Management and Medical Care for Individuals with Type 1 Diabetes Running a Marathon
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
- A diagnosis of T1DM for at least 1 year;
- Age ≥ 18 years;
- Written informed consent and adherence to the study protocol.
- Pregnancy;
- Use of medications significantly affecting metabolism or exercise performance;
- The presence of chronic medical or psychiatric conditions limiting safe participation;
- The presence of advanced chronic complications of diabetes: proliferative retinopathy, diabetic kidney disease in stages III–V, cardiovascular diseases;
- The inability to safely complete maximal exercise testing.
2.2. Baseline Assessment
2.3. Pre-Race Preparations
2.4. Marathon Monitoring and Support
2.5. Cardiopulmonary Exercise Testing (CPET)
2.6. Data Analysis
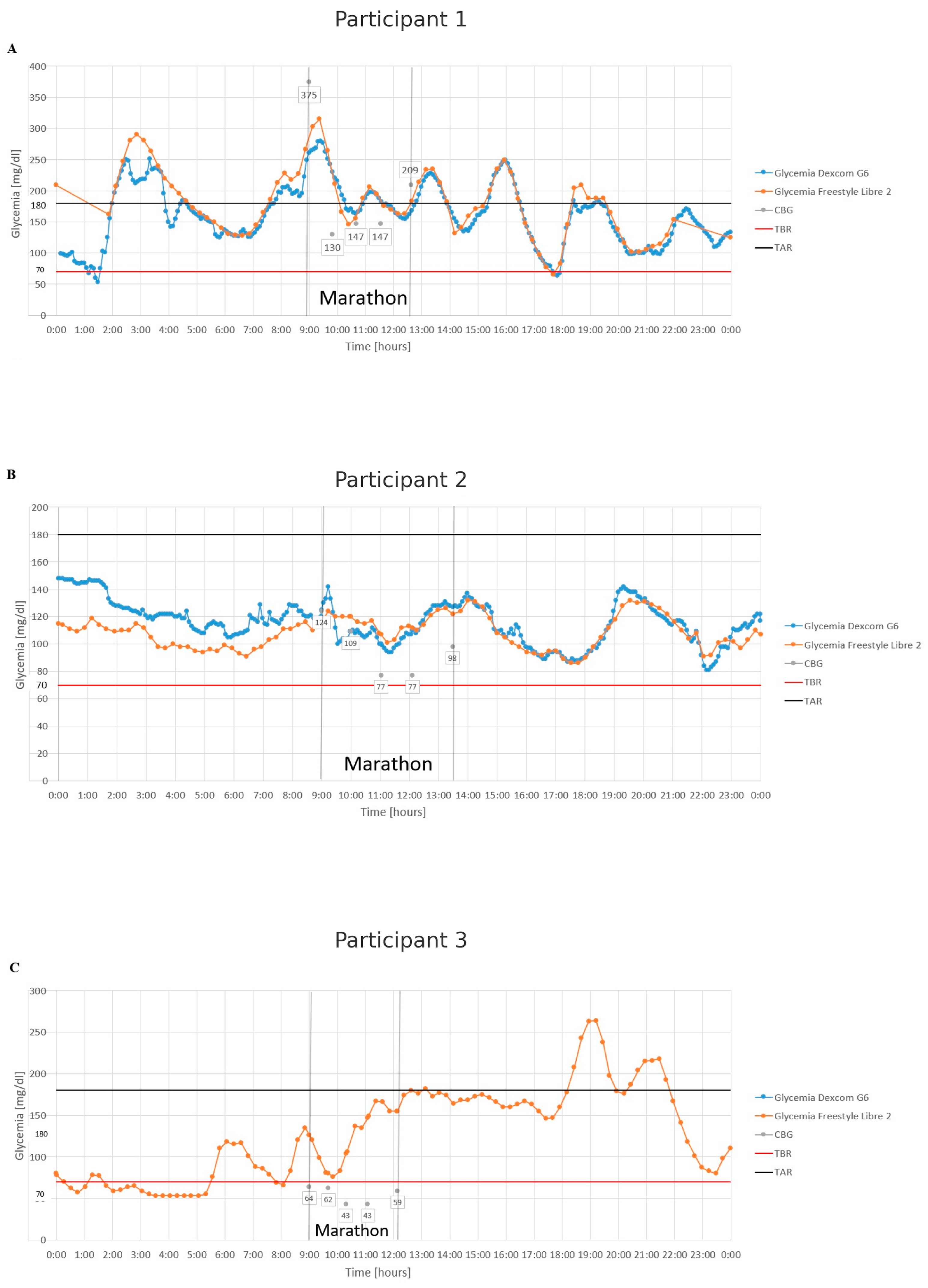
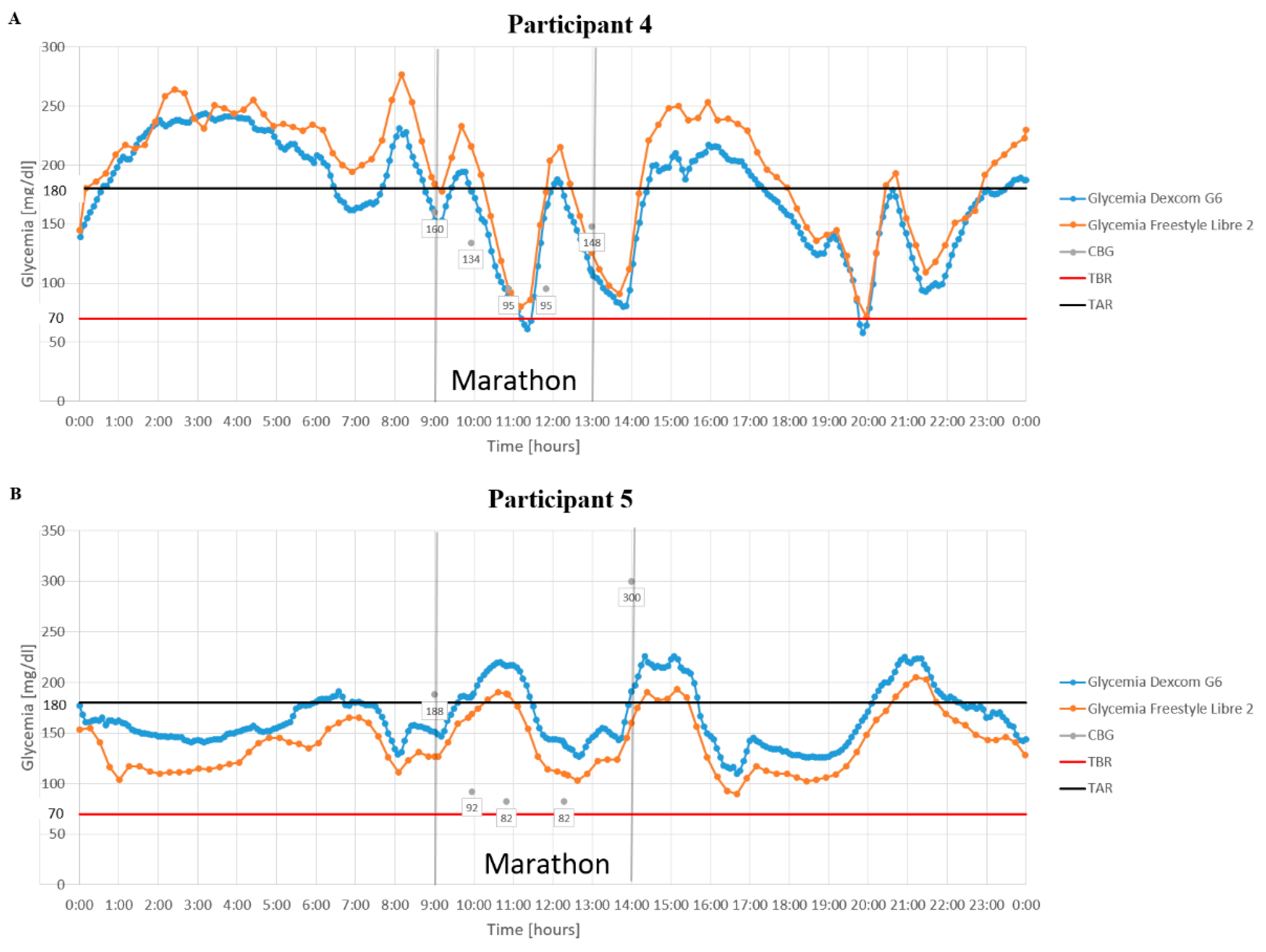
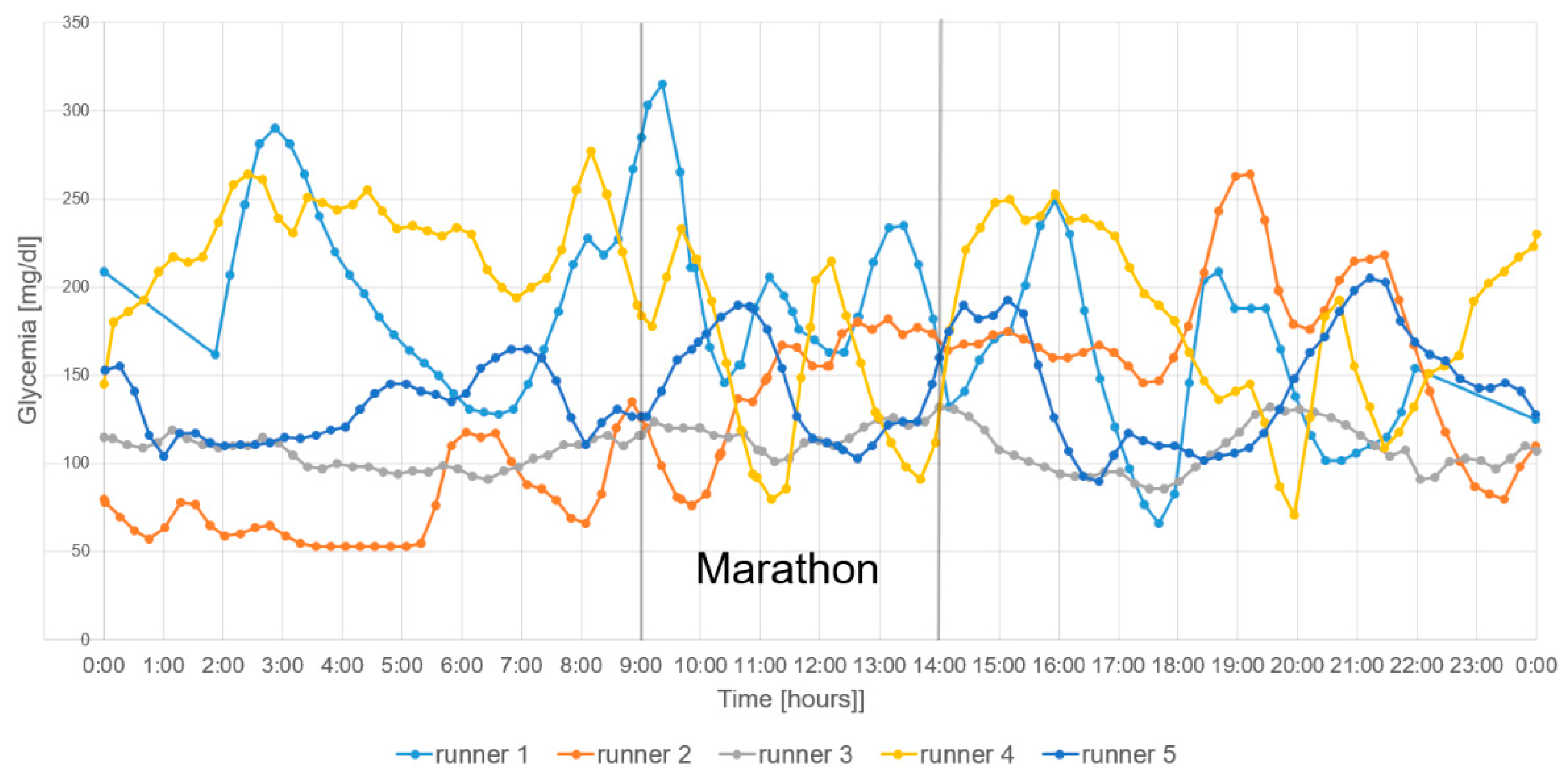
3. Results
4. Discussion
4.1. Marathon with Type 1 Diabetes
4.2. Glycemia During the Marathon
4.3. Continuous Glucose Monitoring
4.4. Insulin Pumps
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADA | American Diabetes Association |
| BMI | Body Mass Index |
| CGM | Continuous Glucose Monitoring |
| CPET | Cardiopulmonary Exercise Testing |
| CSII | Continuous Subcutaneous Insulin Infusion |
| DG6 | Dexcom G6 |
| EASD | European Association for the Study of Diabetes |
| FSL2 | FreeStyle Libre 2 |
| HbA1c | Hemoglobin A1c |
| ISPAD | International Society for Pediatric and Adolescent Diabetes |
| MDI | Multiple Daily Injections |
| SMBG | Self-Monitoring of Blood Glucose |
| T1DM | Type 1 Diabetes Mellitus |
| TIR | Time in Range |
| VO2max | Maximal Oxygen Uptake |
References
- Chimen, M.; Kennedy, A.; Nirantharakumar, K.; Pang, T.T.; Andrews, R.; Narendran, P. What are the health benefits of physical activity in type 1 diabetes mellitus? A literature review. Diabetologia 2012, 55, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Araszkiewicz, A.; Bandurska-Stankiewicz, E.; Borys, S.; Budzyński, A.; Cyganek, K.; Cypryk, K.; Czech, A.; Czupryniak, L.; Drzewoski, J.; Dzida, G.; et al. 2023 guidelines on the management of patients with diabetes—A position of Diabetes Poland. Curr. Top. Diabetes 2023, 3, 1–133. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef]
