Rituximab, Apremilast, and Upadacitinib as Selected Biosimilar and Targeted Synthetic Disease-Modifying Antirheumatic Drugs with Diverse Mechanisms of Action: Their Current Use in Slowing Down the Progression of Disease
Abstract
1. Introduction
2. Biosimilar Disease-Modifying Antirheumatic Drugs (bsDMARDs)
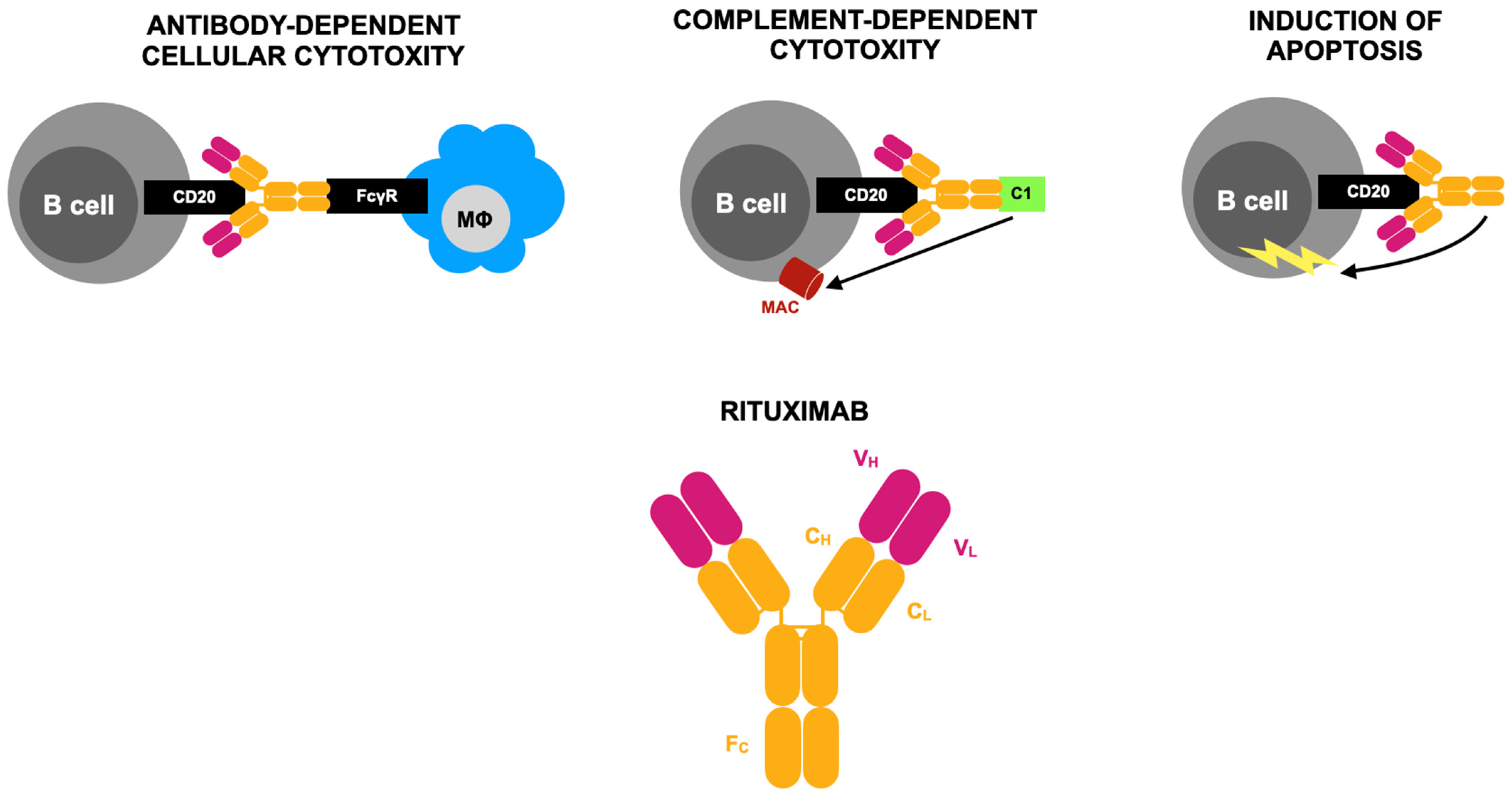
Anti-CD20–Mediated B-Cell Depletion Agent—Rituximab
3. Targeted Synthetic Disease-Modifying Antirheumatic Drugs (tsDMARDS)
3.1. PDE4 Inhibitor—Apremilast

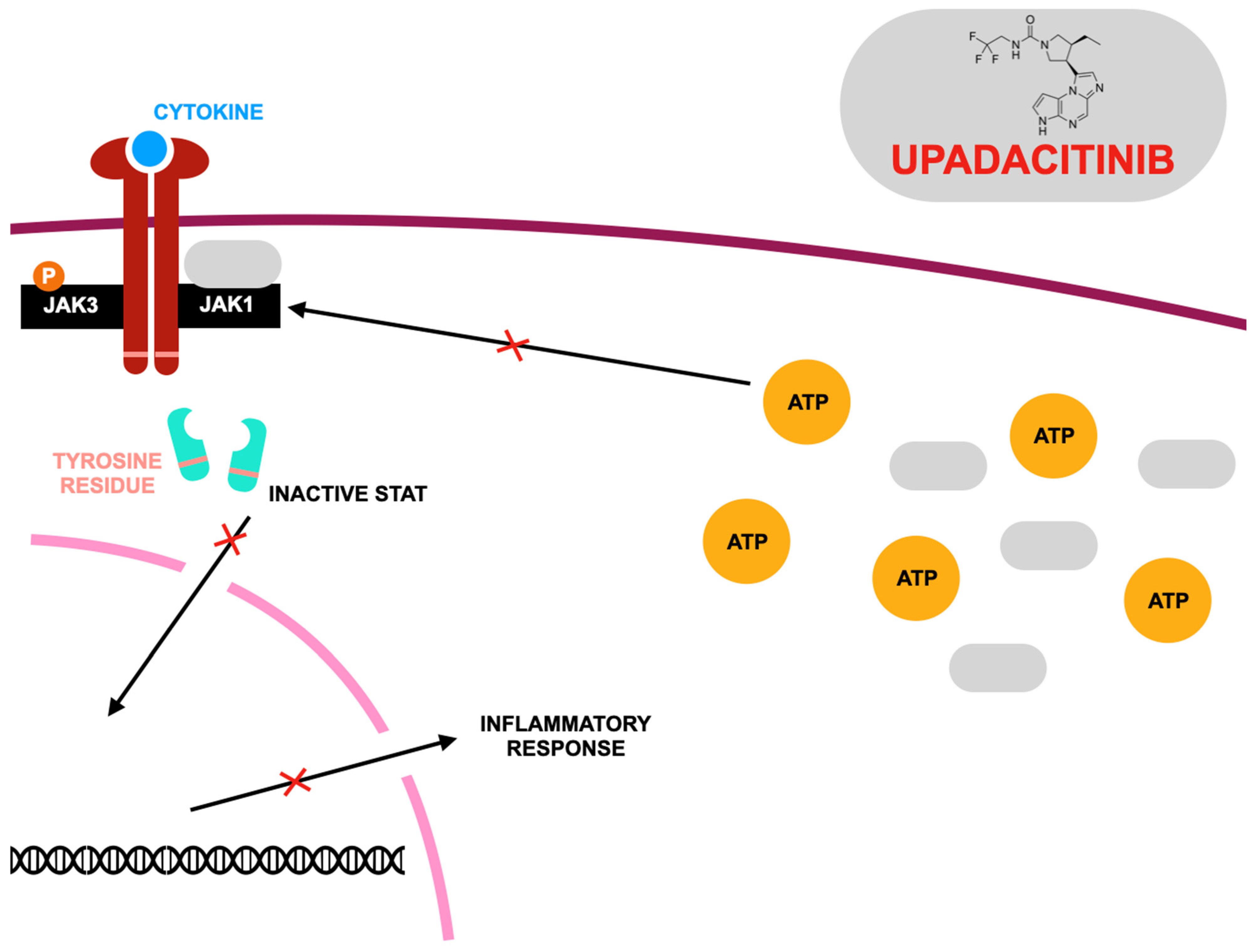
3.2. JAK1 Inhibitor—Upadacitinib
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arthritis and Rheumatic Diseases. Available online: https://www.niams.nih.gov/health-topics/arthritis-and-rheumatic-diseases (accessed on 24 February 2025).
