Role of Artificial Intelligence in Thyroid Cancer Diagnosis
Abstract
1. Introduction
2. Materials and Methods
3. Artificial Intelligence in Thyroid Nodule Diagnostics
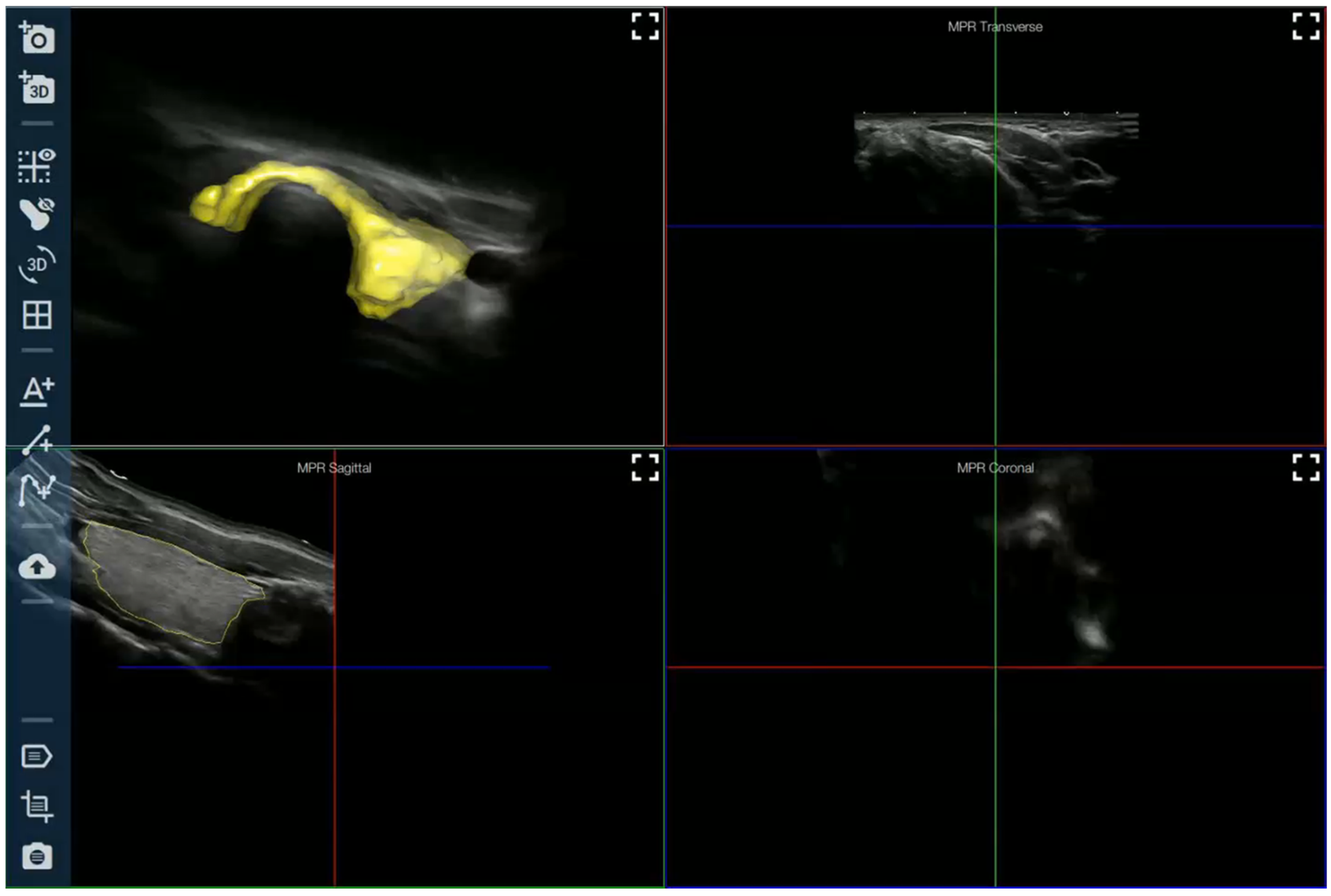
AI Applied to Ultrasound Imaging
4. AI in Thyroid Nodule Classification
5. Limitations
6. AI Applications in Pathological Thyroid Diagnosis
7. Applications of Artificial Intelligence in the Molecular Evaluation of Thyroid Nodules
8. Content-Based Image Retrieval (CBIR) and with Enhanced Saliency (SE-CBIR)
9. Restore-Generative Adversarial Network
10. Vision Transformers and Application in Thyroid Diagnostics
11. Conclusions
- -
- Advancement in diagnosis with AI: The integration of artificial intelligence (AI), particularly through machine learning (ML) and deep learning (DL) algorithms, represents a significant step forward in the diagnosis and prognosis of thyroid nodules.
