Incidence and Risk Factors of Dysphagia After Cardiac Surgery: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Criteria of Studies
2.2. Extracted Primary and Secondary Clinical Outcomes
3. Results
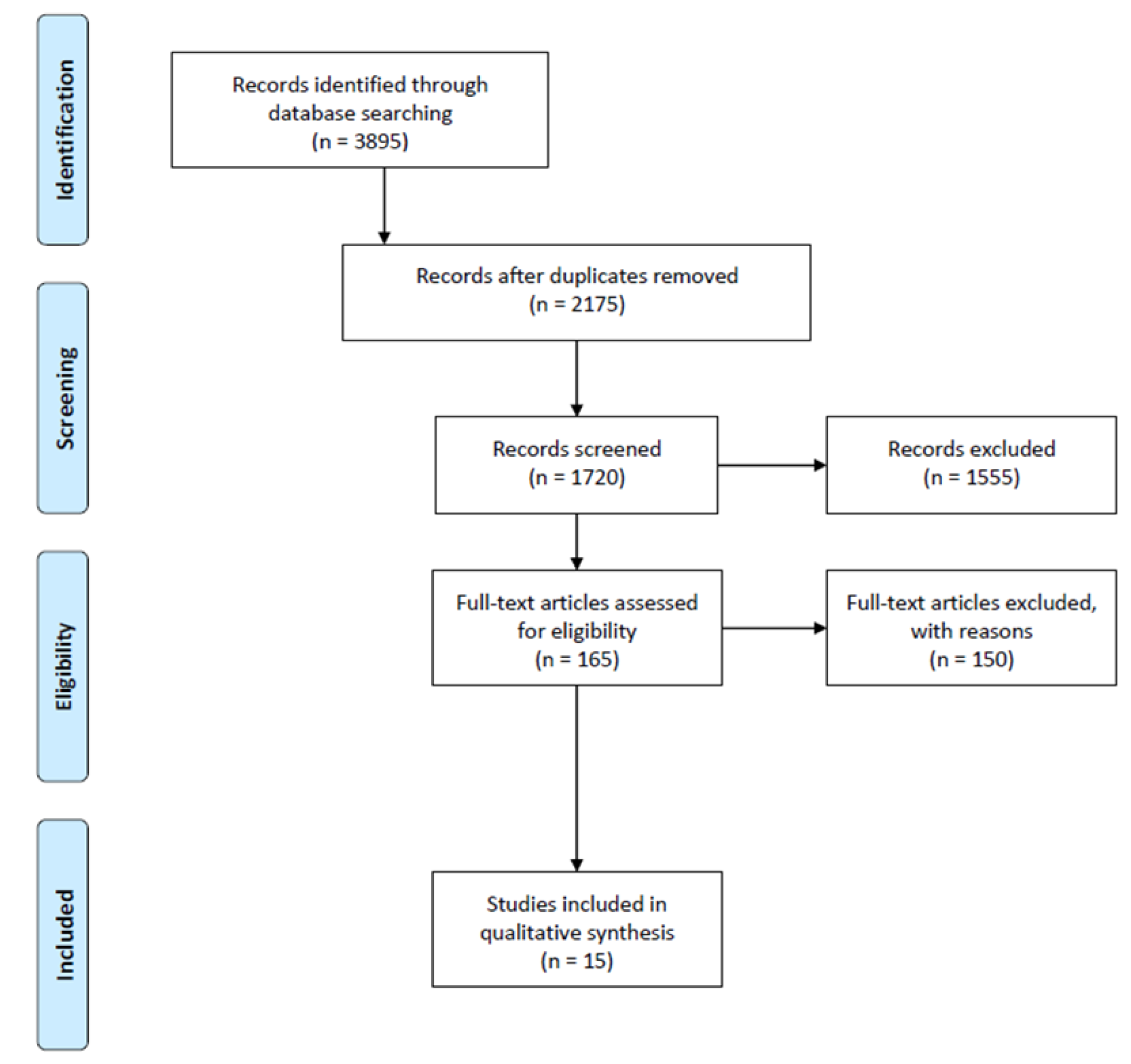
3.1. Search Results
3.2. Incidence of Dysphagia Across Studies
3.3. Risk Factors Related to Patient Characteristics
3.4. Procedural and Intra-Operative Risk Factors
3.5. Post-Operative Complications and Prognosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| QoL | Quality of life |
| LoS | Length of stay |
| PoD | Post-operative dysphagia |
| CABG | Coronary artery bypass grafting |
| FEES | Fiberoptic endoscopic evaluation of swallowing |
| VFSS | Videofluoroscopic swallow studies |
| TEE | Transesophageal echocardiography |
| LVEF | Left ventricular ejection fraction |
| PED | Post-extubation dysphagia |
References
- Karunaratne, T.B.; Clavé, P.; Ortega, O. Complications of oropharyngeal dysphagia in older individuals and patients with neurological disorders: Insights from Mataró hospital, Catalonia, Spain. Front. Neurol. 2024, 15, 1355199. [Google Scholar] [CrossRef]
- Al Rjoob, M.; Hassan, N.F.H.N.; Aziz, M.A.A.; Zakaria, M.N.; Mustafar, M.F.B.M. Quality of life in stroke patients with dysphagia: A systematic review. Tunis. Med. 2022, 100, 664–669. [Google Scholar] [PubMed]
- Song, J.M. Dysphagia and quality of life: A narrative review. Ann. Clin. Nutr. Metab. 2024, 16, 43–48. [Google Scholar] [CrossRef]
- Nguyen, S.; Zhu, A.; Toppen, W.; Ashfaq, A.; Davis, J.; Shemin, R.; Mendelsohn, A.H.; Benharash, P. Dysphagia after cardiac operations is associated with increased length of stay and costs. Am. Surg. 2016, 82, 890–893. [Google Scholar] [CrossRef]
- Barker, J.; Martino, R.; Reichardt, B.; Hickey, E.J.; Ralph-Edwards, A. Incidence and impact of dysphagia in patients receiving prolonged endotracheal intubation after cardiac surgery. Can. J. Surg. 2009, 52, 119–124. [Google Scholar] [PubMed]
- Plowman, E.K.; Anderson, A.; York, J.D.; DiBiase, L.; Vasilopoulos, T.; Arnaoutakis, G.; Beaver, T.; Martin, T.; Jeng, E.I. Dysphagia after cardiac surgery: Prevalence, risk factors, and associated outcomes. J. Thorac. Cardiovasc. Surg. 2023, 165, 737–746.e3. [Google Scholar] [CrossRef]
- Verma, A.; Hadaya, J.; Tran, Z.; Dobaria, V.; Madrigal, J.; Xia, Y.; Sanaiha, Y.; Mendelsohn, A.H.; Benharash, P. Incidence and outcomes of laryngeal complications following adult cardiac surgery: A national analysis. Dysphagia 2022, 37, 1142–1150. [Google Scholar] [CrossRef]
- McDonagh, D.L.; Berger, M.; Mathew, J.P.; Graffagnino, C.; Milano, C.A.; Newman, M.F. Neurological complications of cardiac surgery. Lancet Neurol. 2014, 13, 490–502. [Google Scholar] [CrossRef]