- Sharma, A.; Goel, A. Pathogenesis of rheumatoid arthritis and its treatment with anti-inflammatory natural products. Mol. Biol. Rep. 2023, 50, 4687–4706. [Google Scholar] [CrossRef] [PubMed]
- Khurana, R.; Berney, S.M. Clinical aspects of rheumatoid arthritis. Pathophysiology 2005, 12, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Brock, J.; Basu, N.; Schlachetzki, J.C.M.; Schett, G.; McInnes, I.B.; Cavanagh, J. Immune mechanisms of depression in rheumatoid arthritis. Nat. Rev. Rheumatol. 2023, 19, 790–804. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, C.-E.; Popescu, C.C.; Agache, M.; Dinache, G.; Codreanu, C. Depression in Rheumatoid Arthritis: A Narrative Review-Diagnostic Challenges, Pathogenic Mechanisms and Effects. Medicina 2022, 58, 1637. [Google Scholar] [CrossRef]
- Bungau, S.G.; Behl, T.; Singh, A.; Sehgal, A.; Singh, S.; Chigurupati, S.; Vijayabalan, S.; Das, S.; Palanimuthu, V.R. Targeting Probiotics in Rheumatoid Arthritis. Nutrients 2021, 13, 3376. [Google Scholar] [CrossRef]
- Neumann, E.; Frommer, K.; Diller, M.; Müller-Ladner, U. Rheumatoid arthritis. Z. Rheumatol. 2018, 77, 769–775. [Google Scholar] [CrossRef]
- Adawi, M.; Firas, S.; Blum, A. Rheumatoid Arthritis and Atherosclerosis. Isr. Med. Assoc. J. 2019, 21, 460–463. [Google Scholar]
- England, B.R.; Thiele, G.M.; Anderson, D.R.; Mikuls, T.R. Increased cardiovascular risk in rheumatoid arthritis: Mechanisms and implications. BMJ 2018, 361, k1036. [Google Scholar] [CrossRef]
- Gupta, N.; Kanwar, N.; Arora, A.; Khatri, K.; Kanwal, A. The inter-play of rheumatoid arthritis and osteoporosis: Exploring the pathogenesis and pharma-cological approaches. Clin. Rheumatol. 2024, 43, 1421–1433. [Google Scholar] [CrossRef]
- Mueller, A.-L.; Payandeh, Z.; Mohammadkhani, N.; Mubarak, S.M.H.; Zakeri, A.; Alagheband Bahrami, A.; Brocmueller, A.; Shakibaei, M. Recent Advances in Understanding the Pathogenesis of Rheumatoid Arthritis: New Treat-ment Strategies. Cells 2021, 10, 3017. [Google Scholar] [CrossRef]
- Jang, S.; Kwon, E.-J.; Lee, J.J. Rheumatoid Arthritis: Pathogenic Roles of Diverse Immune Cells. Int. J. Mol. Sci. 2022, 23, 905. [Google Scholar] [CrossRef] [PubMed]
- Karimi, K.; Azar, P.S.; Kadkhodayi, M.; Tandorost, A.; Vajdi, M.; Shoorei, H.; Farhangi, M.A. A comprehensive insight into effects of resveratrol on molecular mechanism in rheumatoid arthritis: A literature systematic review. Int. J. Rheum. Dis. 2022, 25, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, W.; Ren, L. Emerging roles and mechanism of m6A methylation in rheumatoid arthritis. Biomed. Pharmacother. 2024, 170, 116066. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jiang, P.; Guo, S.; Schrodi, S.J.; He, D. Apoptosis, Autophagy, NETosis, Necroptosis, and Pyroptosis Mediated Programmed Cell Death as Targets for Innovative Therapy in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 809806. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, S.; Schrodi, S.J.; He, D. Molecular and Cellular Heterogeneity in Rheumatoid Arthritis: Mechanisms and Clinical Implications. Front. Immunol. 2021, 12, 790122. [Google Scholar] [CrossRef]
- Mahmood, Z.; Schmalzing, M.; Dörner, T.; Tony, H.-P.; Muhammad, K. Therapeutic Cytokine Inhibition Modulates Activation and Homing Receptors of Peripheral Memory B Cell Subsets in Rheumatoid Arthritis Patients. Front. Immunol. 2020, 11, 572475. [Google Scholar] [CrossRef]
- Di Pietro, C.; Falcone, M. The role of invariant NKT cells in organ-specific autoimmunity. Front. Biosci. 2014, 19, 1240–1250. [Google Scholar] [CrossRef]
- Tan, Y.; Buch, M.H. ‘Difficult to treat’ rheumatoid arthritis: Current position and considerations for next steps. RMD Open 2022, 8, e002387. [Google Scholar] [CrossRef]
- Lee, D.M.; Weinblatt, M.E. Rheumatoid arthritis. Lancet 2001, 358, 903–911. [Google Scholar] [CrossRef]
- Huang, J.; Fu, X.; Chen, X.; Li, Z.; Huang, Y.; Liang, C. Promising Therapeutic Targets for Treatment of Rheumatoid Arthritis. Front. Immunol. 2021, 12, 686155. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biolog-ical disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 2023, 82, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Araújo, F.; Gonçalves, J.; Fonseca, J.E. Biosimilar DMARDs: What Does the Future Hold? Drugs 2016, 76, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Pescovitz, M.D. Rituximab, an anti-cd20 monoclonal antibody: History and mechanism of action. Am. J. Transplant. 2006, 6, 859–866. [Google Scholar] [CrossRef] [PubMed]
- De Vita, S.; Quartuccio, L. Treatment of rheumatoid arthritis with rituximab: An update and possible indications. Autoimmun. Rev. 2006, 5, 443–448. [Google Scholar] [CrossRef]
- Smolen, J.S.; Keystone, E.C.; Emery, P.; Breedveld, F.C.; Betteridge, N.; Burmester, G.R.; Dougados, M.; Ferraccioli, G.; Jaeger, U.; Klareskog, L.; et al. Working Group on the Rituximab Consensus Statement Consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2007, 66, 143–150. [Google Scholar] [CrossRef]
- Taylor, R.P.; Lindorfer, M.A. Drug insight: The mechanism of action of rituximab in autoimmune disease--the immune complex decoy hypothesis. Nat. Clin. Pract. Rheumatol. 2007, 3, 86–95. [Google Scholar] [CrossRef]
- Sibilia, J.; Gottenberg, J.-E.; Mariette, X. Rituximab: A new therapeutic alternative in rheumatoid arthritis. Jt. Bone Spine 2008, 75, 526–532. [Google Scholar] [CrossRef]
- Korhonen, R.; Moilanen, E. Anti-CD20 antibody rituximab in the treatment of rheumatoid arthritis. Basic. Clin. Pharmacol. Toxicol. 2010, 106, 13–21. [Google Scholar] [CrossRef]
- Boumans, M.J.; Tak, P.P. Rituximab treatment in rheumatoid arthritis: How does it work? Arthritis Res. Ther. 2009, 11, 134. [Google Scholar] [CrossRef]