- -
- Comparable and superior performance: The studies analyzed in this review show that AI-based systems have achieved levels of accuracy, sensitivity, and specificity comparable to, and in some cases superior to, those achieved by experienced practitioners in the fields of ultrasound, cytopathological, and molecular diagnostics.
- -
- Improvement in diagnostic efficiency: artificial intelligence algorithms have been successfully tested in the evaluation of ultrasound images of thyroid nodules, the classification of lesions, and the prediction of malignancy, showing the ability to improve diagnostic efficiency and reduce evaluation time.
- -
- Clinically approved systems: Systems such as Am-CAD-UT, Koios DS Thyroid, MEDOThyroid, and S-Detect have obtained significant approvals for clinical use, highlighting the reliability and usefulness of these tools in supporting clinicians, especially less experienced ones.
- -
- Improvements in cytopathological and molecular diagnostics: In the field of cytopathological and molecular diagnostics, the use of artificial intelligence has improved accuracy in differentiating between benign and malignant lesions and in predicting genetic mutations, contributing to more precise risk stratification and personalization of therapies.
- -
- Future evolution of AI systems: Looking forward, the continued evolution of artificial intelligence systems, including new models such as enhanced saliency CBIR (SE-CBIR), Restore-Generative Adversarial Network (GAN) models, and Vision Transformers (ViTs), promises to further expand the applications of AI in thyroid diagnostics.
- -
- Benefits for patients and healthcare systems: These advancements not only improve the quality of diagnosis but also open the way to new possibilities in the prevention and personalized management of thyroid cancer, with potentially significant benefits for patients and the healthcare system.
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Pizzato, M.; Li, M.; Vignat, J.; Laversanne, M.; Singh, D.; La Vecchia, C.; Vaccarella, S. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol. 2022, 10, 264–272. [Google Scholar] [PubMed]
- AME NEWS No. 66-August 2022; European Society of Endocrinology: Bristol, UK, 2022.
- Locati, L.D.; Bernardo, G. Thyroid. In Cancer Numbers in Italy 2022; AIOM; AIRT; AIOM Foundation; ONS; SIAPEC-IAP; National Centre for Disease Prevention and Health Promotion: Milan, Italy, 2022; p. 85. [Google Scholar]
- Barp, S.; Grani, G. Thyroid Cancer Guidelines, 2017 ed.; Artificial intelligence in the diagnosis of the thyroid nodule; updated October 2019; AIOM: Milan, Italy, 2017. [Google Scholar]
- Sollini, M.; Cozzi, L.; Chiti, A.; Kirienko, M. Texture analysis and machine learning to characterise suspected thyroid nodules and differentiated thyroid cancer: Where do we stand? Eur. J. Radiol. 2018, 99, 1–8. [Google Scholar] [PubMed]
- Bini, F.; Pica, A.; Azzimonti, L.; Giusti, A.; Ruinelli, L.; Marinozzi, F.; Trimboli, P. Artificial intelligence in the thyroid field—A comprehensive review. Cancers 2021, 13, 4740. [Google Scholar] [CrossRef]
- Liang, J.; Huang, X.; Hu, H.; Liu, Y.; Zhou, Q.; Cao, Q.; Wang, W.; Liu, B.; Zheng, Y.; Li, X.; et al. Predicting malignancy in thyroid nodules: Radiomics score versus 2017 American College of Radiology Thyroid Imaging, Reporting and Data System. Thyroid 2018, 28, 1024–1033. [Google Scholar] [PubMed]
- Zhang, C.; Liu, D.; Huang, L.; Zhao, Y.; Chen, L.; Guo, Y. Classification of thyroid nodules by using deep learning radiomics based on ultrasound dynamic video. J. Ultrasound Med. 2022, 41, 2993–3002. [Google Scholar]
- Yang, J.; Page, L.C.; Wagner, L.; Wildman-Tobriner, B.; Bisset, L.; Frush, D.; Mazurowski, M.A. Thyroid nodules on ultrasound in children and young adults: Comparison of diagnostic performance of radiologists’ impressions, ACR TI-RADS, and a deep learning algorithm. Am. J. Roentgenol. 2023, 220, 408–417. [Google Scholar]
