Effectiveness of Chest Compression-Synchronized Ventilation in Patients with Cardiac Arrest
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population
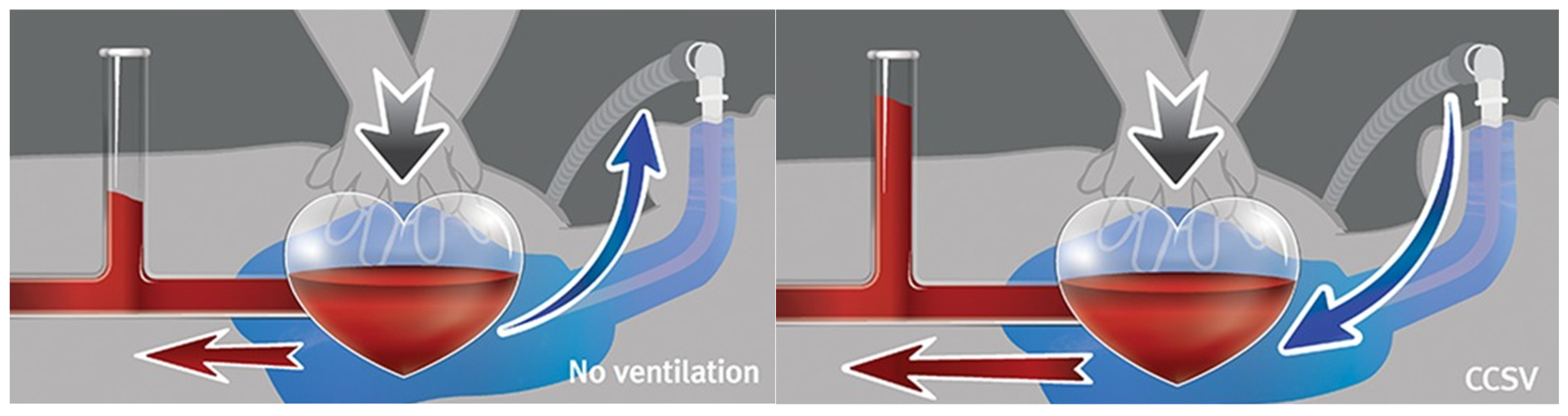
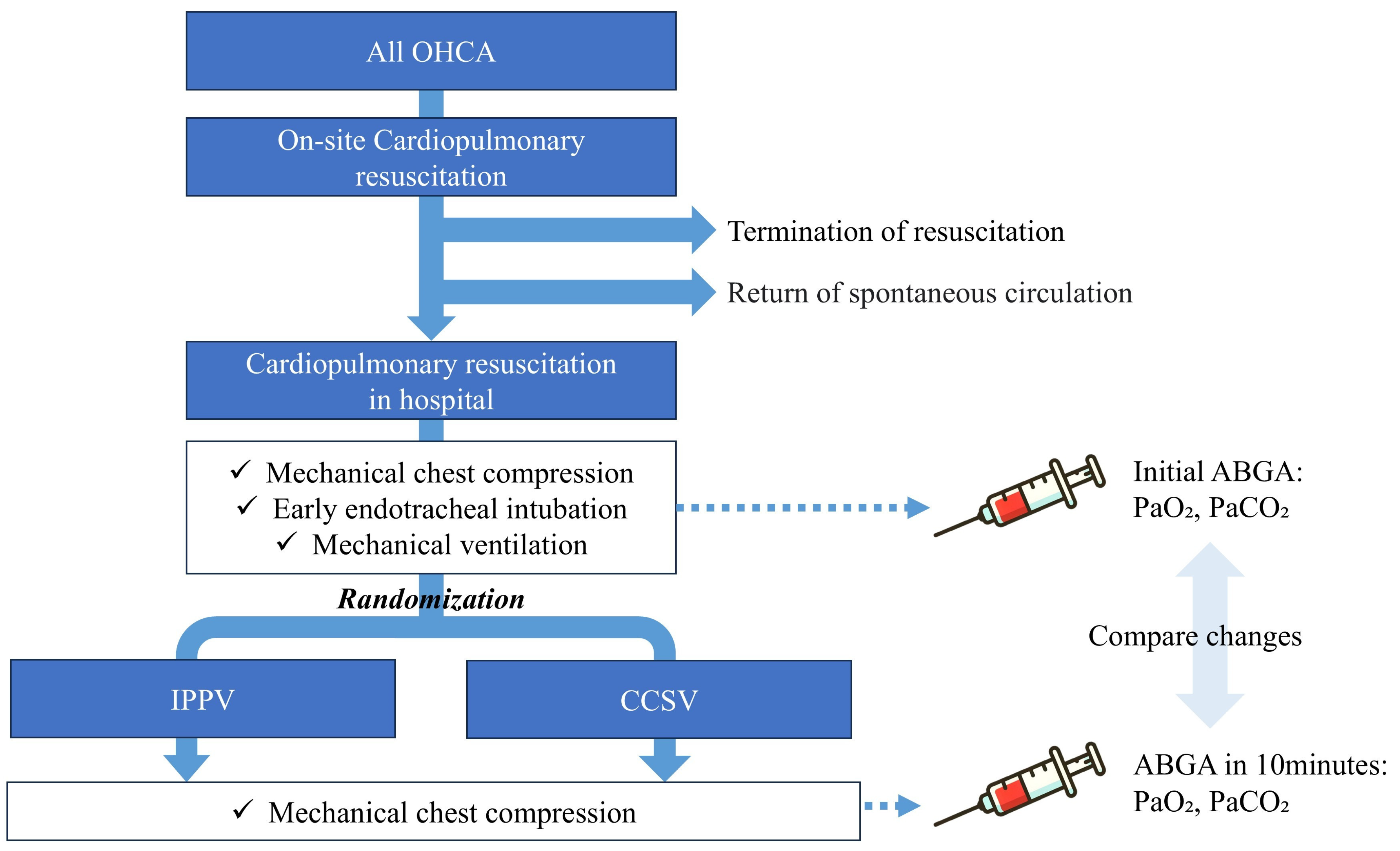
2.3. Study Protocol
2.4. Sample Size
2.5. Variables
2.6. Statistical Analysis
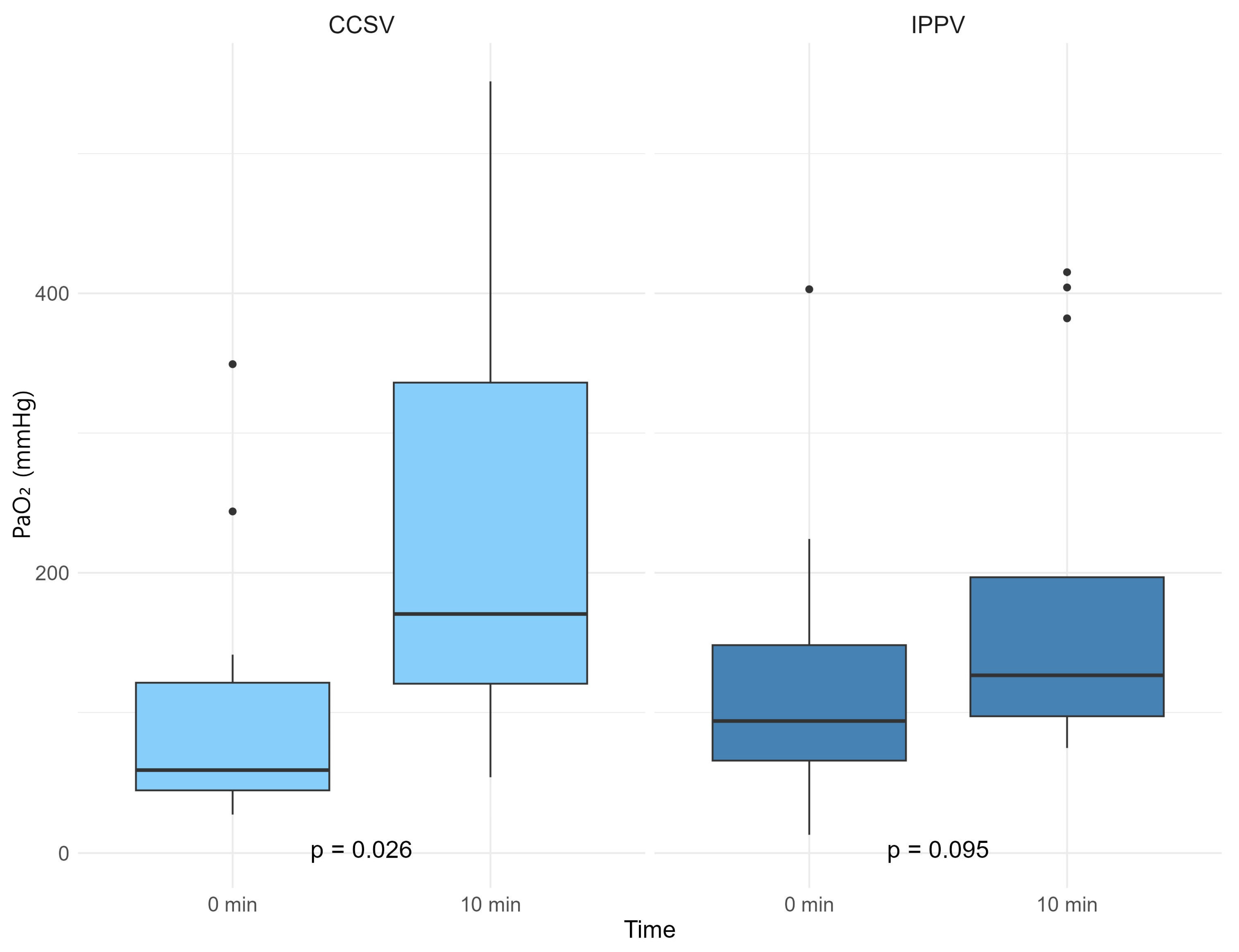
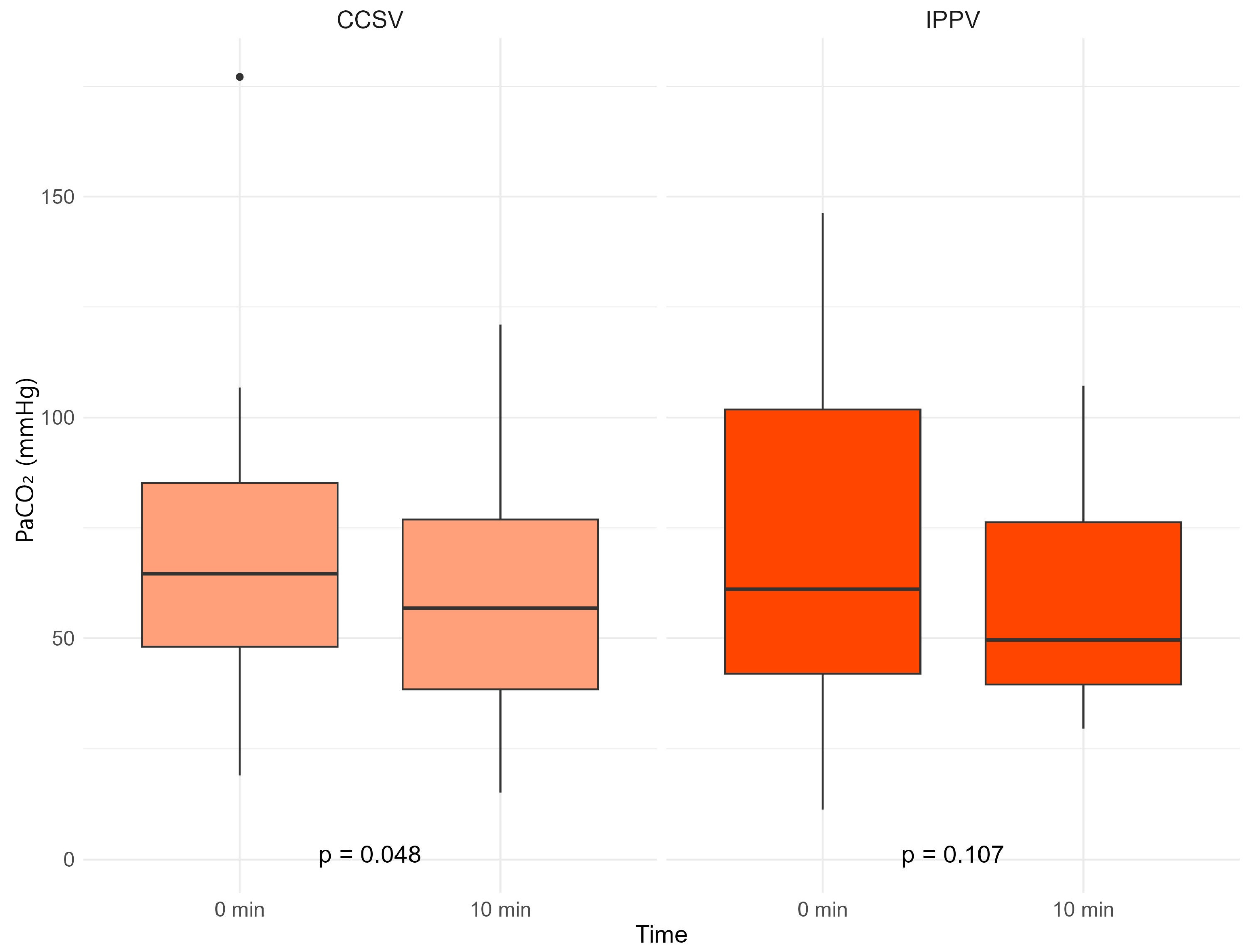
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CPR | cardiopulmonary resuscitation |
| CCSV | chest compression-synchronized ventilation |
| IPPV | intermittent positive-pressure ventilation |
| OHCA | out-of-hospital cardiac arrest |
| ROSC | return of spontaneous circulation |
| ABGA | arterial blood gas analysis |
References
- Cardiac Arrest Incidence and Survival Outcomes: Chronic Disease Health Statistics; Korea Disease Control and Prevention Agency: Cheongju, Republic of Korea, 2023. Available online: https://www.kdca.go.kr/injury/biz/injury/recsroom/statsSmMain.do (accessed on 21 March 2025).
- Corp, A.; Thomas, C.; Adlam, M. The cardiovascular effects of positive pressure ventilation. BJA Educ. 2021, 21, 202–209. [Google Scholar] [CrossRef]
- Panchal, A.R.; Bartos, J.A.; Cabañas, J.G.; Donnino, M.W.; Drennan, I.R.; Hirsch, K.G.; Kudenchuk, P.J.; Kurz, M.C.; Lavonas, E.J.; Morley, P.T.; et al. Adult Basic and Ad-vanced Life Support Writing Group. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020, 142, S366–S468. [Google Scholar] [CrossRef] [PubMed]