- Weiner, G.J. Rituximab: Mechanism of action. Semin. Hematol. 2010, 47, 115–123. [Google Scholar] [CrossRef]
- Boumans, M.J.H.; Thurlings, R.M.; Gerlag, D.M.; Vos, K.; Tak, P.P. Response to rituximab in patients with rheumatoid arthritis in different compartments of the immune system. Arthritis Rheum. 2011, 63, 3187–3194. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Olivo, M.A.; Amezaga Urruela, M.; McGahan, L.; Pollono, E.N.; Suarez-Almazor, M.E. Rituximab for rheumatoid arthritis. Cochrane Database Syst. Rev. 2015, 1, CD007356. [Google Scholar] [CrossRef] [PubMed]
- Paul, F.; Cartron, G. Infusion-related reactions to rituximab: Frequency, mechanisms and predictors. Expert Rev. Clin. Immunol. 2019, 15, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Li, K.H.; Kaker, M.; Lau, J.; Noonan, K.; Zhang, S.; McDermott, C.L.; Lockhart, C.M. The landscape of real-world evidence of rituximab utilization and clinical outcomes in patients with cancer, rheumatoid arthritis, and multiple sclerosis: A scoping review. J. Manag. Care Spec. Pharm. 2024, 30, 480–489. [Google Scholar] [CrossRef]
- Dierickx, D.; Beke, E.; Devos, T.; Delannoy, A. The use of monoclonal antibodies in immune-mediated hematologic disorders. Med. Clin. North Am. 2012, 96, 583–619. [Google Scholar] [CrossRef]
- Dierickx, D.; Kentos, A.; Delannoy, A. The role of rituximab in adults with warm antibody autoimmune hemolytic anemia. Blood 2015, 125, 3223–3229. [Google Scholar] [CrossRef]
- Barešić, M.; Ježić, I.; Simetić, L.; Herceg, D.; Anić, B. Rituximab as a treatment option in a patient with rheumatoid arthritis and a history of malignancy—Intracranial chondrosarcoma/osteochondroma—Case based review. Rheumatol. Int. 2021, 41, 463–468. [Google Scholar] [CrossRef]
- Lobbes, H.; Dervout, C.; Toussirot, E.; Felten, R.; Sibilia, J.; Wendling, D.; Gombert, B.; Ruivard, M.; Grobost, V.; Saraux, A.; et al. Rituximab for rheumatoid arthritis-associated large granular lymphocytic leukemia, a retrospective case series. Semin. Arthritis Rheum. 2020, 50, 1109–1113. [Google Scholar] [CrossRef]
- Summers, K.M.; Kockler, D.R. Rituximab treatment of refractory rheumatoid arthritis. Ann. Pharmacother. 2005, 39, 2091–2095. [Google Scholar] [CrossRef]
- Suzuki, K.; Akiyama, M.; Takei, H.; Kaneko, Y. Successful rituximab treatment in a seronegative rheumatoid arthritis patient with concurrent cold agglutinin syndrome and immune thrombocytopenia. Rheumatol. Int. 2024, 45, 2. [Google Scholar] [CrossRef]
- Soliman, M.M.; Hyrich, K.L.; Lunt, M.; Watson, K.D.; Symmons, D.P.M.; Ashcroft, D.M.; British Society for Rheumatology Biologics Register. Effectiveness of rituximab in patients with rheumatoid arthritis: Observational study from the British Society for Rheumatology Biologics Register. J. Rheumatol. 2012, 39, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Tavakolpour, S.; Alesaeidi, S.; Darvishi, M.; GhasemiAdl, M.; Darabi-Monadi, S.; Akhlaghdoust, M.; Elikaei Behjati, S.; Jafarieh, A. A comprehensive review of rituximab therapy in rheumatoid arthritis patients. Clin. Rheumatol. 2019, 38, 2977–2994. [Google Scholar] [CrossRef] [PubMed]
- Bergantini, L.; d’Alessandro, M.; Cameli, P.; Vietr, L.; Vagaggini, C.; Perrone, A.; Sestini, P.; Frediani, B.; Bargagli, E. Effects of rituximab therapy on B cell differentiation and depletion. Clin. Rheumatol. 2020, 39, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Pollastro, S.; Musters, A.; Balzaretti, G.; Niewold, I.; van Schaik, B.; Hässler, S.; Verhoef, C.M.; Pallardy, M.; van Kampen, A.; Mariette, X.; et al. Sensitive B-cell receptor repertoire analysis shows repopulation correlates with clinical response to rituximab in rheumatoid arthritis. Arthritis Res. Ther. 2024, 26, 70. [Google Scholar] [CrossRef]
- Bensalem, A.; Mulleman, D.; Thibault, G.; Azzopardi, N.; Goupille, P.; Paintaud, G.; Ternant, D. CD4+ count-dependent concentration-effect relationship of rituximab in rheumatoid arthritis. Br. J. Clin. Pharmacol. 2019, 85, 2747–2758. [Google Scholar] [CrossRef]
- Kumar, S.S.; Bagepally, B.S.; Sasidharan, A. Cost Effectiveness of Rituximab Therapy for Rheumatoid Arthritis: A Systematic Review and Meta-Analysis of Cost-Utility Studies. Clin. Drug. Investig. 2023, 43, 97–108. [Google Scholar] [CrossRef]
- Pugashetti, J.V.; Lee, J.S. Overview of Rheumatoid Arthritis-Associated Interstitial Lung Disease and Its Treatment. Semin. Respir. Crit. Care Med. 2024, 45, 329–341. [Google Scholar] [CrossRef]
- Matson, S.M.; Baqir, M.; Moua, T.; Marll, M.; Kent, J.; Iannazzo, N.S.; Boente, R.D.; Donatelli, J.M.; Dai, J.; Diaz, F.J.; et al. Treatment Outcomes for Rheumatoid Arthritis-Associated Interstitial Lung Disease: A Real-World, Multisite Study of the Impact of Immunosuppression on Pulmonary Function Trajectory. Chest 2023, 163, 861–869. [Google Scholar] [CrossRef]
- Taylan, A. Rituximab therapy in pericarditis associated with rheumatoid arthritis. Rheumatol. Int. 2022, 42, 1843–1847. [Google Scholar] [CrossRef]
- Karmachary, P.; Poudel, D.R.; Pathak, R.; Donato, A.A.; Ghimire, S.; Giri, S.; Aryal, M.R.; Bingham, C.O., 3rd. Rituximab-induced serum sickness: A systematic review. Semin. Arthritis Rheum. 2015, 45, 334–340. [Google Scholar] [CrossRef]
- Dammacco, R.; Guerriero, S.; Alessio, G.; Dammacco, F. Natural and iatrogenic ocular manifestations of rheumatoid arthritis: A systematic review. Int. Ophthalmol. 2022, 42, 689–711. [Google Scholar] [CrossRef] [PubMed]
- Kougkas, N.; Dara, A.; Pagkopoulou, E.; Dimitriadou, A.; Papadimitriou, E.; Avdelidou, E.; Garyfallos, A.; Dimitroulas, T. Methotrexate induced neurotoxicity in a patient with rheumatoid arthritis on rituximab therapy: A case-based review. Rheumatol. Int. 2022, 42, 1849–1854. [Google Scholar] [CrossRef] [PubMed]