- Gomez, A.M.; Gomez, C.; Aschner, P.; Veloza, A.; Muñoz, O.; Rubio, C.; Vallejo, S. Effects of performing morning versus afternoon exercise on glycemic control and hypoglycemia frequency in type 1 diabetes patients on sensor-augmented insulin pump therapy. J. Diabetes Sci. Technol. 2015, 9, 619–624. [Google Scholar] [CrossRef]
- Moser, O.; Riddell, M.C.; Eckstein, M.L.; Adolfsson, P.; Rabasa-Lhoret, R.; van den Boom, L.; Gillard, P.; Nørgaard, K.; Oliver, N.S.; Zaharieva, D.P.; et al. Glucose management for exercise using continuous glucose monitoring (CGM) and intermittently scanned CGM (isCGM) systems in type 1 diabetes: Position statement of the European Association for the Study of Diabetes (EASD) and of the International Society for Pediatric and Adolescent Diabetes (ISPAD) endorsed by JDRF and supported by the American Diabetes Association (ADA). Diabetologia 2020, 63, 2501–2520. [Google Scholar] [CrossRef]
- Adolfsson, P.; Taplin, C.E.; Zaharieva, D.P.; Pemberton, J.; Davis, E.A.; Riddell, M.C.; McGavock, J.; Moser, O.; Szadkowska, A.; Lopez, P.; et al. ISPAD clinical practice consensus guidelines 2022: Exercise in children and adolescents with diabetes. Pediatr. Diabetes 2022, 23, 1341–1372. [Google Scholar] [CrossRef] [PubMed]
- Riddell, M.C.; Gallen, I.W.; Smart, C.E.; Taplin, C.E.; Adolfsson, P.; Lumb, A.N.; Kowalski, A.; Rabasa-Lhoret, R.; McCrimmon, R.J.; Hume, C.; et al. Exercise management in type 1 diabetes: A consensus statement. Lancet Diabetes Endocrinol. 2017, 5, 377–390. [Google Scholar] [CrossRef]
- Gargallo-Fernández, M.; Escalada San Martín, J.; Gómez-Peralta, F.; Rozas Moreno, P.; Marco Martínez, A.; Botella-Serrano, M.; Tejera Pérez, C.; López Fernández, J.; en representación del Grupo de Trabajo de Diabetes Mellitus de la Sociedad Española de Endocrinología y Nutrición (SEEN). Clinical recommendations for sport practice in diabetic patients (RECORD guide). Diabetes Mellitus Working Group of the Spanish Society of Endocrinology and Nutrition (SEEN). Endocrinol. Nutr. 2015, 62, e73–e93. [Google Scholar] [CrossRef]