- Henlin, T.; Michalek, P.; Tyll, T.; Hinds, J.D.; Dobias, M. Oxygenation, Ventilation, and Airway Management in Out-of-hospital Cardiac Arrest: A Review. BioMed Res. Int. 2014, 2014, 376871. [Google Scholar] [CrossRef]
- van Eijk, J.A.; Doeleman, L.C.; Loer, S.A.; Koster, R.W.; van Schuppen, H.; Schober, P. Ventilation during cardiopulmonary resuscitation: A narrative review. Resuscitation 2024, 203, 110366. [Google Scholar] [CrossRef]
- Wyckoff, M.H.; Singletary, E.M.; Soar, J.; Olasveengen, T.M.; Greif, R.; Liley, H.G.; Zideman, D.; Bhanji, F.; Andersen, L.W.; Avis, S.R.; et al. 2021 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations: Summary From the Basic Life Support; Advanced Life Support; Neonatal Life Support; Education, Implementation, and Teams; First Aid Task Forces; and the COVID-19 working group. Resuscitation 2021, 169, 229–311. [Google Scholar] [CrossRef]
- Serin, S.; Caglar, B. The Effect of Different Personal Protective Equipment Masks on Health Care Workers’ Cardiopulmonary Resuscitation Performance During the Covid-19 Pandemic. J. Emerg. Med. 2021, 60, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Edelson, D.P.; Sasson, C.; Chan, P.S.; Atkins, D.L.; Aziz, K.; Becker, L.B.; Berg, R.A.; Bradley, S.M.; Brooks, S.C.; Cheng, A.; et al. Interim Guidance for Basic and Advanced Life Support in Adults, Children, and Neonates With Suspected or Confirmed COVID-19: From the Emergency Cardiovascular Care Committee and Get With The Guidelines-Resuscitation Adult and Pediatric Task Forces of the American Heart Association. Circulation 2020, 141, e933–e943. [Google Scholar] [CrossRef]