- Opdam, M.A.A.; Campisi, L.M.; de Leijer, J.H.; Ten Cate, D.; den Broeder, A. Hypogammaglobulinemia in rheumatoid arthritis patients on rituximab: Prevalence and risk factors. Rheumatology 2024, 63, e1–e2. [Google Scholar] [CrossRef] [PubMed]
- Cabral, V.P.; Andrade, C.A.; Passos, S.R.; Martins, M.F.; Hökerberg, Y.H. Severe infection in patients with rheumatoid arthritis taking anakinra, rituximab, or abatacept: A systematic review of observational studies. Rev. Bras. Reumatol. Engl. Ed. 2016, 56, 543–550. [Google Scholar] [CrossRef]
- Shi, Y.; Wu, Y.; Ren, Y.; Jiang, Y.; Chen, Y. Infection risks of rituximab versus non-rituximab treatment for rheumatoid arthritis: A systematic review and meta-analysis. Int. J. Rheum. Dis. 2019, 22, 1361–1370. [Google Scholar] [CrossRef]
- Mariette, X.; Barone, F.; Baldini, C.; Bootsma, H.; Clark, K.L.; De Vita, S.; Gardner, D.H.; Henderson, R.B.; Herdman, M.; Lerang, K.; et al. A randomized, phase II study of sequential belimumab and rituximab in primary Sjögren’s syndrome. JCI Insight 2022, 7, e163030. [Google Scholar] [CrossRef]
- Wang, Z.; Bao, H.-W.; Ji, Y. A systematic review and meta-analysis of rituximab combined with methotrexate versus methotrexate alone in the treatment of rheumatoid arthritis. Medicine 2020, 99, e19193. [Google Scholar] [CrossRef]
- González-Vacarezza, N.; Alemán, A.; González, G.; Pérez, A. Rituximab and tocilizumab for the treatment of rheumatoid arthritis. Int. J. Technol. Assess. Health Care 2014, 30, 282–288. [Google Scholar] [CrossRef]
- Bertsias, A.; Avgoustidis, N.; Papalopoulos, I.; Repa, A.; Kougkas, N.; Kalogiannaki, E.; Bertsias, G.; Flouri, I.; Sidiropoulos, P. Rheumatoid arthritis patients initiating rituximab with low number of previous bDMARDs failures may effectively reduce rituximab dose and experience fewer serious adverse events than patients on full dose: A 5-year cohort study. Arthritis Res. Ther. 2022, 24, 132. [Google Scholar] [CrossRef]
- Humby, F.; Durez, P.; Buch, M.H.; Lewis, M.J.; Rizvi, H.; Rivellese, F.; Nerviani, A.; Giorli, G.; Mahto, A.; Montecucco, C.; et al. Rituximab versus tocilizumab in anti-TNF inadequate responder patients with rheumatoid arthritis (R4RA): 16-week outcomes of a stratified, biopsy-driven, multicentre, open-label, phase 4 randomised controlled trial. Lancet 2021, 397, 305–317. [Google Scholar] [CrossRef]
- Rivellese, F.; Surace, A.E.A.; Goldman, K.; Sciacca, E.; Çubuk, C.; Giorli, G.; Joh, C.R.; Nerviani, A.; Fossati-Jimack, L.; Thorborn, G.; et al. Rituximab versus tocilizumab in rheumatoid arthritis: Synovial biopsy-based biomarker analysis of the phase 4 R4RA randomized trial. Nat. Med. 2022, 28, 1256–1268. [Google Scholar] [CrossRef]
- Frazzei, G.; Musters, A.; de Vries, N.; Tas, S.W.; van Vollenhoven, R.F. Prevention of rheumatoid arthritis: A systematic literature review of preventive strategies in at-risk individuals. Autoimmun. Rev. 2023, 22, 103217. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.H.; Suh, C.-H.; Shim, S.C.; Lee, S.J.; Kim, S.H.; Park, W. A pharmacokinetic evaluation of the rituximab biosimilar CT-P10 in the treatment of rheumatoid arthritis. Expert Opin. Drug Metab. Toxicol. 2020, 16, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, H.; Kim, E. Comparative Efficacy and Safety of Biosimilar Rituximab and Originator Rituximab in Rheumatoid Arthritis and Non-Hodgkin’s Lymphoma: A Systematic Review and Meta-analysis. BioDrugs 2019, 33, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Montoya, L.; Villota-Eraso, C.; Yusof, M.Y.M.; Vital, E.M.; Emery, P. Lessons for rituximab therapy in patients with rheumatoid arthritis. Lancet Rheumatol. 2020, 2, e497–e509. [Google Scholar] [CrossRef]
- Jurczak, W.; Długosz-Danecka, M.; Rivas Navarro, F. The rationale for combination therapy in patients with aggressive B-cell non-Hodgkin lymphoma: Ten questions. Future Oncol. 2019, 15, 305–317. [Google Scholar] [CrossRef]
- Chihara, D.; Arons, E.; Stetler-Stevenson, M.; Yuan, C.M.; Wang, H.W.; Zhou, H.; Raffeld, M.; Xi, L.; Steinberg, S.M.; Feurtado, J.; et al. Randomized phase II study of first-line cladribine with concurrent or delayed rituximab in patients with hairy cell leukemia. J. Clin. Oncol. 2020, 38, 1032–1041. [Google Scholar] [CrossRef]
- Mankikian, J.; Caille, A.; Reynaud-Gaubert, M.; Agier, M.S.; Bermudez, J.; Bonniaud, P.; Borie, R.; Brillet, P.Y.; Cadranel, J.; Court-Fortune, I.; et al. Rituximab and mycophenolate mofetil combination in patients with interstitial lung disease (EVER-ILD): A double-blind, randomised, placebo-controlled trial. Eur. Respir. J. 2023, 61, 2202071. [Google Scholar] [CrossRef]
- Li, X.; Armon, C.; Barkhaus, P.; Barnes, B.; Benatar, M.; Bertorini, T.; Bromberg, M.; Carter, G.T.; Crayle, J.; Cudkowicz, M.; et al. ALSUntangled #67: Rituximab. Amyotroph. Lateral Scler. Frontotemporal Degener. 2023, 24, 544–547. [Google Scholar] [CrossRef]
- Kaegi, C.; Wuest, B.; Schreiner, J.; Steiner, U.C.; Vultaggio, A.; Matucci, A.; Crowley, C.; Boyman, O. Systematic Review of Safety and Efficacy of Rituximab in Treating Immune-Mediated Disorders. Front Immunol. 2019, 10, 1990. [Google Scholar] [CrossRef]
- Caso, F.; Navarini, L.; Ruscitti, P.; Chimenti, M.S.; Girolimetto, N.; Del Puente, A.; Giacomelli, R.; Scarpa, R.; Costa, L. Targeted synthetic pharmacotherapy for psoriatic arthritis: State of the art. Expert Opin. Pharmacother. 2020, 21, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Sparks, J.A. Rheumatoid Arthritis. Ann. Intern. Med. 2019, 170, ITC1–ITC16. [Google Scholar] [CrossRef] [PubMed]
