri, A.; Giannetti, A.; Indolfi, C.; Mori, F.; Marseglia, G.L.; Peroni, D. Epidemiology of Allergic Rhinitis in Children: A Systematic Review and Meta-Analysis. J. Allergy Clin. Immunol. Pract. 2023, 11, 2547–2556. [Google Scholar] [CrossRef]
- Shin, S.; Lee, J.Y.; Cho, H.; Kim, M.; Kim, S.; Jang, S.; Song, J.; Kim, J.; Kim, S.; Ahn, K. Prevalence of Severe Atopic Dermatitis and Comorbid Chronic Systemic Diseases Is Increasing in Korean Children and Adolescents. Allergy Asthma Immunol. Res. 2024, 16, 300–307. [Google Scholar] [CrossRef]
- Alvaro-Lozano, M.; Akdis, C.A.; Akdis, M.; Alviani, C.; Angier, E.; Arasi, S.; Arzt-Gradwohl, L.; Barber, D.; Bazire, R.; Cavkaytar, O.; et al. EAACI Allergen Immunotherapy User’s Guide. Pediatr. Allergy Immunol. 2020, 31 (Suppl. S25), 1–101. [Google Scholar] [CrossRef]
- Caffarelli, C.; Mastrorilli, C.; Procaccianti, M.; Santoro, A. Use of Sublingual Immunotherapy for Aeroallergens in Children with Asthma. J. Clin. Med. 2020, 9, 3381. [Google Scholar] [CrossRef]
- Kruppert, S.; Vogelberg, C.; Klimek, L.; Becker, S. TARGET—Real-World-Evidence Study on the Long-Term Benefits of MCT®—Associated Pollen Allergoid SCIT on AR and Asthma; Authorea: New York, NY, USA, 2022. [Google Scholar]
- Durham, S.R.; Emminger, W.; Kapp, A.; de Monchy, J.G.; Rak, S.; Scadding, G.K.; Wurtzen, P.A.; Andersen, J.S.; Tholstrup, B.; Riis, B.; et al. SQ-standardized sublingual grass immunotherapy: Confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J. Allergy Clin. Immunol. 2012, 129, 717–725.e5. [Google Scholar] [CrossRef]
- Didier, A.; Malling, H.; Worm, M.; Horak, F.; Sussman, G.L. Prolonged efficacy of the 300IR 5-grass pollen tablet up to 2 years after treatment cessation, as measured by a recommended daily combined score. Clin. Transl. Allergy 2015, 5, 12. [Google Scholar] [CrossRef]