- Rapoport, B.I. Metabolic factors limiting performance in marathon runners. PLoS Comput. Biol. 2010, 6, e1000960. [Google Scholar] [CrossRef]
- Riddell, M.C.; Scott, S.N.; Fournier, P.A.; Colberg, S.R.; Gallen, I.W.; Moser, O.; Stettler, C.; Yardley, J.E.; Zaharieva, D.P.; Adolfsson, P.; et al. The competitive athlete with type 1 diabetes. Diabetologia 2020, 63, 1475–1490. [Google Scholar] [CrossRef]
- Grimm, J.-J.; Muchnick, S. Type I diabetes and marathon running. Diabetes Care 1993, 16, 1624. [Google Scholar] [CrossRef] [PubMed]
- Thuillier, P.; Domun, N.; Sonnet, E.; Le Ven, F.; Roudaut, C.; Kergus, A.; Kerlan, V.; Roudaut, N. Prevention of exercise-induced hypoglycemia in 12 patients with type 1 diabetes running the Paris Marathon using continuous glucose monitoring: A prospective, single-center observational study. Diabetes Metab. 2022, 48, 101321. [Google Scholar] [CrossRef]
- Brazeau, A.-S.; Rabasa-Lhoret, R.; Strychar, I.; Mircescu, H. Barriers to physical activity among patients with type 1 diabetes. Diabetes Care 2008, 31, 2108–2109. [Google Scholar] [CrossRef]
- Friedman, J.G.; Cardona Matos, Z.; Szmuilowicz, E.D.; Aleppo, G. Use of continuous glucose monitors to manage type 1 diabetes mellitus: Progress, challenges, and recommendations. Pharmgenom. Pers. Med. 2023, 16, 263–276. [Google Scholar] [CrossRef]
- Houlder, S.K.; Yardley, J.E. Continuous glucose monitoring and exercise in type 1 diabetes: Past, present and future. Biosensors 2018, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Murillo, S.; Brugnara, L.; Novials, A. One year follow-up in a group of half-marathon runners with type-1 diabetes treated with insulin analogues. J. Sports Med. Phys. Fitness 2010, 50, 506–510. [Google Scholar]
- Mourot, L.; Fornasiero, A.; Rakobowchuk, M.; Skafidas, S.; Brighenti, A.; Stella, F.; Zignoli, A.; Savoldelli, A.; Pellegrini, B.; Danese, E.; et al. Similar cardiovascular and autonomic responses in trained type 1 diabetes mellitus and healthy participants in response to half marathon. Diabetes Res. Clin. Pract. 2020, 160, 107995. [Google Scholar] [CrossRef] [PubMed]