- Kill, C.; Galbas, M.; Neuhaus, C.; Hahn, O.; Wallot, P.; Kesper, K.; Wulf, H.; Dersch, W. Chest Compression Synchronized Ventilation versus Intermitted Positive Pressure Ventilation during Cardiopulmonary Resuscitation in a Pig Model. PLoS ONE 2015, 10, e0127759. [Google Scholar] [CrossRef]
- Kill, C.; Paul, C.; Manegold, R.K.; Holzner, C.; Blomeyer, R.; Risse, J. Mechanical Positive Pressure Ventilation during Resus-citation in Out-of-hospital Cardiac Arrest with Chest Compression Synchronized Ventilation (CCSV). Resuscitation 2019, 142, e42. [Google Scholar] [CrossRef]
- Bobrow, B.J.; Clark, L.L.; Ewy, G.A.; Chikani, V.; Sanders, A.B.; Berg, R.A.; Richman, P.B.; Kern, K.B. Minimally interrupted cardiac resuscitation by emergency medical services for out-of-hospital cardiac arrest. JAMA 2008, 299, 1158–1165. [Google Scholar] [CrossRef]
- Chang, M.P.; Lu, Y.; Leroux, B.; Aramendi Ecenarro, E.; Owens, P.; Wang, H.E.; Idris, A.H. Association of Ventilation with Outcomes from Out-of-hospital Cardiac Arrest. Resuscitation 2019, 141, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Nichol, G.; Leroux, B.; Wang, H.; Callaway, C.W.; Sopko, G.; Weisfeldt, M.; Stiell, I.; Morrison, L.J.; Aufderheide, T.P.; Cheskes, S.; et al. Trial of Continuous or Interrupted Chest Compressions during CPR. N. Engl. J. Med. 2015, 373, 2203–2214. [Google Scholar] [CrossRef] [PubMed]