- Bufe, A.; Eberle, P.; Franke-Beckmann, E.; Funck, J.; Kimmig, M.; Klimek, L.; Knecht, R.; Stephan, V.; Tholstrup, B.; Weißhaar, C.; et al. Safety and efficacy in children of an SQ-standardized grass allergen tablet for sublingual immunotherapy. J. Allergy Clin. Immunol. 2009, 123, 167–173.e7. [Google Scholar] [CrossRef]
- Wahn, U.; Tabar, A.; Kuna, P.; Halken, S.; Montagut, A.; de Beaumont, O.; Le Gall, M. Efficacy and safety of 5-grass-pollen sublingual immunotherapy tablets in pediatric allergic rhinoconjunctivitis. J. Allergy Clin. Immunol. 2009, 123, 160–166.e3. [Google Scholar] [CrossRef]
- Pfaar, O.; Agache, I.; Bergmann, K.; Bindslev-Jensen, C.; Bousquet, J.; Creticos, P.S.; Devillier, P.; Durham, S.R.; Hellings, P.; Kaul, S.; et al. Placebo effects in allergen immunotherapy—An EAACI Task Force Position Paper. Allergy 2021, 76, 629–647. [Google Scholar] [CrossRef]
- De Kam, P.-J.; Kramer, M.F.; Shamji, M.H.; Oluwayi, K.; Heath, M.D.; Jensen-Jarolim, E.; Berger, M.H.; Berger, U.E.; Graessel, A.; Sellwood, F.; et al. Dogmas, challenges, and promises in phase III allergen immunotherapy studies. World Allergy Organ. J. 2021, 14, 100578. [Google Scholar] [CrossRef]
- Eng, P.A.; Reinhold, M.; Gnehm, H.E. Long-term efficacy of preseasonal grass pollen immunotherapy in children. Allergy 2002, 57, 306–312. [Google Scholar] [CrossRef]
- Eng, P.A.; Borer-Reinhold, M.; Heijnen, I.A.F.M.; Gnehm, H.P.E. Twelve-year follow-up after discontinuation of preseasonal grass pollen immunotherapy in childhood. Allergy 2006, 61, 198–201. [Google Scholar] [CrossRef]
- Leibson, C.L.; Rocca, W.A.; Hanson, V.A.; Cha, R.; Kokmen, E.; O’Brien, P.C.; Palumbo, P.J. Risk of dementia among persons with diabetes mellitus: A population-based cohort study. Am. J. Epidemiol. 1997, 145, 301–308. [Google Scholar] [CrossRef]