- Barp, S.; Grani, G. Intelligenza artificiale nella diagnostica del nodulo tiroideo. L’Endocrinologo 2023, 24, 385–390. [Google Scholar]
- Xue, Y.; Zhou, Y.; Wang, T.; Chen, H.; Wu, L.; Ling, H.; Wang, H.; Qiu, L.; Ye, D.; Wang, B. Accuracy of ultrasound diagnosis of thyroid nodules based on artificial intelligence-assisted diagnostic technology: A systematic review and meta-analysis. Int. J. Endocrinol. 2022, 2022, 9492056. [Google Scholar]
- Li, Y.; Liu, Y.; Xiao, J.; Yan, L.; Yang, Z.; Li, X.; Zhang, M.; Luo, Y. Clinical value of artificial intelligence in thyroid ultrasound: A prospective study from the real world. Eur. Radiol. 2023, 33, 4513–4523. [Google Scholar] [CrossRef]
- Fresilli, D.; Grani, G.; De Pascali, M.L.; Alagna, G.; Tassone, E.; Ramundo, V.; Ascoli, V.; Bosco, D.; Biffoni, M.; Bononi, M.; et al. Computer- aided diagnostic system for thyroid nodule sonographic evaluation outperforms the specificity of less experienced examiners. J. Ultrasound 2020, 23, 169–174. [Google Scholar]
- Swan, K.Z.; Thomas, J.; Nielsen, V.E.; Jespersen, M.L.; Bonnema, S.J. External validation of AIBx, an artificial intelligence model for risk stratification, in thyroid nodules. Eur. Thyroid J. 2022, 11, e21012. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Ledger, G.A.; Mamillapalli, C.K. Use of artificial intelligence and machine learning for estimating malignancy risk of thyroid nodules. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 345–350. [Google Scholar] [CrossRef]
- Guan, Q.; Wang, Y.; Ping, B.; Li, D.; Du, J.; Qin, Y.; Lu, H.; Wan, X.; Xiang, J. Deep convolutional neural network VGG16 model for differential diagnosing of papillary thyroid carcinomas in cytological images: A pilot study. J. Cancer 2019, 10, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, M.; Spitale, A.; Faquin, W.C.; Mazzucchelli, L.; Baloch, Z.W. The Bethesda System for Reporting Thyroid Cytopathology: A meta-analysis. Acta Cytol. 2012, 56, 333–339. [Google Scholar] [CrossRef]
- Sanyal, P.; Mukherjee, T.; Barui, S.; Das, A.; Gangopadhyay, P. Artificial intelligence in cytopathology: A neural network to identify papillary carcinoma on thyroid fine-needle aspiration cytology smears. J. Pathol. Inform. 2018, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Elliott Range, D.D.; Dov, D.; Kovalsky, S.Z.; Henao, R.; Carin, L.; Cohen, J. Application of a machine learning algorithm to predict malignancy in thyroid cytopathology. Cancer Cytopathol. 2020, 128, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.; Zandvakili, A.; Gera, S.; Khutti, S.D.; Gersten, A.; Khader, S.N. Differentiating noninvasive follicular thyroid neoplasm with papillary-like nuclear features from classic papillary thyroid carcinoma: Analysis of cytomorphologic descriptions using a novel machinelearning approach. J. Pathol. Inform. 2019, 10, 29. [Google Scholar] [CrossRef]
- Fu, Y.; Jung, A.W.; Torne, R.V.; Gonzalez, S.; Vöhringer, H.; Shmatko, A.; Yates, L.R.; Jimenez-Linan, M.; Moore, L.; Gerstung, M. Pan-cancer computational histopathology reveals mutations, tumor composition and prognosis. Nat. Cancer 2020, 1, 800–810. [Google Scholar]
- Girolami, I.; Marletta, S.; Pantanowitz, L.; Torresani, E.; Ghimenton, C.; Barbareschis, M.; Scarpa, A.; Brunelli, M.; Barresi, V.; Trimboli, P.; et al. Impact of image analysis and artificial intelligence in thyroid pathology, with particular reference to cytological aspects. Cytopathology 2020, 31, 432–444. [Google Scholar] [CrossRef]
- Bongiovanni, M.; Bellevicine, C.; Troncone, G.; Sykiotis, G.P. Approach to cytological indeterminate thyroid nodules. Gland. Surg. 2019, 8 (Suppl. S2), S98–S104. [Google Scholar] [CrossRef]
- Diggans, J.; Kim, S.Y.; Hu, Z.; Pankratz, D.; Wong, M.; Reynolds, J.; Tom, E.; Pagan, M.; Monroe, R.; Rosai, J.; et al. Machine learning from concept to clinic: Reliable detection of BRAF V600E DNA mutations in thyroid nodules using highdimensional RNA expression data. Pac. Symp. Biocomput. 2015, 20, 371–382. [Google Scholar]