- Gilbey, T.; Milne, B.; de Somer, F.; Kunst, G. Neurologic complications after cardiopulmonary bypass—A narrative review. Perfusion 2023, 38, 1545–1559. [Google Scholar] [CrossRef]
- Mao, L.; Wang, J.; Li, Y.; Zheng, J.; Fan, D.; Wei, S.; Wu, X.; Yang, X.; Wang, D. Risk factors for dysphagia in patients with acute and chronic ischemic stroke: A retrospective cohort study. Heliyon 2024, 10, e24582. [Google Scholar] [CrossRef]
- Yang, C.; Pan, Y. Risk factors of dysphagia in patients with ischemic stroke: A meta-analysis and systematic review. PLoS ONE 2022, 17, e0270096. [Google Scholar] [CrossRef] [PubMed]
- Regala, M.; Marvin, S.; Ehlenbach, W.J. Association Between Postextubation Dysphagia and Long-Term Mortality Among Critically Ill Older Adults. J. Am. Geriatr. Soc. 2019, 67, 1895–1901. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Hogue, C.W., Jr.; Lappas, G.D.; Creswell, L.L.; Ferguson, T.B., Jr.; Sample, M.; Pugh, D.; Balfe, D.; Cox, J.L.; Lappas, D.G. Swallowing dysfunction after cardiac operations. Associated adverse outcomes and risk factors including intraoperative transesophageal echocardiography. J. Thorac. Cardiovasc. Surg. 1995, 110, 517–522. [Google Scholar] [CrossRef]
- Rousou, J.A.; Tighe, D.A.; Garb, J.L.; Krasner, H.; Engelman, R.M.; Flack, J.E., III; Deaton, D.W. Risk of dysphagia after transesophageal echocardiography during cardiac operations. Ann. Thorac. Surg. 2000, 69, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Grimm, J.C.; Magruder, J.T.; Ohkuma, R.; Dungan, S.P.; Hayes, A.; Vose, A.K.; Orlando, M.; Sussman, M.S.; Cameron, D.E.; Whitman, G.J. A novel risk score to predict dysphagia after cardiac surgery procedures. Ann. Thorac. Surg. 2015, 100, 568–574. [Google Scholar] [CrossRef]
- Daly, E.; Miles, A.; Scott, S.; Gillham, M. Finding the red flags: Swallowing difficulties after cardiac surgery in patients with prolonged intubation. J. Crit. Care 2016, 31, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Bowles, B.J.; Puntil-Sheltman, J. Is dysphagia after cardiac operations a “preexisting condition”? Ann. Thorac. Surg. 2016, 101, 1450–1453. [Google Scholar] [CrossRef][Green Version]
- Miles, A.; McLellan, N.; Machan, R.; Vokes, D.; Hunting, A.; McFarlane, M.; Holmes, J.; Lynn, K. Dysphagia and laryngeal pathology in post-surgical cardiothoracic patients. J. Crit. Care 2018, 45, 121–127. [Google Scholar] [CrossRef]
- Zhou, X.D.; Dong, W.H.; Zhao, C.H.; Feng, X.F.; Wen, W.W.; Tu, W.Y.; Cai, M.X.; Xu, T.C.; Xie, Q.L. Risk scores for predicting dysphagia in critically ill patients after cardiac surgery. BMC Anesthesiol. 2019, 19, 7. [Google Scholar] [CrossRef]