- Sawicka-Gutaj, N.; Gruszczyński, D.; Guzik, P.; Mostowska, A.; Walkowiak, J. Publication ethics of human studies in the light of the Declaration of Helsinki—A mini-review. J. Med. Sci. 2022, 91, e700. [Google Scholar] [CrossRef]
- Hansen, J.E.; Sue, D.Y.; Wasserman, K. Predicted values for clinical exercise testing. Am. Rev. Respir. Dis. 1984, 129, S49–S55. [Google Scholar] [CrossRef]
- Neder, J.A.; Phillips, D.B.; Marillier, M.; Bernard, A.-C.; Berton, D.C.; O’Donnell, D.E. Clinical interpretation of cardiopulmonary exercise testing: Current pitfalls and limitations. Front. Physiol. 2021, 12, 552000. [Google Scholar] [CrossRef]
- Di Molfetta, S.; Rossi, A.; Assaloni, R.; Cherubini, V.; Consoli, A.; Di Bartolo, P.; Guardasole, V.; Laurenzi, A.; Lombardo, F.; Maffeis, C.; et al. A guide for the use of LibreView digital diabetes platform in clinical practice: Expert paper of the Italian Working Group on Diabetes and Technology. Diabetes Res. Clin. Pract. 2022, 187, 109867. [Google Scholar] [CrossRef]
- Akturk, H.K.; Dowd, R.; Shankar, K.; Derdzinski, M. Real-world evidence and glycemic improvement using Dexcom G6 features. Diabetes Technol. Ther. 2021, 23, S-21. [Google Scholar] [CrossRef]
- Davey, R.J.; Low, C.; Jones, T.W.; Fournier, P.A. Contribution of an intrinsic lag of continuous glucose monitoring systems to differences in measured and actual glucose concentrations changing at variable rates in vitro. J. Diabetes Sci. Technol. 2010, 4, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.H.; Darre, E.; Holmich, P.; Jahnsen, F. Insulin-dependent diabetes mellitus and marathon running. Br. J. Sports Med. 1987, 21, 51. [Google Scholar] [CrossRef][Green Version]
- Gawrecki, A.; Zozulinska-Ziolkiewicz, D.; Matejko, B.; Hohendorff, J.; Malecki, M.T.; Klupa, T. Safe completion of a trail running ultramarathon by four men with type 1 diabetes. Diabetes Technol. Ther. 2018, 20, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Weiss, K.; Thuany, M.; Scheer, V.; Ouerghi, N.; Andrade, M.S.; Nikolaidis, P.T.; Ćuk, I.; Knechtle, B. How to end up on the podium after running a 6-days-run with type 1 diabetes mellitus—A case study and literature review. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 88–97. [Google Scholar] [CrossRef]