- Dwivedi, V.; Kopanja, S.; Schmidthaler, K.; Sieber, J.; Bannert, C.; Szépfalusi, Z. Preventive allergen immunotherapy with inhalant allergens in children. Allergy 2024, 79, 2065–2087. [Google Scholar] [CrossRef]
- Parashar, S.; Pandya, A.; Portnoy, J.M. Pediatric subcutaneous allergen immunotherapy. Allergy Asthma Proc. 2022, 43, 286–291. [Google Scholar] [CrossRef]


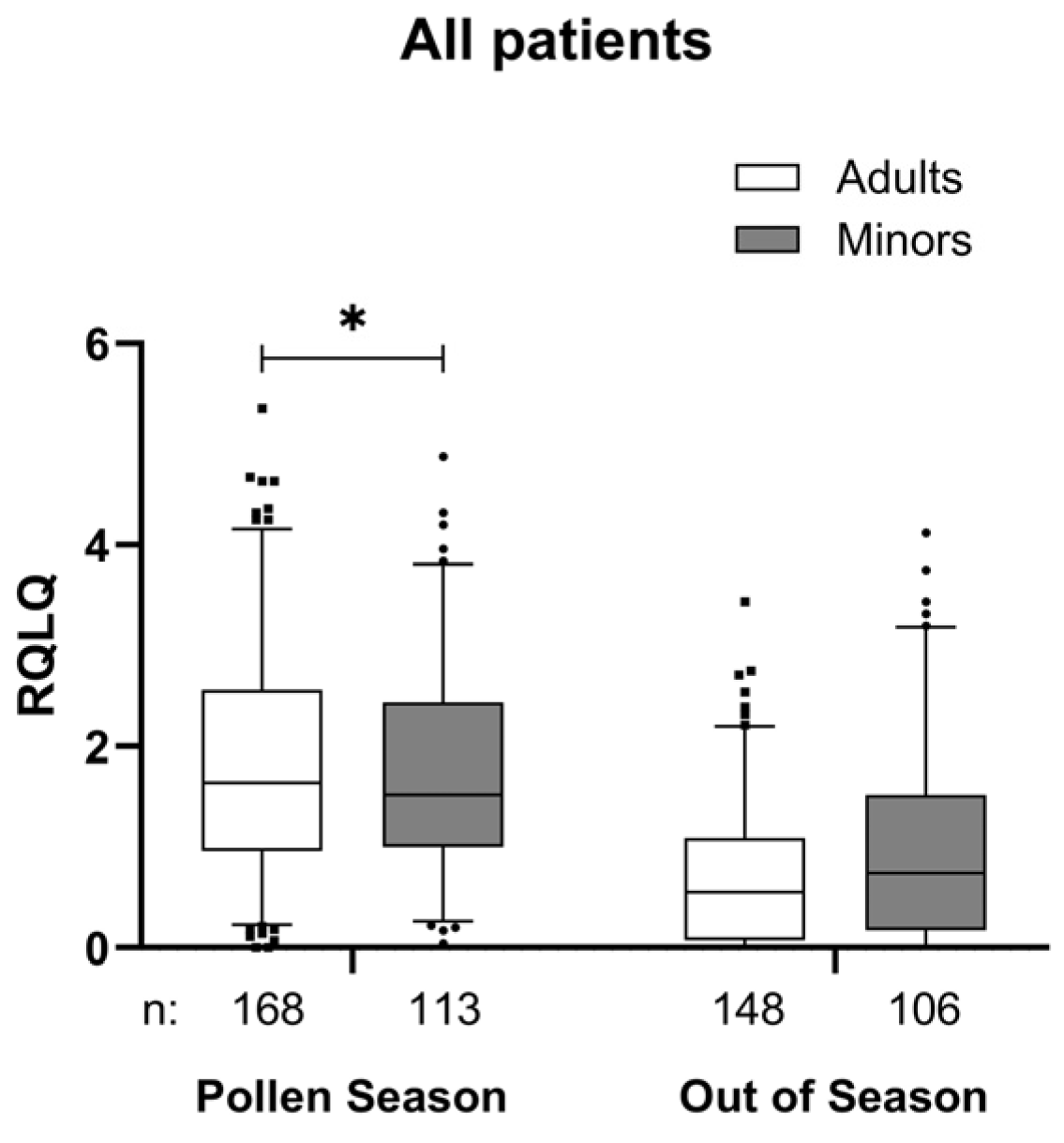
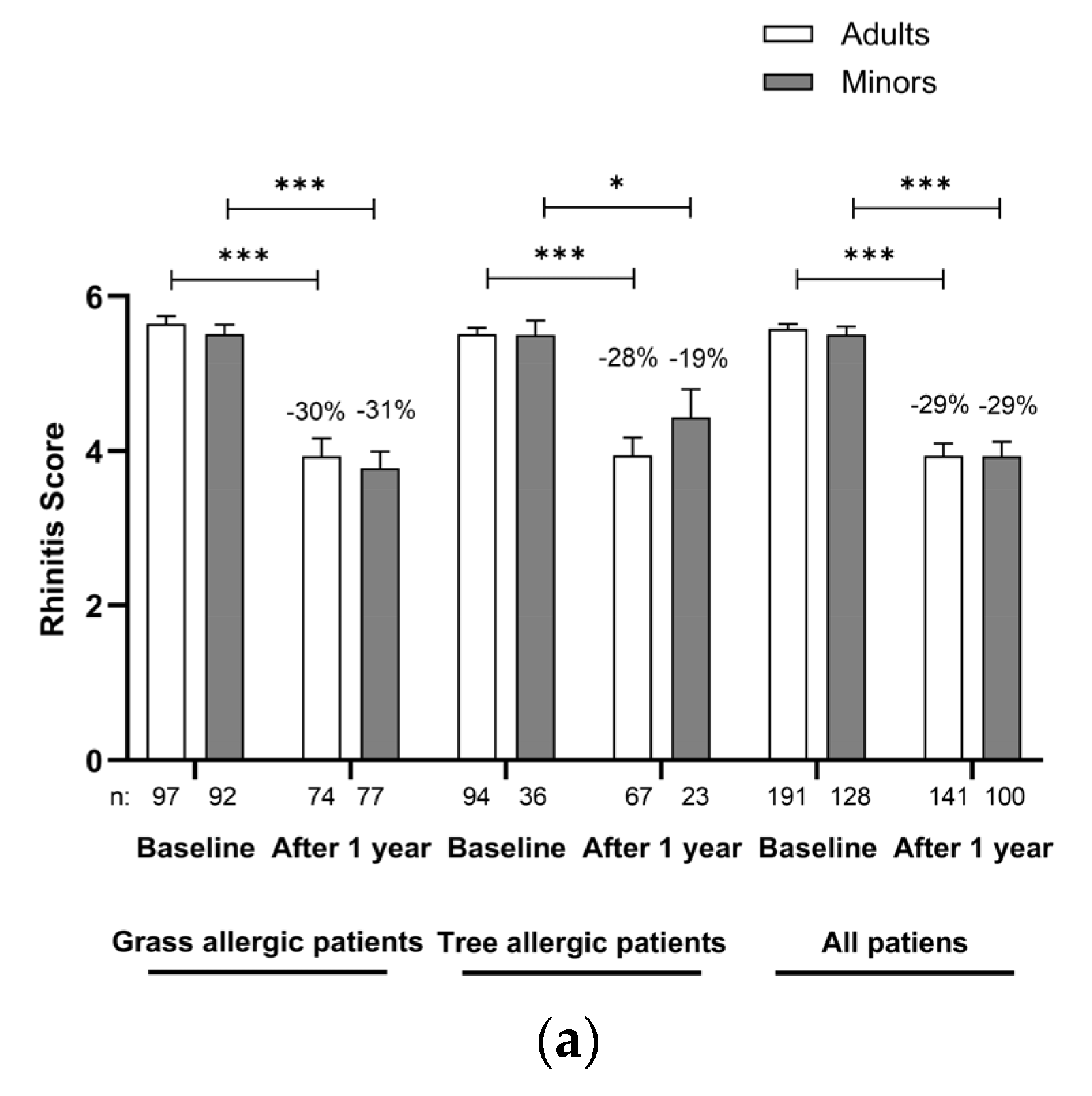

| Age Group | Minors | Adults | Total | ||||
|---|---|---|---|---|---|---|---|
| Count | % | Count | % | Count | % | ||
| Allergic diseases | |||||||
| Allergic rhinitis Severity | Mild | 9 | 7.1% | 9 | 4.7% | 18 | 5.7% |
| Moderate | 54 | 42.5% | 88 | 46.1% | 142 | 44.7% | |
| Severe | 64 | 50.4% | 94 | 49.2% | 158 | 49.7% | |
| Total | 127 | 100.0% | 191 | 100.0% | 318 | 100.0% | |
| Allergic conjunctivitis | |||||||
| Severity | Mild | 19 | 16.7% | 43 | 25.9% | 62 | 22.1% |
| Moderate | 50 | 43.9% | 78 | 47.0% | 128 | 45.7% | |
| Severe | 45 | 39.5% | 45 | 27.1% | 90 | 32.1% | |
| Total | 114 | 100.0% | 166 | 100.0% | 280 | 100.0% | |
| Allergic asthma | |||||||
| Severity | Mild | 18 | 42.9% | 31 | 49.2% | 49 | 46.7% |
| Moderate | 16 | 38.1% | 20 | 31.7% | 36 | 34.3% | |
| Severe | 8 | 19.0% | 12 | 19.0% | 20 | 19.0% | |
| Total | 42 | 100.0% | 63 | 100.0% | 105 | 100.0% | |
| Sensitisation Profiles | |||||||
| Monosensitised | 51 | 39.8% | 74 | 38.9% | 125 | 39.3% | |
| Polysensitised | 77 | 60.2% | 116 | 61.1% | 193 | 60.7% | |
| Total | 128 | 100.0% | 190 | 100.0% | 318 | 100.0% | |
| Main allergen + grasses | 11 | 14.3% | 33 | 28.4% | 44 | 22.8% | |
| Main allergen + trees | 26 | 33.8% | 15 | 12.9% | 41 | 21.2% | |
| Main allergen + animal epithelia | 26 | 33.8% | 33 | 28.4% | 59 | 30.6% | |
