The Oral Findings and Dental Management of Patients with West Syndrome: A Case Series and Literature Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- West, W. On a peculiar form of infantile convulsions. Lancet 1841, 35, 724–725. [Google Scholar] [CrossRef]
- Pies, N.J.; Beardsmore, C.W. West & West syndrome—A historical sketch about the eponymous doctor, his work and his family. Brain Dev. 2003, 25, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, Y. History of clinical identification of West syndrome—In quest after the classic. Brain Dev. 2001, 23, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Zuberi, S.M.; Wirrell, E.; Yozawitz, E.; Wilmshurst, J.M.; Specchio, N.; Riney, K.; Pressler, R.; Auvin, S.; Samia, P.; Hirsch, E.; et al. ILAE classification and definition of epilepsy syndromes with onset in neonates and infants: Position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia 2002, 63, 1349–1397. [Google Scholar] [CrossRef]
- Lux, A.L.; Osborne, J.P. A Proposal for case definitions and outcome measures in studies of infantile spasms and West syndrome: Consensus statement of the West Delphi group. Epilepsia 2004, 45, 1416–1428. [Google Scholar] [CrossRef]
- Jia, J.L.; Chen, S.; Sivarajah, V.; Stephens, D.; Cortez, M.A. Latitudinal differences on the global epidemiology of infantile spasms: Systematic review and meta-analysis. Orphanet J. Rare Dis. 2018, 13, 216. [Google Scholar] [CrossRef]
- Smith, M.S.; Matthews, R.; Rajnik, M.; Mukherji, P. Infantile Epileptic Spasms Syndrome (West Syndrome) [Updated 2024 Feb 1]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024; Available online: https://www.ncbi.nlm.nih.gov/books/NBK537251/ (accessed on 1 December 2024).
- Fusco, L.; Vigevano, F. Ictal clinical electroencephalographic findings of spasms in West syndrome. Epilepsia 1993, 34, 671–678. [Google Scholar] [CrossRef]
- Wheless, J.W.; A Gibson, P.; Rosbeck, K.L.; Hardin, M.; O’dell, C.; Whittemore, V.; Pellock, J.M. Infantile spasms (West syndrome): Update and resources for pediatricians and providers to share with parents. BMC Pediatr. 2012, 12, 108. [Google Scholar] [CrossRef]
- Berg, A.T.; Berkovic, S.F.; Brodie, M.J.; Buchhalter, J.; Cross, J.H.; van Emde Boas, W.; Engel, J.; French, J.; Glauser, T.A.; Mathern, G.W.; et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 2010, 51, 676–685. [Google Scholar] [CrossRef]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshé, S.L.; et al. ILAE classification of the epilepsies: Position paper of the ILAE commission for classification and terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef]