- Prasanna, S.; Barua, S.; Siller, A.F.; Johnson, J.J.; Sabharwal, A.; DeSalvo, D.J. Hypoglycemia risk with physical activity in type 1 diabetes: A data-driven approach. Front. Digit. Health 2023, 5, 1142021. [Google Scholar] [CrossRef]
- Hiromatsu, C.; Kasahara, N.; Lin, C.-A.; Wang, F.; Goto, K. Continuous monitoring of interstitial fluid glucose responses to endurance exercise with different levels of carbohydrate intake. Nutrients 2023, 15, 4746. [Google Scholar] [CrossRef]
- Moser, O.; Mueller, A.; Eckstein, M.L.; Ziko, H.; Aberer, F.; Treiber, G.; Unteregger, C.; Kojzar, H.; Mader, J.K.; Sourij, C.; et al. Improved glycaemic variability and basal insulin dose reduction during a running competition in recreationally active adults with type 1 diabetes—A single-centre, prospective, controlled observational study. PLoS ONE 2020, 15, e0239091. [Google Scholar] [CrossRef]
- Eckstein, M.L.; Weilguni, B.; Tauschmann, M.; Zimmer, R.T.; Aziz, F.; Sourij, H.; Moser, O. Time in range for closed-loop systems versus standard of care during physical exercise in people with type 1 diabetes: A systematic review and meta-analysis. J. Clin. Med. 2021, 10, 2445. [Google Scholar] [CrossRef]
- Yardley, J.E.; Iscoe, K.E.; Sigal, R.J.; Kenny, G.P.; Perkins, B.A.; Riddell, M.C. Insulin pump therapy is associated with less post-exercise hyperglycemia than multiple daily injections: An observational study of physically active type 1 diabetes patients. Diabetes Technol. Ther. 2013, 15, 84–88. [Google Scholar] [CrossRef]
- van den Boom, L.; Ziko, H.; Mader, J.K. Safely finishing a half marathon by an adult with type 1 diabetes using a commercially available hybrid closed-loop system. J. Diabetes Investig. 2021, 12, 450. [Google Scholar] [CrossRef] [PubMed]