| Main allergen + house dust mite | 39 | 50.6% | 54 | 46.6% | 93 | 48.2% | |
| Main allergen + mould fungi | 7 | 9.1% | 16 | 13.8% | 23 | 11.9% | |
| Main allergen + other | 13 | 16.9% | 36 | 31.0% | 49 | 25.4% | |
| Concomitant medications | |||||||
| Cases | 114 | 88.4% | 153 | 80.1% | 267 | 83.4% | |
| Eye drops | 72 | 55.8% | 64 | 33.5% | 136 | 42.5% | |
| Antihistamines: oral | 95 | 73.6% | 128 | 67.0% | 223 | 69.7% | |
| Corticosteroids: oral | 5 | 3.9% | 6 | 3.1% | 11 | 3.4% | |
| Corticosteroids: nasal | 47 | 36.4% | 49 | 25.7% | 96 | 30.0% | |
| Antihistamines: nasal | 38 | 29.5% | 30 | 15.7% | 68 | 21.3% | |
| Corticosteroids: inhaled | 20 | 15.5% | 24 | 12.6% | 44 | 13.8% | |
| ß-sympathomimetics: inhaled | 26 | 20.2% | 17 | 8.9% | 43 | 13.4% | |
| Other | 6 | 4.7% | 18 | 9.4% | 24 | 7.5% | |
| Concomitant diseases | |||||||
| Cases | 48 | 37.2% | 66 | 34.6% | 114 | 35.6% | |
| Atopic dermatitis | 28 | 21.7% | 11 | 5.8% | 39 | 12.2% | |
| Food allergy/intolerance | 20 | 15.5% | 25 | 13.1% | 45 | 14.1% | |
| Urticaria | 5 | 3.9% | 0 | 0.0% | 5 | 1.6% | |
| Nasal polyposis | 3 | 2.3% | 2 | 1.0% | 5 | 1.6% | |
| ASA intolerance | 0 | 0.0% | 1 | 0.5% | 1 | 0.3% | |
| Age Group | Main Allergen | Frequency | Percentage |
|---|---|---|---|
| Children (5–11 years) | Grasses | 56 | 71.8 |
| Trees | 22 | 28.2 | |
| Total | 78 | 100 | |
| Adolescents (12–17 years) | Grasses | 37 | 72.5 |
| Trees | 14 | 27.5 | |
| Total | 51 | 100 | |
| Adults (18–75 years) | Grasses | 97 | 50.8 |
| Trees | 94 | 49.2 | |
| Total | 191 | 100 | |
| Total | Grasses | 190 | 59.4 |
| Trees | 130 | 40.6 | |
| Total | 320 | 100 |
| Total Patients with at Least One ADR (n = 320) | Minors (n = 129) | Adults (n = 191) | p-Value |
|---|---|---|---|
| None | 101 (78.3%) | 168 (88.0%) | 0.021 * |