- Palfreeman, A.C.; McNamee, K.E.; McCann, F.E. New developments in the management of psoriasis and psoriatic arthritis: A focus on apremilast. Drug Des. Devel. Ther. 2013, 7, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Varada, S.; Tintle, S.J.; Gottlieb, A.B. Apremilast for the treatment of psoriatic arthritis. Expert Rev. Clin. Pharmacol. 2014, 7, 239–250. [Google Scholar] [CrossRef]
- Forchhammer, S.; Ghoreschi, K. Update on the treatment of psoriasis and psoriatic arthritis—Role of apremilast. Psoriasis 2015, 5, 117–124. [Google Scholar] [CrossRef]
- Schett, G. Apremilast in psoriatic arthritis. Clin. Exp. Rheumato. 2015, 33, S98–S100. [Google Scholar]
- Deeks, E.D. Apremilast: A Review in Psoriasis and Psoriatic Arthritis. Drugs 2015, 75, 1393–1403. [Google Scholar] [CrossRef]
- Hernández-Flórez, D.; Valor, L. Selective Phosphodiesterase Inhibitors: A New Therapeutic Option in Inflammation and Autoimmunity. Reumatol. Clin. 2016, 12, 303–306. [Google Scholar] [CrossRef]
- Torres, T.; Puig, L. Apremilast: A Novel Oral Treatment for Psoriasis and Psoriatic Arthritis. Am. J. Clin. Dermatol. 2018, 19, 23–32. [Google Scholar] [CrossRef]
- Li, H.; Zuo, J.; Tang, W. Phosphodiesterase-4 Inhibitors for the Treatment of Inflammatory Diseases. Front. Pharmacol. 2018, 17, 1048. [Google Scholar] [CrossRef]
- Crocetti, L.; Floresta, G.; Cilibrizzi, A.; Giovannoni, M.P. An Overview of PDE4 Inhibitors in Clinical Trials: 2010 to Early 2022. Molecules 2022, 27, 4964. [Google Scholar] [CrossRef] [PubMed]
- Ayan, G.; Ribeiro, A.; Macit, B.; Proft, F. Pharmacologic Treatment Strategies in Psoriatic Arthritis. Clin. Ther. 2023, 45, 826–840. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.M.; Ballantyne, A.D. Apremilast: First Global Approval. Drugs 2014, 74, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.; Crosbie, D. Apremilast in the treatment of psoriatic arthritis: A perspective review. Ther. Adv. Musculoskelet. Dis. 2017, 9, 45–53. [Google Scholar] [CrossRef]
- Carrascosa, J.M.; Del-Alcazar, E. Apremilast for psoriasis treatment. G. Ital. Dermatol. Venereol. 2020, 155, 421–433. [Google Scholar] [CrossRef]
- Torre Alonso, J.C.; Almodóvar González, R.; Montilla Morales, C.; Sanz Sanz, J.; Díaz González, F.; Pascual Alfonso, E.; Gratacós, J. Expert recommendations for the use of apremilast in psoriatic arthritis. Reumatol. Clin. 2023, 19, 34–44. [Google Scholar] [CrossRef]
- Kerschbaumer, A.; Smolen, J.S.; Ferreira, R.J.O.; Bertheussen, H.; Baraliakos, X.; Aletaha, D.; McGonagle, D.G.; van der Heijde, D.; McInnes, I.B.; Esbensen, B.A.; et al. Efficacy and safety of pharmacological treatment of psoriatic arthritis: A systematic literature research informing the 2023 update of the EULAR recommendations for the management of psoriatic arthritis. Ann. Rheum. Dis. 2024, 83, 760–774. [Google Scholar] [CrossRef]
- Wendling, D.; Prati, C. Targeted synthetic disease-modifying antirheumatic drugs in spondyloarthritis. Immunotherapy 2017, 9, 221–223. [Google Scholar] [CrossRef]
- Nassim, D.; Alajmi, A.; Jfri, A.; Pehr, K. Apremilast in dermatology: A review of literature. Dermatol. Ther. 2020, 33, e14261. [Google Scholar] [CrossRef]
- Sideris, E.; Corbett, M.; Palmer, S.; Woolacott, N.; Bojke, L. The Clinical and Cost Effectiveness of Apremilast for Treating Active Psoriatic Arthritis: A Critique of the Evidence. Pharmacoeconomics 2016, 34, 1101–1110. [Google Scholar] [CrossRef]
- Torrente-Segarra, V.; Bonet, M. Apremilast Use in Oligoarticular Psoriatic Arthritis. J. Rheumatol. 2022, 49, 1293–1294. [Google Scholar] [CrossRef] [PubMed]
- Cauli, A.; Porru, G.; Piga, M.; Vacca, A.; Dessole, G.; Mathieu, A. Clinical potential of apremilast in the treatment of psoriatic arthritis. Immunotargets Ther. 2014, 3, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Sbidian, E.; Chaimani, A.; Garcia-Doval, I.; Do, G.; Hua, C.; Mazaud, C.; Droitcourt, C.; Hughes, C.; Ingram, J.R.; Naldi, L.; et al. Systemic pharmacological treatments for chronic plaque psoriasis: A network meta-analysis. Cochrane Database Syst. Rev. 2017, 12, CD011535. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Mease, P.J. Apremilast: A Phosphodiesterase 4 Inhibitor for the Treatment of Psoriatic Arthritis. Rheumatol. Ther. 2014, 1, 1–20. [Google Scholar] [CrossRef]
- Busa, S.; Kavanaugh, A. Drug safety evaluation of apremilast for treating psoriatic arthritis. Expert Opin. Drug Saf. 2015, 14, 979–985. [Google Scholar] [CrossRef]
- Souto, S.; Gómez-Reino, J.J. Apremilast for the treatment of psoriatic arthritis. Expert Rev. Clin. Immunol. 2015, 11, 1281–1290. [Google Scholar] [CrossRef]
- Martin, B.C.; Thomas, L.W.; Dann, F.J. Apremilast for the treatment of psoriatic arthritis. Dermatol. Online J. 2017, 23, 1–12. [Google Scholar] [CrossRef]
- Qu, X.; Zhang, S.; Tao, L.; Song, Y. A meta-analysis of apremilast on psoriatic arthritis long-term assessment of clinical efficacy (PALACE). Expert Rev. Clin. Pharmacol. 2016, 9, 799–805. [Google Scholar] [CrossRef]
- Sandhu, V.K.; Eder, L.; Yeung, J. Apremilast and its role in psoriatic arthritis. G. Ital. Dermatol. Venereol. 2020, 155, 386–399. [Google Scholar] [CrossRef]
- Azuaga, A.B.; Ramírez, J.; Cañete, J.D. Psoriatic Arthritis: Pathogenesis and Targeted Therapies. Int. J. Mol. Sci. 2023, 24, 4901. [Google Scholar] [CrossRef]
- Fukushima-Nomura, A.; Takamiyagi, S.; Kakuta, R.; Ito, Y.; Hirai, I.; Umemoto, J.; Hanaoka, H.; Kaneko, Y.; Tanese, K. Apremilast is a potentially useful treatment for severe palmoplantar pustulosis with extra-palmoplantar symptoms. Skin Health Dis. 2024, 4, e336. [Google Scholar] [CrossRef] [PubMed]