- Vissers, G.; Soar, J.; Monsieurs, K.G. Ventilation Rate in Adults with a Tracheal Tube during Cardiopulmonary Resuscitation: A systematic review. Resuscitation 2017, 119, 5–12. [Google Scholar] [CrossRef]
- Safar, P.; Brown, T.C.; Holtey, W.J.; Wilder, R.J. Ventilation and Circulation with Closed-chest Cardiac Massage in Man. JAMA 1961, 176, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Deakin, C.D.; O’Neill, J.F.; Tabor, T. Does compression-only cardiopulmonary resuscitation generate adequate passive ventilation during cardiac arrest? Resuscitation 2007, 75, 53–59. [Google Scholar] [CrossRef]
- McDannold, R.; Bobrow, B.J.; Chikani, V.; Silver, A.; Spaite, D.W.; Vadeboncoeur, T. Quantification of ventilation volumes produced by compressions during emergency department cardiopulmonary resuscitation. Am. J. Emerg. Med. 2018, 36, 1640–1644. [Google Scholar] [CrossRef]
- Hernández-Tejedor, A.; González Puebla, V.; Corral Torres, E.; Benito Sánchez, A.; Pinilla López, R.; Galán Calategui, M.D. Ventilatory improvement with mechanical ventilator versus bag in non-traumatic out-of-hospital cardiac arrest: SYMEVECA study, phase 1. Resuscitation 2023, 192, 109965. [Google Scholar] [CrossRef]
- Zhang, X.; Hartmann, P. How to calculate sample size in animal and human studies. Front. Med. 2023, 10, 1215927. [Google Scholar] [CrossRef]
- Kill, C.; Hahn, O.; Dietz, F.; Neuhaus, C.; Schwarz, S.; Mahling, R.; Wallot, P.; Jerrentrup, A.; Steinfeldt, T.; Wulf, H.; et al. Mechanical Ventilation during Cardiopulmonary Resuscitation with Intermittent Positive-Pressure Ventilation, Bilevel Ventila-tion, or Chest Compression Synchronized Ventilation in a Pig Model. Crit. Care Med. 2014, 42, e89–e95. [Google Scholar] [CrossRef]