- Black, R.J.; Bogaardt, H.; McCabe, P.; Glanville, A.R.; MacDonald, P.; Madill, C. Clinical predictors for oropharyngeal dysphagia and laryngeal dysfunction after lung and heart transplantation. Int. J. Lang. Commun. Disord. 2019, 54, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Satomi-Kobayashi, S.; Yoshida, N.; Komaki, K.; Izawa, K.P.; Hamaguchi, M.; Inoue, T.; Sakai, Y.; Hirata, K.I.; Okada, K. Impact of frailty on postoperative dysphagia in patients undergoing elective cardiovascular surgery. JACC Asia 2022, 2, 104–113. [Google Scholar] [CrossRef]
- Ogawa, M.; Satomi-Kobayashi, S.; Hamaguchi, M.; Komaki, K.; Izawa, K.P.; Miyahara, S.; Inoue, T.; Sakai, Y.; Hirata, K.I.; Okada, K. Postoperative dysphagia as a predictor of functional decline and prognosis after undergoing cardiovascular surgery. Eur. J. Cardiovasc. Nurs. 2023, 22, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Satomi-Kobayashi, S.; Hamaguchi, M.; Komaki, K.; Kusu, H.; Izawa, K.P.; Miyahara, S.; Sakai, Y.; Hirata, K.I.; Okada, K. Impact of maximum phonation time on postoperative dysphagia and prognosis after cardiac surgery. JTCVS Open 2024, 18, 123–137. [Google Scholar] [CrossRef]
- Colton House, J.; Noordzij, J.P.; Murgia, B.; Langmore, S. Laryngeal injury from prolonged intubation: A prospective analysis of contributing factors. Laryngoscope 2011, 121, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Shinn, J.R.; Kimura, K.S.; Campbell, B.R.; Sun Lowery, A.; Wootten, C.T.; Garrett, C.G.; Francis, D.O.; Hillel, A.T.; Du, L.; Casey, J.D.; et al. Incidence and outcomes of acute laryngeal injury after prolonged mechanical ventilation. Crit. Care Med. 2019, 47, 1699–1706. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, Y.; Ren, Z.; Xie, M. Transesophageal echocardiography related complications. Front. Cardiovasc. Med. 2024, 11, 1410594. [Google Scholar] [CrossRef]
- Mathur, S.K.; Singh, P. Transoesophageal echocardiography related complications. Indian J. Anaesth. 2009, 53, 567–574. [Google Scholar]
- Macht, M.; White, S.D.; Moss, M. Swallowing dysfunction after critical illness. Chest 2014, 146, 1681–1689. [Google Scholar] [CrossRef]
- Rassameehiran, S.; Klomjit, S.; Mankongpaisarnrung, C.; Rakvit, A. Postextubation dysphagia. Bayl. Univ. Med. Cent. Proc. 2015, 28, 18–20. [Google Scholar] [CrossRef]
- Ambrosino, N.; Clini, E. Long-term mechanical ventilation and nutrition. Respir. Med. 2004, 98, 413–420. [Google Scholar] [CrossRef]