- Hao, Y.; Choi, Y.; Babiarz, J.E.; Kloos, R.T.; Kennedy, G.C.; Huang, J.; Walsh, P.S. Analytical Verification Performance of A firma Genomic Sequencing Classifier in the Diagnosis of Cytologically Indeterminate Thyroid Nodules. Front. Endocrinol. 2019, 10, 438. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Duh, Q.-Y.; Kloos, R.T.; Babiarz, J.; Harrell, R.M.; Traweek, S.T.; Kim, S.Y.; Fedorowicz, G.; Walsh, P.S.; Sadow, P.M.; et al. Identification of Hürthle cell cancers: Solving a clinical challenge with genomic sequencing and a trio of machine learning algorithms. BMC Syst. Biol. 2019, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Steward, D.L.; Carty, S.E.; Sippel, R.S.; Yang, S.P.; Sosa, J.A.; Sipos, J.A.; Figge, J.J.; Mandel, S.; Haugen, B.R.; Burman, K.D.; et al. Performance of a Multigene Genomic Classifier in Thyroid Nodules with Indeterminate Cytology: A Prospective Blinded Multicenter Study. JAMA Oncol. 2019, 5, 204–212. [Google Scholar] [CrossRef]
- Mallick, U.K.; Mazzaferri, E.L.; Harmer, C.; Kendall-Taylor, P. Practical management of thyroid cancer: A multidisciplinary approach. In Practical Management of Thyroid Cancer: A Multidisciplinary Approach; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Gassner, M.; Barranco Garcia, J.; Tanadini-Lang, S.; Bertoldo, F.; Fröhlich, F.; Guckenberger, M.; Haueis, S.; Pelzer, C.; Reyes, M.; Schmithausen, P.; et al. Saliency-Enhanced ContentBased Image Retrieval for Diagnosis Support in Dermatology Consultation: Reader Study. JMIR Dermatol. 2023, 6, e42129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rong, R.; Wang, S.; Zhang, X.; Wen, Z.; Cheng, X.; Jia, L.; Yang, D.M.; Xie, Y.; Zhan, X.; Xiao, G. Enhanced Pathology Image Quality with Restore-Generative Adversarial Network. Am. J. Pathol. 2023, 193, 404–416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ayana, G.; Choe, S.W. BUViTNet: Breast Ultrasound Detection via Vision Transformers. Diagnostics 2022, 12, 2654. [Google Scholar] [CrossRef]
- Sun, J.; Wu, B.; Zhao, T.; Gao, L.; Xie, K.; Lin, T.; Sui, J.; Li, X.; Wu, X.; Ni, X. Classification for thyroid nodule using ViT with contrastive learning in ultrasound images. Comput. Biol. Med. 2022, 152, 106444. [Google Scholar] [CrossRef] [PubMed]
| CAD SYSTEM | SOURCE DATA | NODULE RECOGNITION | TYPE OF APPLICATION |
|---|---|---|---|
| AmCAD-UT (AmCad BioMed Corporation, Taipei City, Taiwan); | Single image | Semi-automatic | Local computer |
| Koios DS Thyroid (Koios Medical, Inc., New York, NY, USA); | Double projection image | Manual | Local server |
| MEDO-Thyroid (MEDO AI, Edmonton, AB, Canada); | Video | Manual | Cloud |
| S-Detect (Samsung Medison Co., Ltd., Seoul, Republic of Korea) | Single image | Manual | Installed in the ultrasound machine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cece, A.; Agresti, M.; De Falco, N.; Sperlongano, P.; Moccia, G.; Luongo, P.; Miele, F.; Allaria, A.; Torelli, F.; Bassi, P.; et al. Role of Artificial Intelligence in Thyroid Cancer Diagnosis. J. Clin. Med. 2025, 14, 2422. https://doi.org/10.3390/jcm14072422
Cece A, Agresti M, De Falco N, Sperlongano P, Moccia G, Luongo P, Miele F, Allaria A, Torelli F, Bassi P, et al. Role of Artificial Intelligence in Thyroid Cancer Diagnosis. Journal of Clinical Medicine. 2025; 14(7):2422. https://doi.org/10.3390/jcm14072422
Chicago/Turabian StyleCece, Alessio, Massimo Agresti, Nadia De Falco, Pasquale Sperlongano, Giancarlo Moccia, Pasquale Luongo, Francesco Miele, Alfredo Allaria, Francesco Torelli, Paola Bassi, and et al. 2025. "Role of Artificial Intelligence in Thyroid Cancer Diagnosis" Journal of Clinical Medicine 14, no. 7: 2422. https://doi.org/10.3390/jcm14072422
APA StyleCece, A., Agresti, M., De Falco, N., Sperlongano, P., Moccia, G., Luongo, P., Miele, F., Allaria, A., Torelli, F., Bassi, P., Sciarra, A., Avenia, S., Della Monica, P., Colapietra, F., Di Domenico, M., Docimo, L., & Parmeggiani, D. (2025). Role of Artificial Intelligence in Thyroid Cancer Diagnosis. Journal of Clinical Medicine, 14(7), 2422. https://doi.org/10.3390/jcm14072422