- Koo, B.; Hwang, P. Localization of focal cortical lesions influences age of onset of infantile spasms. Epilepsia 1996, 37, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Baram, T.Z.; Mitchell, W.G.; Brunson, K.; Haden, E. Infantile spasms: Hypothesis-driven therapy and pilot human infant experiments using corticotropin-releasing hormone receptor antagonists. Dev. Neurosci. 1999, 21, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, E.; Go, C.; McCoy, B.; Snead, O.C. Neurodevelopmental outcome of infantile spasms: A systematic review and meta-analysis. Epilepsy Res. 2015, 109, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Song, J.M.; Hahn, J.; Kim, S.H.; Chang, M.J. Efficacy of treatments for infantile spasms: A systematic review. Clin. Neuropharmacol. 2017, 40, 63–84. [Google Scholar] [CrossRef]
- Wilmshurst, J.M.; Gaillard, W.D.; Vinayan, K.P.; Tsuchida, T.N.; Plouin, P.; Van Bogaert, P.; Carrizosa, J.; Elia, M.; Craiu, D.; Jovic, N.J.; et al. Summary of recommendations for the management of infantile seizures: Task Force Report for the ILAE Commission of Pediatrics. Epilepsia 2015, 56, 1185–1197. [Google Scholar] [CrossRef]
- O’Callaghan, F.J.K.; Edwards, S.W.; Alber, F.D.; Hancock, E.; Johnson, A.L.; Kennedy, C.R.; Likeman, M.; Lux, A.L.; Mackay, M.; A Mallick, A.; et al. Safety and effectiveness of hormonal treatment versus hormonal treatment with vigabatrin for infantile spasms (ICISS): A randomized, multicenter, open-label trial. Lancet Neurol. 2017, 16, 33–42. [Google Scholar] [CrossRef]
- Prezioso, G.; Carlone, G.; Zaccara, G.; Verrotti, A. Efficacy of ketogenic diet for infantile spasms: A systematic review. Acta Neurol. Scand. 2018, 137, 4–11. [Google Scholar] [CrossRef]
- Regis, R.R.; Rocha, C.T.; Torres, C.P.; Queiroz, I.F.; De Queiroz, A.M. Oral findings and dental treatment in a child with West syndrome. Spéc. Care Dent. 2009, 29, 259–263. [Google Scholar] [CrossRef]
- Bussell, R.M.; Deery, C. Case report: Blue chromogenic dental staining in child with West syndrome. Eur. Arch. Paediatr. Dent. 2010, 11, 298–300. [Google Scholar] [CrossRef]
- Khatri, A.; Kalra, N.; Tyagi, R.; Baweja, M.; Khandelwal, D. Dental findings in patients with West syndrome: A report of two cases. J. Indian Soc. Pedod Prev. Dent. 2014, 32, 168–171. [Google Scholar] [CrossRef]
- Dantas-Neta, N.B.; Carvalho e Souza, C.H.; Mendes Alencar, S.M.; Prado Júnior, R.R.; Mendes, R.F. Dental findings in children with West syndrome. Spéc. Care Dent. 2014, 34, 291–294. [Google Scholar] [CrossRef]
- Badnaware, S.D.; Dedhia, S.P.; Desai, R.; Kakade, A. Oral findings in West syndrome—A case report. Braz. Dent. Sci. 2017, 20, 138–142. [Google Scholar] [CrossRef]
- Nacamura, C.A.; Trize, D.d.M.; Cabello, L.R.C.; Franzolin, S.d.O.B.; Marta, S.N. West syndrome: Report of clinical case: 9 Years of follow-up. RGO Rev. GaúchaOdontol 2018, 66, 369–374. [Google Scholar] [CrossRef]