- Robison, R.; Focht Garand, K.L.; Affoo, R.; Yeh, C.K.; Chin, N.; McArthur, C.; Pulia, M.; Rogus-Pulia, N. New horizons in understanding oral health and swallowing function within the context of frailty. Age Ageing 2023, 52, afac276. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Y.; Yang, A.Y.; Chen, Y.C.; Lee, S.D.; Lee, S.H.; Chen, J.W. Association between dysphagia and frailty in older adults: A systematic review and meta-analysis. Nutrients 2022, 14, 1812. [Google Scholar] [CrossRef] [PubMed]
- Elpern, E.H.; Scott, M.G.; Petro, L.; Ries, M.H. Pulmonary aspiration in mechanically ventilated patients with tracheostomies. Chest 1994, 105, 563–566. [Google Scholar] [CrossRef]
- Mirzakhani, H.; Williams, J.N.; Mello, J.; Joseph, S.; Meyer, M.J.; Waak, K.; Schmidt, U.; Kelly, E.; Eikermann, M. Muscle weakness predicts pharyngeal dysfunction and symptomatic aspiration in long-term ventilated patients. Anesthesiology 2013, 119, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Alfares, F.A.; Hynes, C.F.; Ansari, G.; Chounoune, R.; Ramadan, M.; Shaughnessy, C.; Reilly, B.K.; Zurakowski, D.; Jonas, R.A.; Nath, D.S. Outcomes of recurrent laryngeal nerve injury following congenital heart surgery: A contemporary experience. J. Saudi Heart Assoc. 2016, 28, 1–6. [Google Scholar] [CrossRef]
- Dimarakis, I.; Protopapas, A.D. Vocal cord palsy as a complication of adult cardiac surgery: Surgical correlations and analysis. Eur. J. Cardiothorac. Surg. 2004, 26, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, A.L.; Moukarbel, R.V.; Farhat, F.; Obeid, M. Vocal cord paralysis after open-heart surgery. Eur. J. Cardiothorac. Surg. 2002, 21, 671–674. [Google Scholar] [CrossRef]
- Wallace, S.; McGrath, B.A. Laryngeal complications after tracheal intubation and tracheostomy. BJA Educ. 2021, 21, 250–257. [Google Scholar] [CrossRef]
- Black, R.J.; Novakovic, D.; Plit, M.; Miles, A.; MacDonald, P.; Madill, C. Swallowing and laryngeal complications in lung and heart transplantation: Etiologies and diagnosis. J. Heart Lung Transplant. 2021, 40, 1483–1494. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, J.; Zheng, L.; Li, X.; Hao, Y. Implementation strategies to improve evidence-based practice for post-stroke dysphagia identification and management: A before-and-after study. Int. J. Nurs. Sci. 2022, 9, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Rosenvinge, S.K.; Starke, I.D. Improving care for patients with dysphagia. Age Ageing 2005, 34, 587–593. [Google Scholar] [CrossRef] [PubMed]
| Study | Year | Type of Study | Number of Patients (N) | Males, n (%) | Age (Years) | BMI (kg/m2) | Type of Surgery | Parameters of Interest |
|---|---|---|---|---|---|---|---|---|