- van Weenen, E.; Banholzer, N.; Föll, S.; Zueger, T.; Fontana, F.Y.; Skroce, K.; Hayes, C.; Kraus, M.; Feuerriegel, S.; Lehmann, V.; et al. Glycaemic patterns of male professional athletes with type 1 diabetes during exercise, recovery and sleep: Retrospective, observational study over an entire competitive season. Diabetes Obes. Metab. 2023, 25, 2616–2625. [Google Scholar] [CrossRef] [PubMed]
- Adolfsson, P.; Mattsson, S.; Jendle, J. Evaluation of glucose control when a new strategy of increased carbohydrate supply is implemented during prolonged physical exercise in type 1 diabetes. Eur. J. Appl. Physiol. 2015, 115, 2599–2607. [Google Scholar] [CrossRef] [PubMed]
- Iscoe, K.E.; Corcoran, M.; Riddell, M.C. High rates of nocturnal hypoglycemia in a unique sports camp for athletes with type 1 diabetes: Lessons learned from continuous glucose monitoring systems. Can. J. Diabetes 2008, 32, 182–189. [Google Scholar] [CrossRef]
- Muñoz Fabra, E.; Díez, J.-L.; Bondia, J.; Laguna Sanz, A.J. A comprehensive review of continuous glucose monitoring accuracy during exercise periods. Sensors 2021, 21, 479. [Google Scholar] [CrossRef]
- Gamarra, E.; Careddu, G.; Fazi, A.; Turra, V.; Morelli, A.; Camponovo, C.; Trimboli, P. Continuous glucose monitoring and recreational scuba diving in type 1 diabetes: Head-to-head comparison between Free Style Libre 3 and Dexcom G7 performance. Diabetes Technol. Ther. 2024, 26, 829–841. [Google Scholar] [CrossRef]
- Moser, O.; Mader, J.K.; Tschakert, G.; Mueller, A.; Groeschl, W.; Pieber, T.R.; Koehler, G.; Messerschmidt, J.; Hofmann, P. Accuracy of continuous glucose monitoring (CGM) during continuous and high-intensity interval exercise in patients with type 1 diabetes mellitus. Nutrients 2016, 8, 489. [Google Scholar] [CrossRef]
- Rilstone, S.; Oliver, N.; Godsland, I.; Tanushi, B.; Thomas, M.; Hill, N. A randomized controlled trial assessing the impact of continuous glucose monitoring with a predictive hypoglycemia alert function on hypoglycemia in physical activity for people with type 1 diabetes (PACE). Diabetes Technol. Ther. 2024, 26, 95–102. [Google Scholar] [CrossRef]