- della Vella, F.; Contaldo, M.; Fucile, R.; Panza, F.; Dibello, V.; Kalemaj, Z.; Ninivaggi, R.; Petruzzi, M.; Serpico, R. ORO-dental manifestations in West syndrome. Curr. Top. Med. Chem. 2019, 19, 2824–2828. [Google Scholar] [CrossRef]
- Akbar, T.; Hassan, S.; Kiani, A.Z.; Arooj, S.; Haq, A.; Elahi, A.S. Dental findings in patient with West Syndrome: A case report. Merit Res. J. Med. Med. Sci. 2020, 8, 481–484. [Google Scholar] [CrossRef]
- Ferreira do Amaral, C.O.; Lobo Nogueira, B.; Ferreira do Amaral, M.O. West syndrome: Medical considerations and stomatologic aspects. Int. J. Dev. Res. 2020, 10, 41958–41962. [Google Scholar] [CrossRef]
- Goswami, M.; Sharma, S. “West syndrome—Infantile spasms”: A pediatric case report. Int. J. Clin. Pediatr. Dent. 2021, 14, 323–326. [Google Scholar] [CrossRef]
- Mehrotra, D.; Nayak, P.P.; Naik, S.S.; Krishna, N. Role of pediatric dentist in West syndrome rehabilitation: A case report. Spec. Care Dent. 2024, 44, 1615–1620. [Google Scholar] [CrossRef]
- Cornacchio, A.L.; Burneo, J.G.; Aragon, C.E. The effects of antiepileptic drugs on oral health. J. Can Dent. Assoc. 2011, 77, b140. [Google Scholar]
- Ransford, N.; Soryal, I.; McCorry, D.; Sander, J.W.; Duncan, F.; Huggins, N. Specialist management of routine dental procedures in adults with refractory epilepsy. Br. Dent. J. 2014, 216, 403–407. [Google Scholar] [CrossRef]
- Vozza, I.; Cavallè, E.; Corridore, D.; Ripari, F.; Spota, A.; Brugnoletti, O.; Guerra, F. Preventive strategies in oral health for special needs patients. Ann. Stomatol. 2015, 6, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Madaan, P.; Chand, P.; Linn, K.; Wanigasinghe, J.; Mynak, M.L.; Poudel, P.; Riikonen, R.; Kumar, A.; Dhir, P.; Negi, S.; et al. Management practices for West syndrome in South Asia: A survey study and meta-analysis. Epilepsia Open 2020, 5, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Aramanadka, R.; Sahu, J.K.; Madaan, P.; Sankhyan, N.; Malhi, P.; Singhi, P. Epilepsy and neurodevelopmental outcomes in a cohort of West Syndrome beyond two years of age. Indian J. Pediatr. 2022, 89, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Riahi-Zanjani, B.; Delirrad, M.; Fazeli-Bakhtiyari, R.; Sadeghi, M.; Zare-Zardini, H.; Jafari, A.; Ghorani-Azam, A. Hematological consequences of valproic acid in pediatric patients: A systematic review with a mechanistic approach. CNS Neurol. Disord.—Drug Targets 2022, 21, 316–325. [Google Scholar] [CrossRef]
- Verrotti, A.; Moavero, R.; Panzarino, G.; Di Paolantonio, C.; Rizzo, R.; Curatolo, P. The challenge of pharmacotherapy in children and adolescents with epilepsy-ADHD comorbidity. Clin. Drug Investig. 2018, 38, 1–8. [Google Scholar] [CrossRef]
- Zahan, S.; Sahu, J.K.; Madaan, P.; Suthar, R.; Pattanaik, S.; Saini, A.G.; Saini, L.; Kumar, A.; Sankhyan, N. Effectiveness and safety of nitrazepam in children with resistant West Syndrome. Indian J. Pediatr. 2022, 89, 37–44. [Google Scholar] [CrossRef]