| Hogue CW Jr et al. [14] | 1995 | Prospective cohort study | Total: 838 SD: 34 No-SD: 835 | Total: 557 (66.5) SD: 20 (58.8) No-SD: 537 (64.3) | SD: 71.0 ± 2.0 No-SD: 63.0 ± 0.4 | NA | SD: CABG 21 (62%), CABG/valvular procedure 3 (9%), arrhythmia surgery 2 (6%), valvular surgery 1 (3%), other 7 (21%) No-SD: CABG 621 (75%), CABG/valvular procedure 40 (5%), arrhythmia surgery 47 (6%), valvular surgery 35 (4%), other 90 (11%) |
|
| Rousou JA et al. [15] | 2000 | Prospective cohort study | Total: 838 TEE: 126 No-TEE: 712 | Total: 561 (67) TEE: 79 (62.7) No-TEE: 412 (67.7) | TEE: 66.3 ± 12 No-TEE: 66.9 ± 0.4 | NA | TEE: CABG 39.7%, Non-CABG 60.3% No-TEE: CABG 79.3%, Non-CABG 20.7% |
|
| Barker J et al. [5] | 2009 | Retrospective study | Total: 254 DYS: 130 No-DYS: 124 | Total: 176 (69.3) DYS: 91 (70.0) No-DYS: 85 (68.5) | Total: 64.5 ± 12.6 DYS: 65.4 ± 12.1 No-DYS: 63.5 ± 13.0 | NA | Total: CABG 123 (48.4%), valve 59 (23.2%), other 72 (28.3%) DYS: CABG 57 (43.8%), valve 37 (28.5%), other 36 (27.7%) No-DYS: CABG 66 (53.2%), valve 22 (17.7%), other 36 (29.0%) |
|
| Grimm JC et al. [16] | 2015 | Prospective cohort study | Total: 1314 DYS: 115 No-DYS: 1199 | Total: 852 (64.8) DYS: 83 (72.2) No-DYS: 769 (64.1) | DYS: 65 (53–74) * No-DYS: 61 (52–71) * | DYS: 27.0 ± 7.1 No-DYS: 29.4 ± 7.3 | DYS: CABG 23 (20.0%), valve 24 (20.9%), VAD or heart transplant 17 (14.8%), other 6 (5.2%), combination 45 (39.1%) No-DYS: CABG 392 (32.7%), valve 234 (19.5%), VAD or heart transplant 57 (4.8%), other 70 (5.8%), combination 446 (37.2%) |
|
| Daly E et al. [17] | 2016 | Retrospective study | Total: 190 DYS: 33 No-DYS: 157 | NA | Total: 61.3 ± 15.0 DYS: 63.5 ± 17.6 No-DYS: 61.2 ± 14.4 | NA | Total: CABG 99 (52.1%), valve 58 (30.5%), cardiac transplant 9 (4.7%), other 24 (12.6%) DYS: CABG 18 (54.5%), valve 10 (30.3%), cardiac transplant 1 (3.0%), other 4 (12.1%) No-DYS: CABG 81 (51.6%), valve 48 (30.6%), cardiac transplant 8 (5.1%), other 20 (12.7%) |
|
| Bowles BJ et al. [18] | 2016 | Prospective cohort study | 176 | 132 (75.0) | 73.5 ± 5.2 | NA | CABG 96 (54.5), aortic valve replacement 20 (11.4), mitral valve repair or replacement 11 (6.3), CABG + single valve 23 (13.1), other 26 (14.8) |
|
| Nguyen S et al. [4] | 2016 | Prospective cohort study | Total: 354 DYS: 56 No-DYS: 298 | 230 (65) | DYS: 73 ± 12.5 No-DYS: 64 ± 13.6 | DYS: 25.0 ± 4.7 No-DYS: 27.6 ± 6.0 | Non-emergent, non-transplant cardiac operations |
|
| Miles A et al. [19] | 2018 | Retrospective study | 106 | 72 (69) | 63 ± 15.16 | NA | CABG 59 (56%), valve 37 (35%), cardiac transplant 2 (2%), other (infection, aneurysm) 8 (8%) |
|
| Zhou XD et al. [20] | 2019 | Prospective cohort study | 395 | 258 (73) | 61.7 ± 12.8 | 23.4 ± 3.3 | CABG |
|