- Takamura, S.; Sugai, S.; Taguchi, R.; Teraki, Y. Combination therapy of apremilast and biologics in patients with psoriasis showing biologic fatigue. J. Dermatol. 2020, 47, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Persson, R.; Cordey, M.; Paris, M.; Jick, S. Safety of Apremilast in Patients with Psoriasis and Psoriatic Arthritis: Findings from the UK Clinical Practice Research Datalink. Drug Saf. 2022, 45, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Malara, G.; Politi, C.; Trifirò, C.; Verduci, C.; D’Arrigo, G.; Testa, A.; Tripepi, G. Effectiveness of Apremilast in Real Life in Patients with Psoriasis: A Longitudinal Study. Acta Derm. Venereol. 2021, 101, 1–6. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Y.; Yu, Q.; Shi, Y. Biologic and Small-Molecule Therapies for Moderate-to-Severe Psoriasis: Focus on Psoriasis Comorbidities. BioDrugs 2023, 37, 35–55. [Google Scholar] [CrossRef]
- Sfikakis, P.P.; Vassilopoulos, D.; Katsifi, G.; Vosvotekas, G.; Dimitroulas, T.; Sidiropoulos, P.; Vounotrypidis, P.; Bogdanos, D.P.; Georgountzos, A.I.; Bounas, A.G.; et al. Apremilast for biologic-naïve, peripheral psoriatic arthritis, including patients with early disease: Results from the APROACH observational prospective study. Rheumatol. Int. 2023, 43, 889–902. [Google Scholar] [CrossRef]
- Mease, P.J.; Hatemi, G.; Paris, M.; Cheng, S.; Maes, P.; Zhang, W.; Shi, R.; Flower, A.; Picard, H.; Stein Gold, L. Apremilast Long-Term Safety Up to 5 Years from 15 Pooled Randomized, Placebo-Controlled Studies of Psoriasis, Psoriatic Arthritis, and Behçet’s Syndrome. Am. J. Clin Dermatol. 2023, 24, 809–820. [Google Scholar] [CrossRef]
- Well, A.F.; Edwards, C.J.; Kivitz, A.J.; Bird, P.; Guerette, B.; Delev, N.; Paris, M.; Teng, L.; Aelion, J.A. Apremilast monotherapy for long-term treatment of active psoriatic arthritis in DMARD-naïve patients. Rheumatology 2022, 61, 1035–1043. [Google Scholar] [CrossRef]
- Haddad, A.; Stein, N.; Lavi, I.; Shynkar, L.; Bergman, I.; Feldhamer, I.; Cohen, A.D.; Saliba, W.; Zisman, D. Treatment Persistence of Apremilast Among Patients with Psoriatic Arthritis. Biologics 2023, 17, 129–136. [Google Scholar] [CrossRef]
- Koskivirta, I.; Ruotsalainen, J.; Kurki, S.; Lakkakorpi, P.; Salminen-Mankonen, H.; Pirilä, L.; Harvima, R.; Palomäki, A. Real-world registry-based study on apremilast use in psoriasis and psoriatic arthritis in Finland. Scand. J. Rheumatol. 2023, 52, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Gratacós-Masmitja, J.; Beltrán Catalán, E.; Álvarez Vega, J.L.; Urruticoechea-Arana, A.; Fito, C.; Maceiras, F.; Belzunegui Otano, J.M.; Fernández Melón, J.; Chamizo Carmona, E.; Abad Hernández, M.A.; et al. Real-world apremilast use in biologic-naïve psoriatic arthritis patients. Data from Spanish clinical practice. Reumatol. Clin. 2024, 20, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Chandran, V.; Bessette, L.; Thorne, C.; Sheriff, M.; Rahman, P.; Gladman, D.D.; Anwar, S.; Jelley, J.; Gaudreau, A.-J.; Chohan, M.; et al. Use of Apremilast to Achieve Psoriatic Arthritis Treatment Goals and Satisfaction at 1 Year in the Canadian Real-World APPRAISE Study. Rheumatol. Ther. 2024, 11, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Alharthy, R.F.; Alharthy, J.M.; Bawazir, R.O.; Katib, R.I.; Alharthy, F.S. The Efficacy and Safety of Apremilast in the Management of Psoriatic Arthritis: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e55773. [Google Scholar] [CrossRef]
- Gossec, L.; Coates, L.C.; Gladman, D.D.; Aelion, J.A.; Vasandani, J.; Pinter, A.; Merola, J.F.; Kavanaugh, A.; Reddy, J.; Wang, R.; et al. Treatment of early oligoarticular psoriatic arthritis with apremilast: Primary outcomes at week 16 from the FOREMOST randomised controlled trial. Ann. Rheum. Dis. 2024, 83, 1480–1488. [Google Scholar] [CrossRef]
- Papp, K.; Reich, K.; Leonardi, C.L.; Kircik, L.; Chimenti, S.; Langley, R.G.; Hu, C.; Stevens, R.M.; Day, R.M.; Gordon, K.B.; et al. Apremilast in moderate to severe plaque psoriasis: Results of a phase III randomized controlled trial (ESTEEM 1). J. Am. Acad. Dermatol. 2015, 73, 37–49. [Google Scholar] [CrossRef]
- Cather, P.J.; Gooderham, M.; Poulin, Y.; Mrowietz, U.; Ferrandiz, C.; Crowley, J.; Hu, C.; Stevens, R.M.; Shah, K.; Day, R.M.; et al. Efficacy and safety of apremilast in plaque psoriasis: 52-week results from ESTEEM 2. Br. J. Dermatol. 2015, 173, 1387–1399. [Google Scholar] [CrossRef]
- Papadavid, E.; Rompoti, N.; Theodoropoulos, K.; Kokkalis, G.; Rigopoulos, D. Real-world data on the efficacy and safety of apremilast in plaque psoriasis. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1173–1179. [Google Scholar] [CrossRef]
- Hatemi, G.; Mahr, A.; Ishigatsubo, Y.; Song, Y.W.; Takeno, M.; Kim, D.; Melikoğlu, M.; Cheng, S.; McCue, S.; Paris, M.; et al. Apremilast for oral ulcers in Behçet’s syndrome: A randomized controlled trial. N. Engl. J. Med. 2019, 381, 1918–1928. [Google Scholar] [CrossRef]
- Parmentier, J.M.; Voss, J.; Graff, C.; Schwartz, A.; Argiriadi, M.; Friedman, M.; Camp, H.S.; Padley, R.J.; George, J.S.; Hyland, D.; et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494). BMC Rheumatol. 2018, 2, 23. [Google Scholar] [CrossRef]
- Fleischmann, R.; Pangan, A.L.; Song, I.-H.; Mysler, E.; Bessette, L.; Peterfy, C.; Durez, P.; Ostor, A.J.; Li, Y.; Zhou, Y.; et al. Upadacitinib Versus Placebo or Adalimumab in Patients with Rheumatoid Arthritis and an Inadequate Response to Methotrexate: Results of a Phase III, Double-Blind, Randomized Controlled Trial. Arthritis Rheumatol. 2019, 71, 1788–1800. [Google Scholar] [CrossRef] [PubMed]