- Go, C.; Mackay, M.; Weiss, S.; Stephens, D.; Adams-Webber, T.; Ashwal, S.; Snead, O. Evidence-based guideline update: Medical treatment of infantile spasms. Report of the Guideline Development Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2012, 78, 1974–1980. [Google Scholar] [CrossRef]
- Matsuo, A.; Matsuzaka, T.; Tsuru, A.; Moriuchi, H.; Nakashita, Y.; Tanaka, S.; Baba, C.; Tomimasu, K. Epidemiological and clinical studies of West syndrome in Nagasaki Prefecture, Japan. Brain Dev. 2001, 23, 575–579. [Google Scholar] [CrossRef]
- Hatahira, H.; Abe, J.; Hane, Y.; Matsui, T.; Sasaoka, S.; Motooka, Y.; Hasegawa, S.; Fukuda, A.; Naganuma, M.; Ohmori, T.; et al. Drug-induced gingival hyperplasia: A retrospective study using spontaneous reporting system databases. J. Pharm. Health Care Sci. 2017, 3, 19. [Google Scholar] [CrossRef]
- Zieliński, G.; Pająk, A.; Wójcicki, M. Global Prevalence of sleep bruxism and awake bruxism in pediatric and adult populations: A systematic review and meta-analysis. J. Clin. Med. 2024, 13, 4259. [Google Scholar] [CrossRef]
- Rezazadeh, A.; Uddin, M.; Snead, O.C., 3rd; Lira, V.; Silberberg, A.; Weiss, S.; Donner, E.J.; Zak, M.; Bradbury, L.; Scherer, S.W.; et al. STXBP1 encephalopathy is associated with awake bruxism. Epilepsy Behav. 2019, 92, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Elkhayat, H.A.; Hassanein, S.M.; Tomoum, H.Y.; Abd-Elhamid, I.A.; Asaad, T.; Elwakkad, A.S. Melatonin and sleep-related problems in children with intractable epilepsy. Pediatr. Neurol. 2010, 42, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Camfield, C.; Camfield, P. Injuries from seizures are a serious, persistent problem in childhood onset epilepsy: A population-based study. Seizure 2015, 27, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Pooya, A.A.; Nikseresht, A.; Yaghoubi, E.; Nei, M. Physical injuries in patients with epilepsy and their associated risk factors. Seizure 2012, 21, 165–168. [Google Scholar] [CrossRef]
- Mahdi, S.S.; Jafri, H.A.; Allana, R.; Amenta, F.; Khawaja, M.; Qasim, S.S.B. Oral manifestations of Rett syndrome—A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 1162. [Google Scholar] [CrossRef]
- Conley, Z.R.; Hague, M.; Kurosaka, H.; Dixon, J.; Dixon, M.J.; Trainor, P.A. A quantitative method for defining high-arched palate using the Tcof1(+/-) mutant mouse as a model. Dev. Biol. 2016, 415, 296–305. [Google Scholar] [CrossRef]
- Redman, R.S.; Shapiro, B.L.; Gorlin, R.J. Measurement of normal and reportedly malformed palatal vaults. II. Normal juvenile measurements. J. Dent. Res. 1996, 45, 266–269. [Google Scholar] [CrossRef]
- Grant, E.; Carlson, G.; Cullen-Erickson, M. Oral health for people with intellectual disability and high support needs: Positive outcomes. Spec. Care Dent. 2004, 24, 70–79. [Google Scholar] [CrossRef]