- Hong, S.-I.; Kim, J.-S.; Kim, Y.-J.; Kim, W.Y. Dynamic changes in arterial blood gas during cardiopulmonary resuscitation in out-of-hospital cardiac arrest. Sci. Rep. 2021, 11, 23165. [Google Scholar] [CrossRef]
- Spindelboeck, W.; Gemes, G.; Strasser, C.; Toescher, K.; Kores, B.; Metnitz, P.; Haas, J.; Prause, G. Arterial blood gases during and their dynamic changes after cardiopulmonary resuscitation: A prospective clinical study. Resuscitation 2016, 106, 24–29. [Google Scholar] [CrossRef] [PubMed]
- SOS-KANTO Study Group. Relationship between the hemoglobin level at hospital arrival and post-cardiac arrest neurologic outcome. Am. J. Emerg. Med. 2012, 30, 770–774. [Google Scholar] [CrossRef] [PubMed]

| MV Type | CCSV (N = 15) | IPPV (N = 15) | p | |
|---|---|---|---|---|
| Age (years) | 70.0 [60.5; 80.5] | 63.0 [56.5; 79.5] | 0.901 | |
| Sex | Male | 12 (80.0%) | 10 (66.7%) | 0.680 |
| Female | 3 (20.0%) | 5 (33.3%) | ||
| Medical history | HTN | 9 (60.0%) | 7 (46.7%) | 0.714 |
| DM | 6 (40.0%) | 6 (40.0%) | 1.000 | |
| Heart disease | 3 (20.0%) | 2 (13.3%) | 1.000 | |
| CRF | 0 (0.0%) | 1 (6.7%) | 1.000 | |
| Liver | 2 (13.3%) | 1 (6.7%) | 1.000 | |
| Witnessed arrest | Yes | 9 (60.0%) | 11 (73.3%) | 0.699 |
| Bystander CPR | Yes | 8 (53.3%) | 8 (53.3%) | 0.809 |
| Unknown | 2 (13.3%) | 1 (6.7%) | ||
| Initial rhythm | VF | 1 (6.7%) | 2 (13.3%) | 0.395 |
| pVT | 0 (0.0%) | 0 (0.0%) | ||