- Harrington, R.; Al Nokhatha, S.A.; Conway, R. JAK Inhibitors in Rheumatoid Arthritis: An Evidence-Based Review on the Emerging Clinical Data. J. Inflamm. Res. 2020, 13, 519–531. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Anderson, J.K.; Magrey, M.; Merola, J.F.; Liu, Y.; Kishimoto, M.; Jeka, S.; Pacheco-Tena, C.; Wang, X.; Chen, L.; et al. Trial of Upadacitinib and Adalimumab for Psoriatic Arthritis. N. Engl. J. Med. 2021, 384, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- van der Heijde, D.; Baraliakos, X.; Sieper, J.; Deodhar, A.; Inman, R.D.; Kameda, H.; Zeng, X.; Sui, Y.; Bu, X.; Pangan, A.L.; et al. Efficacy and safety of upadacitinib for active ankylosing spondylitis refractory to biological therapy: A double-blind, randomised, placebo-controlled phase 3 trial. Ann. Rheum. Dis. 2022, 81, 1515–1523. [Google Scholar] [CrossRef]
- Caiazzo, G.; Caiazzo, A.; Napolitano, M.; Megna, M.; Potestio, L.; Fornaro, L.; Parisi, M.; Luciano, M.A.; Ruggiero, A.; Testa, A.; et al. The Use of JAK/STAT Inhibitors in Chronic Inflammatory Disorders. J. Clin. Med. 2023, 12, 2865. [Google Scholar] [CrossRef]
- Kiełbowski, K.; Plewa, P.; Bratborska, A.W.; Bakinowska, E.; Pawlik, A. JAK Inhibitors in Rheumatoid Arthritis: Immunomodulatory Properties and Clinical Efficacy. Int. J. Mol. Sci. 2024, 25, 8327. [Google Scholar] [CrossRef]
- Duggan, S.; Keam, S.J. Upadacitinib: First Approval. Drugs 2019, 79, 1819–1828. [Google Scholar] [CrossRef]
- Chaplin, S. Upadacitinib for the treatment of rheumatoid arthritis. Prescriber 2020, 31, 32–34. [Google Scholar] [CrossRef][Green Version]
- Mohamed, M.E.; Bhatnagar, S.; Parmentier, J.M.; Nakasato, P.; Wung, P. Upadacitinib: Mechanism of action, clinical, and translational science. Clin Transl. Sci. 2024, 17, e13688. [Google Scholar] [CrossRef]
- Wang, N.; Yang, Q.; Liu, Y.; Liu, H. Upadacitinib in nail psoriasis: A case report. J. Dermatolog. Treat. 2023, 34, 2246604. [Google Scholar] [CrossRef]
- Tanaka, Y. A review of upadacitinib in rheumatoid arthritis. Mod. Rheumatol. 2020, 30, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Blauvelt, A.; Teixeira, H.D.; Simpson, E.L.; Costanzo, A.; De Bruin-Weller, M.; Barbarot, S.; Prajapati, V.H.; Lio, P.; Hu, X.; Wu, T.; et al. Efficacy and Safety of Upadacitinib vs Dupilumab in Adults with Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2021, 157, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Vermeire, S.; Zhou, W.; Pangan, A.L.; Siffledeen, J.; Greenbloom, S.; Hébuterne, X.; D’Haens, G.; Nakase, H.; Panés, J.; et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: Results from three phase 3, multicentre, double-blind, randomised trials. Lancet 2022, 399, 2113–2128. [Google Scholar] [CrossRef] [PubMed]
- Wodeyar, A.M.; Pansuriya, N.; Saeed, S.; Lakhani, A.; Sartaj, S.; Keerthi, N.S.J.; Guntur Bhuvika Raji, A.; Bhavatharini, S.; Wahane, V.; Thapa, Y.; et al. Upadacitinib in Crohn’s Disease: A Comprehensive Systematic Review of Efficacy and Safety. Cureus 2023, 15, e50657. [Google Scholar] [CrossRef]
- Loftus, E.V., Jr.; Panés, J.; Lacerda, A.P.; Peyrin-Biroulet, L.; D’Haens, G.; Panaccione, R.; Reinisch, W.; Louis, E.; Chen, M.; Nakase, H.; et al. Upadacitinib Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2023, 388, 1966–1980. [Google Scholar] [CrossRef]
- Huang, Z.; Jiang, Q.; Chen, J.; Liu, X.; Gu, C.; Tao, T.; Lv, J.; Li, Z.; Li, Z.; Su, W. Therapeutic Effects of Upadacitinib on Experimental Autoimmune Uveitis: Insights from Single-Cell Analysis. Ophthalmol. Vis. Sci. 2023, 64, 28. [Google Scholar] [CrossRef]
- Smolen, J.S.; Pangan, A.L.; Emery, P.; Rigby, W.; Tanaka, Y.; Vargas, J.I.; Zhang, Y.; Damjanov, N.; Friedman, A.; Othman, A.A.; et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): A randomised, placebo-controlled, double-blind phase 3 study. Lancet 2019, 393, 2303–2311. [Google Scholar] [CrossRef]
- Felson, D.; Smolen, J.S. Trial of Upadacitinib or Abatacept in Rheumatoid Arthritis. N. Engl. J. Med. 2021, 384, 83. [Google Scholar] [CrossRef]
- Mease, P.J.; Lertratanakul, A.; Anderson, J.K.; Papp, K.; Van den Bosch, F.; Tsuji, S.; Dokoupilova, E.; Keiserman, M.; Wang, X.; Zhong, S.; et al. Upadacitinib for psoriatic arthritis refractory to biologics: SELECT-PsA 2. Ann. Rheum. Dis. 2021, 80, 312–320. [Google Scholar] [CrossRef]
- Wan, H.; Jia, H.; Xia, T.; Zhang, D. Comparative efficacy and safety of abrocitinib, baricitinib, and upadacitinib for moderate-to-severe atopic dermatitis: A network meta-analysis. Dermatol. Ther. 2022, 35, e15636. [Google Scholar] [CrossRef]
- Burmester, G.R.; Cohen, S.B.; Winthrop, K.L.; Nash, P.; Irvine, A.D.; Deodhar, A.; Mysler, E.; Tanaka, Y.; Liu, J.; Lacerda, A.P.; et al. Safety profile of upadacitinib over 15 000 patient-years across rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and atopic dermatitis. RMD Open 2023, 9, e002735. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.J.; Karner, C.; Jhita, T.; Barton, S.; Marceniuk, G.; Yiu, Z.Z.N.; Wittmann, M. Abrocitinib, tralokinumab and upadacitinib for treating moderate-to-severe atopic dermatitis. Health Technol. Assess. 2024, 28, 1–113. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.Y.; Luo, J.; Zhang, Y.; Li, Q.; Jiang, Z.Q.; Wen, Z.R.; Wang, Y.Y.; Shi, M.R.; Zhang, S.X. Efficacy and safety of current therapies for difficult-to-treat rheumatoid arthritis: A systematic review and network meta-analysis. J. Transl. Med. 2024, 22, 795. [Google Scholar] [CrossRef] [PubMed]
- Benucci, M.; Li Gobbi, F.; Damiani, A.; Russo, E.; Guiducci, S.; Manfredi, M.; Lari, B.; Grossi, V.; Infantino, M. Real-Life Comparison of Four JAK Inhibitors in Rheumatoid Arthritis (ELECTRA-i Study). J. Clin. Med. 2024, 13, 1821. [Google Scholar] [CrossRef]
- European Medicines Agency. Recommendations on the Use of JAK Inhibitors Following Signal Evaluation. EMA/114007/2023. Published January 2023. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/janus-kinase-inhibitors-jaki (accessed on 24 February 2025).