| Medical Characteristics | Cases (%) | |
|---|---|---|
| Controlled | 2 (14.2) | |
| Uncontrolled | 9 (64.2) | |
| Resolved * | 3 (21.4) | |
| Epilepsy | Neuropsychomotor delay | 13 (92.8) |
| Lennox–Gastaut syndrome | 6 (42.8) | |
| Tetraparesis | 3 (21.4) | |
| Autism spectrum disorder | 2 (14.2) | |
| Comorbidities | Limb atrophy | 1 (7.1) |
| Scoliosis | 1 (7.1) | |
| Anticonvulsants | ||
| Valproic acid | 11 (78.5) | |
| Topiramate | 4 (28.5) | |
| Clobazam | 4 (28.5) | |
| Clonazepam | 3 (21.4) | |
| Phenobarbital | 3 (21.4) | |
| Levetiracetam | 3 (21.4) | |
| Lamotrigine | 1 (7.1) | |
| Vigabatrin | 0 | |
| Other medications | ||
| Baclofen | 1 (7.1) | |
| Risperidone | 1 (7.1) | |
| ACTH | 0 | |
| Corticosteroids | 0 | |
| Oral Findings | Cases (%) | |
|---|---|---|
| Dental anomalies | ||
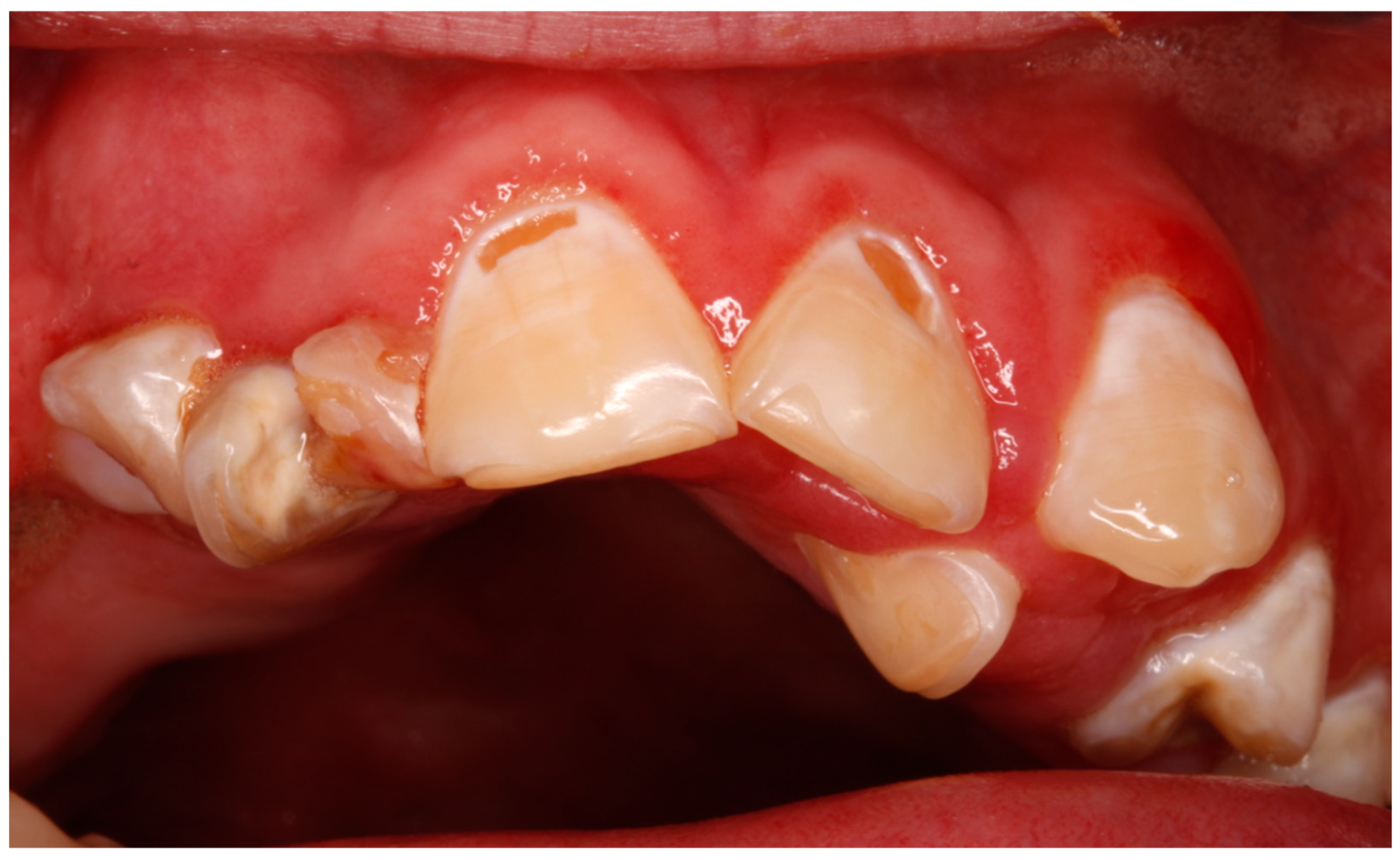
| Poor oral hygiene | 9 (64.2) | |
| Cavities | 8 (57.1) | |
| Bruxism/attrition | 4 (28.5) | |
| Dental malposition | 2 (14.2) | |
| Enamel hypoplasia | 2 (14.2) | |
| White spot lesion | 1 (7.1) | |
| Dental fracture | 1 (7.1) | |
| Abnormal tooth eruption | 0 | |
| Delayed tooth eruption | 0 | |
| Soft tissues anomalies | ||
| Gingivitis | 9 (64.2) | |
| Lingual malposition | 1 (7.1) | |
| Gingival enlargement | 0 | |
| Maxillary bones anomalies | ||
| Anterior open bite | 1 (7.1) | |
| High palate | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limeres-Posse, J.; Muñoz-Navarro, C.; García-Mato, E.; Sande-López, L.; Diniz-Freitas, M.; Diz-Dios, P.; Rivas-Mundiña, B. The Oral Findings and Dental Management of Patients with West Syndrome: A Case Series and Literature Review. J. Clin. Med. 2025, 14, 2494. https://doi.org/10.3390/jcm14072494
Limeres-Posse J, Muñoz-Navarro C, García-Mato E, Sande-López L, Diniz-Freitas M, Diz-Dios P, Rivas-Mundiña B. The Oral Findings and Dental Management of Patients with West Syndrome: A Case Series and Literature Review. Journal of Clinical Medicine. 2025; 14(7):2494. https://doi.org/10.3390/jcm14072494
Chicago/Turabian StyleLimeres-Posse, Jacobo, Carolina Muñoz-Navarro, Eliane García-Mato, Lucía Sande-López, Márcio Diniz-Freitas, Pedro Diz-Dios, and Berta Rivas-Mundiña. 2025. "The Oral Findings and Dental Management of Patients with West Syndrome: A Case Series and Literature Review" Journal of Clinical Medicine 14, no. 7: 2494. https://doi.org/10.3390/jcm14072494
APA StyleLimeres-Posse, J., Muñoz-Navarro, C., García-Mato, E., Sande-López, L., Diniz-Freitas, M., Diz-Dios, P., & Rivas-Mundiña, B. (2025). The Oral Findings and Dental Management of Patients with West Syndrome: A Case Series and Literature Review. Journal of Clinical Medicine, 14(7), 2494. https://doi.org/10.3390/jcm14072494