| PEA | 7 (46.7%) | 9 (60.0%) | ||
| Asystole | 0 (0.0%) | 1 (6.7%) | ||
| Unknown | 2 (13.3%) | 0 (0.0%) | ||
| Prehospital defibrillation | Yes | 2 (13.3%) | 3 (20.0%) | 1.000 |
| Prehospital airway | BVM | 1 (6.7%) | 2 (13.3%) | 0.659 |
| Igel | 13 (86.7%) | 11 (73.3%) | ||
| Unknown | 1 (6.7%) | 2 (13.3%) | ||
| Prehospital no-flow time | 7.0 [2.0; 10.0] | 9.0 [5.0; 11.0] | 0.466 | |
| Prehospital low-flow time | 20.5 [16.5; 27.0] | 25.0 [21.0; 31.0] | 0.210 | |
| Cause of death | Cardiac | 6 (40.0%) | 6 (40.0%) | 0.361 |
| Trauma | 2 (13.3%) | 0 (0.0%) | ||
| Metabolic | 3 (20.0%) | 4 (26.7%) | ||
| Respiratory | 1 (6.7%) | 3 (20.0%) | ||
| Other | 1 (6.7%) | 2 (13.3%) | ||
| Unknown | 2 (13.3%) | 0 (0.0%) | ||
| Any ROSC | Yes | 7 (46.7%) | 13 (86.7%) | 0.053 |
| ED outcome | Death | 9 (60.0%) | 7 (46.7%) | 0.515 |
| Admission | 6 (40.0%) | 7 (46.7%) | ||
| Transfer | 0 (0.0%) | 1 (6.7%) | ||
| TTM | Yes | 0 (0.0%) | 2 (13.3%) | 0.464 |
| ECMO | Yes | 0 (0.0%) | 2 (13.3%) | 0.464 |
| Hospital outcome | Death | 14 (93.3%) | 12 (80.0%) | 0.475 |
| Admission | 0 (0.0%) | 0 (0.0%) | ||
| Transfer | 1 (6.7%) | 2 (13.3%) | ||
| Discharge | 0 (0.0%) | 1 (6.7%) |
| MV Type | CCSV (N = 15) | IPPV (N = 15) | p | |
|---|---|---|---|---|
| ABGA at 0 min | pH | 7.0 [6.9; 7.1] | 6.9 [6.9; 7.1] | 0.494 |
| PCO2 | 64.6 [48.1; 85.2] | 61.1 [42.0; 101.8] | 1.000 | |
| PO2 | 59.0 [43.8; 131.3] | 105.9 [73.6; 184.8] | 0.267 | |
| HCO3 | 16.4 [13.8; 19.4] | 15.5 [12.2; 17.6] | 0.678 | |