| Not serious | 28 (21.7%) | 22 (11.5%) | |
| Serious | 0 (0.0%) | 1 (0.5%) | |
| Local | 26 (20.2%) | 16 (8.4%) | 0.002 * |
| Systemic | 10 (7.8%) | 14 (7.3%) | 0.888 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerstlauer, M.; Hiller, J.; Raab, J.; Birkholz, K.; Tapparo, M.; Neuhof, C.; Day, L.; Rybachuk, A.; Acikel, C.; Sahin, H.; et al. TAPAS—A Prospective, Multicentre, Long-Term Cohort Study in Children, Adolescents and Adults with Seasonal Allergic Rhinitis—Design and Early Results. J. Clin. Med. 2025, 14, 2609. https://doi.org/10.3390/jcm14082609
Gerstlauer M, Hiller J, Raab J, Birkholz K, Tapparo M, Neuhof C, Day L, Rybachuk A, Acikel C, Sahin H, et al. TAPAS—A Prospective, Multicentre, Long-Term Cohort Study in Children, Adolescents and Adults with Seasonal Allergic Rhinitis—Design and Early Results. Journal of Clinical Medicine. 2025; 14(8):2609. https://doi.org/10.3390/jcm14082609
Chicago/Turabian StyleGerstlauer, Michael, Julia Hiller, Jennifer Raab, Katrin Birkholz, Martin Tapparo, Christian Neuhof, Laura Day, Anna Rybachuk, Cengizhan Acikel, Hacer Sahin, and et al. 2025. "TAPAS—A Prospective, Multicentre, Long-Term Cohort Study in Children, Adolescents and Adults with Seasonal Allergic Rhinitis—Design and Early Results" Journal of Clinical Medicine 14, no. 8: 2609. https://doi.org/10.3390/jcm14082609
APA StyleGerstlauer, M., Hiller, J., Raab, J., Birkholz, K., Tapparo, M., Neuhof, C., Day, L., Rybachuk, A., Acikel, C., Sahin, H., Hebbeler, K., Becker, S., Vogelberg, C., Allekotte, S., Kramer, M. F., & the TAPAS Study Group. (2025). TAPAS—A Prospective, Multicentre, Long-Term Cohort Study in Children, Adolescents and Adults with Seasonal Allergic Rhinitis—Design and Early Results. Journal of Clinical Medicine, 14(8), 2609. https://doi.org/10.3390/jcm14082609