- Chetty, T.; Shetty, V.; Fournier, P.A.; Adolfsson, P.; Jones, T.W.; Davis, E.A. Exercise management for young people with type 1 diabetes: A structured approach to the exercise consultation. Front. Endocrinol. 2019, 10, 326. [Google Scholar] [CrossRef]
- Perkins, B.A.; Turner, L.V.; Riddell, M.C. Applying technologies to simplify strategies for exercise in type 1 diabetes. Diabetologia 2024, 67, 2045–2058. [Google Scholar] [CrossRef] [PubMed]
- Moser, O.; Zaharieva, D.P.; Adolfsson, P.; Battelino, T.; Bracken, R.M.; Buckingham, B.A.; Danne, T.; Davis, E.A.; Dovč, K.; Forlenza, G.P.; et al. The use of automated insulin delivery around physical activity and exercise in type 1 diabetes: A position statement of the European Association for the Study of Diabetes (EASD) and the International Society for Pediatric and Adolescent Diabetes (ISPAD). Diabetologia 2025, 68, 255–280. [Google Scholar] [CrossRef] [PubMed]
- Elian, V.; Popovici, V.; Ozon, E.-A.; Musuc, A.M.; Fița, A.C.; Rusu, E.; Radulian, G.; Lupuliasa, D. Current technologies for managing type 1 diabetes mellitus and their impact on quality of life—A narrative review. Life 2023, 13, 1663. [Google Scholar] [CrossRef] [PubMed]
| Blood Glucose Level [mg/dL] | Recommendations | Comment |
|---|---|---|
| 140–200 | Target glycemia before start | |
| <150 | 15–30 g of carbohydrates | Real-time glucose monitoring by a medical team using a cloud-based platform |
| 150–249 | No intervention | |
| 250–299 with ketone level ≥ 0.6 mmol/L (3.49 mg/dL) | Insulin correction factor with a 50% reduction in the correction dose at checkpoints In case of ketone level ≥ 1.5 mmol/L (8.71 mg/dL) → stop running | |
| ≥300 |
| Parameter | Participant 1 | Participant 2 | Participant 3 | Participant 4 | Participant 5 | All Participants (n = 5) |
|---|---|---|---|---|---|---|
| Age [years] | 32 | 34 | 48 | 44 | 55 | 44 (34–48) |
| Diabetes duration [years] | 6 | 10 | 1 | 14 | 42 | 10 (6–14) |
| Diabetes complications | - | - | - | - | Non-proliferative retinopathy | 1/5 |
| Time spent on sports per week [h] | 12 | 6 | 12 | 3.5 | 4 | 6 (4–12) |
| Running time [years] | 8 | 8 | 13 | 5 | 11 | 8 (8–11) |
| Height (m) | 1.8 | 1.83 | 1.72 | 1.89 | 1.77 | 1.8 (1.8–1.8) |