| ABGA at 10 min | pH | 7.1 [6.9; 7.2] | 7.1 [6.9; 7.2] | 0.820 |
| PCO2 | 56.8 [38.5; 76.8] | 49.6 [39.5; 76.3] | 0.967 | |
| PO2 | 166.6 [97.7; 303.6] | 127.9 [97.8; 335.7] | 0.967 | |
| HCO3 | 14.3 [10.4; 16.2] | 14.5 [12.4; 18.7] | 0.507 | |
| ABGA difference | ΔO2 | 76.1 [22.8; 260.3] | 8.8 [−1.6; 113.9] | 0.250 |
| ΔCO2 | −10.3 [−18.3; −2.7] | −11.5 [−36.5; 5.6] | 0.935 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, Y.T.; Lee, C.A.; Park, H.A.; Park, J.; Kim, S.; Park, H.J.; Han, S.; Wang, S.; Kim, J.W. Effectiveness of Chest Compression-Synchronized Ventilation in Patients with Cardiac Arrest. J. Clin. Med. 2025, 14, 2394. https://doi.org/10.3390/jcm14072394
Oh YT, Lee CA, Park HA, Park J, Kim S, Park HJ, Han S, Wang S, Kim JW. Effectiveness of Chest Compression-Synchronized Ventilation in Patients with Cardiac Arrest. Journal of Clinical Medicine. 2025; 14(7):2394. https://doi.org/10.3390/jcm14072394
Chicago/Turabian StyleOh, Young T., Choung A. Lee, Hang A. Park, Juok Park, Sola Kim, Hye J. Park, Sangsoo Han, Soonjoo Wang, and Jong W. Kim. 2025. "Effectiveness of Chest Compression-Synchronized Ventilation in Patients with Cardiac Arrest" Journal of Clinical Medicine 14, no. 7: 2394. https://doi.org/10.3390/jcm14072394
APA StyleOh, Y. T., Lee, C. A., Park, H. A., Park, J., Kim, S., Park, H. J., Han, S., Wang, S., & Kim, J. W. (2025). Effectiveness of Chest Compression-Synchronized Ventilation in Patients with Cardiac Arrest. Journal of Clinical Medicine, 14(7), 2394. https://doi.org/10.3390/jcm14072394