- US Food and Drug Administration. FDA Drug Safety Communication: Safety Trial Finds Increased Risk of Serious Heart-Related Events, Cancer, Blood Clots, and Death with JAK Inhibitors. Updated September 2021. Available online: https://www.fda.gov/drugs (accessed on 5 April 2025).
| Disease | Treatment Regimen | Key Clinical Outcomes | Ref. |
|---|---|---|---|
| Diffuse large B-cell lymphoma (DLBCL) | RTX + CHOP | 10-year overall survival: 43.5% vs. 27.6%; PFS: 36.5% vs. 20.1% | [68] |
| Follicular lymphoma (FL) | RTX + CVP (+ Maintenance) | Median PFS: 10.5 vs. 4.1 years; HR: 0.55 (95% CI: 0.44–0.68); p < 0.0001 | [68] |
| Chronic lymphocytic leukemia (CLL) | RTX + Fludarabine + Cyclophosphamide | CR rate: 97% vs. 24%; MRD-free at 96 months: 94% vs. 12% | [69] |
| Interstitial Lung Disease (EVER-ILD) | RTX + Mycophenolate Mofetil | ΔFVC: +1.60% vs. −2.01%; PFS HR: 0.47 (95% CI: 0.23–0.96); p = 0.03 | [70] |
| Adverse Event | Frequency | Severity | Risk Factors | Clinical Context |
|---|---|---|---|---|
| Infusion-related reactions | Up to 77% (first dose) | Mild to severe; rare anaphylaxis | First cycle, infusion speed | Across all indications |
| Late-onset neutropenia | 10–25% | Grade 3–4 (notable in combination therapy) | Chemoimmunotherapy | Hematologic malignancies |
| Hepatitis B reactivation | <5% | May be fulminant without prophylaxis | Chronic HBV, lack of screening | Immunocompromised patients |
| Progressive multifocal leukoencephalopathy | <0.1% | Often fatal | JC virus reactivation | Long-term treatment |
| Hypogammaglobulinemia | 15–30% (long-term) | Mild to moderate; risk of infections | Cumulative exposure | Autoimmune disorders |
| Disease | Treatment Regimen | Key Clinical Outcomes | Ref. |
|---|---|---|---|
| Moderate-to-severe plaque psoriasis (ESTEEM 1) | Apremilast 30 mg BID | PASI-75 at week 16: 33.1% vs. 5.3% (placebo); PASI-75 at week 52: 61% | [117] |
| Moderate-to-severe plaque psoriasis (ESTEEM 2) | Apremilast 30 mg BID | PASI-75 at week 16: 28.8% vs. 5.8%; PASI-50 at week 52: 80% | [118] |
| Moderate psoriasis (real-world) | Apremilast 30 mg BID | PASI-75 at week 16: 59.3%; PASI-100: 18.5%; discontinuation rate: 28% | [119] |
| Behçet’s disease | Apremilast 30 mg BID | AUC for oral ulcers: 129.5 vs. 222.1; QoL score change: −4.3 vs. −1.2 | [120] |
| Atopic dermatitis, cutaneous sarcoidosis, hidradenitis suppurativa (off-label) | Apremilast 30 mg BID | Case-level improvement in lesion severity and pruritus | [90] |
| Adverse Event | Frequency | Clinical Impact | Ref. |
|---|---|---|---|
| Diarrhea | 17.3% | Mostly mild to moderate; transient | [117] |
| Nausea | 15.7% | Often occurs within first weeks; transient | [117] |
| URTI | 15.5% | Non-severe, self-limiting | [117] |
| Nasopharyngitis | 14.4% | Mild; typically no need for discontinuation | [117] |
| Headache | 6.3–9.0% | Tension-type most frequent | [117] |
| Weight loss | 5–10% | Mild to moderate; may be desired or problematic | [119] |
| Disease | Treatment Dose | Clinical Outcomes | Ref. |
|---|---|---|---|
| Psoriatic arthritis | 15/30 mg QD | ACR20 at week 12: 70.6% (15 mg), 78.5% (30 mg), vs. 65.0% (adalimumab), 36.2% (placebo) | [130] |
| Crohn’s disease (induction) | 45 mg QD | Week 12: remission 49.5% (U-EXCEL), 38.9% (U-EXCEED) vs. 29.1% (U-EXCEL placebo), 21.1% (U-EXCEED placebo) | [136] |
| Crohn’s disease (maintenance) | 30 mg QD | Week 52 remission: 47.6% vs. 15.1% (placebo) | [136] |
| Atopic dermatitis | 30 mg QD | Week 16 EASI-75: 72.4% vs. 62.6% (dupilumab); Week 16 EASI-100: 28.4% vs. 7.9% (dupilumab) | [133] |
| Adverse Event | Frequency | Clinical Impact | Ref. |
|---|---|---|---|
| Acne | 10–15% | Typically mild; more common in atopic dermatitis | [133] |
| URTI | 8–13% | Self-limiting; common across indications | [130,133] |
| Headache | ~10% | Mild to moderate; manageable | [133] |
| Elevated CPK | 2–8% | Asymptomatic in most cases | [133] |
| Herpes zoster (shingles) | 1–3% | Increased risk in elderly or immunocompromised | [136] |
| Property | Rituximab [67,68,69,70,71] | Apremilast [130,133,136,144,145] | Upadacitinib [130,133,136,144,145,146,147] |
|---|---|---|---|
| Mechanism of Action | Anti-CD20 monoclonal antibody depleting B cells | PDE4 inhibitor increasing intracellular cAMP | Selective JAK1 inhibitor modulating cytokine signaling |
| Route of Administration | Intravenous infusion | Oral tablet | Oral tablet |
| Approved Indications | B-cell NHL, CLL, RA, GPA/MPA | Psoriasis, PsA | RA, PsA, AD, CD, UC, AS |
| Key Clinical Outcomes | R-CHOP in DLBCL: 10-year OS 43.5% vs. 27.6% | PASI-75 at week 16: 33.1% (ESTEEM 1) | ACR20 in PsA: up to 78.5%; CD remission: 49.5% at week 12 |
| Common Adverse Events | Infusion reactions, infections | GI symptoms (nausea, diarrhea), weight loss | Acne, URTI, headache, herpes zoster |
| Special Considerations | Risk of hepatitis B reactivation, long B-cell recovery time | Slower onset, moderate efficacy | Risk of MACE, VTE; CYP3A4 drug interactions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawczak, P.; Feszak, I.J.; Bączek, T. Rituximab, Apremilast, and Upadacitinib as Selected Biosimilar and Targeted Synthetic Disease-Modifying Antirheumatic Drugs with Diverse Mechanisms of Action: Their Current Use in Slowing Down the Progression of Disease. J. Clin. Med. 2025, 14, 2605. https://doi.org/10.3390/jcm14082605
Kawczak P, Feszak IJ, Bączek T. Rituximab, Apremilast, and Upadacitinib as Selected Biosimilar and Targeted Synthetic Disease-Modifying Antirheumatic Drugs with Diverse Mechanisms of Action: Their Current Use in Slowing Down the Progression of Disease. Journal of Clinical Medicine. 2025; 14(8):2605. https://doi.org/10.3390/jcm14082605
Chicago/Turabian StyleKawczak, Piotr, Igor Jarosław Feszak, and Tomasz Bączek. 2025. "Rituximab, Apremilast, and Upadacitinib as Selected Biosimilar and Targeted Synthetic Disease-Modifying Antirheumatic Drugs with Diverse Mechanisms of Action: Their Current Use in Slowing Down the Progression of Disease" Journal of Clinical Medicine 14, no. 8: 2605. https://doi.org/10.3390/jcm14082605
APA StyleKawczak, P., Feszak, I. J., & Bączek, T. (2025). Rituximab, Apremilast, and Upadacitinib as Selected Biosimilar and Targeted Synthetic Disease-Modifying Antirheumatic Drugs with Diverse Mechanisms of Action: Their Current Use in Slowing Down the Progression of Disease. Journal of Clinical Medicine, 14(8), 2605. https://doi.org/10.3390/jcm14082605