| Body mass (kg) | 73 | 84 | 65.1 | 78 | 73 | 73 (73–78) |
| BMI [kg/m2] | 22.5 | 25.1 | 22.0 | 21.8 | 23.3 | 22.5 (22–23.3) |
| HbA1c [%] | 5.8 | 6.9 | 5.3 | 5.6 | 6.9 | 5.8 (5.6–6.9) |
| TIR [%] | 70.0 | 60.0 | 100.0 | 91.0 | 67.0 | 70 (65–90) |
| TAR [%] | 17.0 | 37.0 | 0 | 8.0 | 32.0 | 20 (4–34.5) |
| TBR [%] | 13.0 | 3.0 | 0 | 1.0 | 1.0 | 1 (0.5–8) |
| Average glycemia | 118.0 | 158.0 | 107.0 | 130.0 | 163.0 | 130 (118–158) |
| CV [%] | 44.0 | 39.5 | 12.5 | 27.8 | 34.6 | 34.6 (27.8–39.5) |
| Basal insulin per day [U] | 13.0 | 24.0 | 0.0 | 22.0 | 7.0 | 13 (7–22) |
| Prandial insulin per day [U] | 30.0 | 24.0 | 6.0 | 16.5 | 12.0 | 16.5 (12–24) |
| Marathon time [min] | 188.9 | 239.3 | 268.6 | 299.9 | 218.0 | 239.3 (218.0–268.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulecki, M.; Daroszewski, M.; Birula, P.; Bonikowska, A.; Kreczmer, A.; Pietrzak, M.; Adamska, A.; Michalak, M.; Sroczyńska, A.; Michalski, M.; et al. Management and Medical Care for Individuals with Type 1 Diabetes Running a Marathon. J. Clin. Med. 2025, 14, 2493. https://doi.org/10.3390/jcm14072493
Kulecki M, Daroszewski M, Birula P, Bonikowska A, Kreczmer A, Pietrzak M, Adamska A, Michalak M, Sroczyńska A, Michalski M, et al. Management and Medical Care for Individuals with Type 1 Diabetes Running a Marathon. Journal of Clinical Medicine. 2025; 14(7):2493. https://doi.org/10.3390/jcm14072493
Chicago/Turabian StyleKulecki, Michał, Marcin Daroszewski, Paulina Birula, Anita Bonikowska, Anna Kreczmer, Monika Pietrzak, Anna Adamska, Magdalena Michalak, Alicja Sroczyńska, Mateusz Michalski, and et al. 2025. "Management and Medical Care for Individuals with Type 1 Diabetes Running a Marathon" Journal of Clinical Medicine 14, no. 7: 2493. https://doi.org/10.3390/jcm14072493
APA StyleKulecki, M., Daroszewski, M., Birula, P., Bonikowska, A., Kreczmer, A., Pietrzak, M., Adamska, A., Michalak, M., Sroczyńska, A., Michalski, M., Zozulińska-Ziółkiewicz, D., & Gawrecki, A. (2025). Management and Medical Care for Individuals with Type 1 Diabetes Running a Marathon. Journal of Clinical Medicine, 14(7), 2493. https://doi.org/10.3390/jcm14072493