| Black RJ et al. [21] | 2019 | Retrospective study | Total: 284 SP: 68 No-SP: 216 | Total: 159 (56) SP: 40 (58.8) No-SP: 119 (55.1) | Total: 46.7 ± 14.1 SP: 48.3 ± 13.0 No-SP: 46.2 ± 14.4 | NA | Total: bilateral lung transplant 175 (62%), heart transplant 101 (36%), single lung transplant 4 (1%), heart and lung transplant 4 (1%) SP: bilateral lung transplant 42 (62%), heart transplant 25 (37%), heart and lung transplant 1 (1%) |
|
| Ogawa M et al. [22] | 2022 | Retrospective cohort study | Total: 644 PED: 98 No-PED: 566 | Total: 382 (57.5) PED: 52 (53.1) No-PED: 330 (58.3) | Total: 67.72 ± 13.69 PED: 73.62 ± 9.78 No-PED: 66.55 ± 14.12 | Total: 22.86 ± 3.79 PED: 22.30 ± 3.71 No-PED: 22.97 ± 3.80 | Total: aortic 88 (13.3%), CABG 88 (13.3%), valve 434 (65.4%), combination 54 (8.1%) PED: aortic 27 (27.6%), CABG 9 (9.2%), valve 52 (53.1%), combination 10 (10.2%) No-PED: aortic 61 (10.8%), CABG 79 (14.0%), valve 382 (67.5%), combination 44 (7.8%) |
|
| Verma A et al. [7] | 2022 | Retrospective cohort study | Total: 2,319,628 LC: 39,688 No-LC: 2,279,940 | Total: 1,603,477 (69.1) LC: 25,758 (64.9) No-LC: 1,577,719 (69.2) | LC: 72 (63–79) * No-LC: 67 (59–74) * | NA | LC: CABG 52.1%, valve 26.4%, CABG + valve 17.4%, multiple valve 4.0% No-LC: CABG 61.5%, valve 23.8%, CABG + valve 11.9%, multiple valve 2.8% |
|
| Plowman EK et al. [6] | 2023 | Prospective cohort study | Total: 182 ASP: 53 No-ASP: 129 | Total: 122 (67) ASP: 39 (74) No-ASP: 83 (64) | Total: 62.3 ± 13.3 ASP: 61.8 ± 12.8 No-ASP: 63.4 ± 14.6 | Total: 29.7 ± 6.3 ASP: 29.9 ± 6.4 No-ASP: 29.2 ± 6.0 | Total: CABG or valve 72, aortic root 12, arch 40, LVAD or transplant 9, arch and valve 43, other 6 ASP: CABG or valve 46, aortic root 10, arch 26, LVAD or transplant 6, arch and valve 26, other 4 No-ASP: CABG or valve 26, aortic root 2, arch 14, LVAD or transplant 3, arch and valve 17, other 2 |
|
| Ogawa M et al. [23] | 2023 | Retrospective cohort study | Total: 712 Abnormal PED: 104 No-PED: 608 | Total: 408 (57.5) PED: 55 (52.9) No-PED: 353 (58.1) | Total: 67.7 ± 13.7 PED: 73.5 ± 9.8 No-PED: 66.8 ± 14.0 | PED: 22.3 ± 3.6 No-PED: 22.9 ± 3.8 | Total: CABG 58 (9.6%), valve 384 (63%), aortic, combination PED: CABG 11 (10.6%), valve 55 (52.9%), aortic 27 (26%), combination 11 (10.6%) No-PED: CABG 84 (13.8%), valve 411 (67.6%), aortic 61 (10.8%), combination 52 (8.6%) |
|
| Ogawa M et al. [24] | 2024 | Retrospective cohort study | Total: 442 Abnormal MPT: 243 Normal MPT: 199 | Total: 247 (56) Abnormal MPT: 132 (54) Normal MPT: 115 (58) | Total: 71.5 ± 6.4 Abnormal MPT: 72.8 ± 6.5 Normal MPT: 69.9 ± 6.0 | Total: 22.7 ± 3.8 Abnormal MPT: 22.7 ± 3.9 Normal MPT: 22.7 ± 3.8 | Total: CABG 58 (9.6%), valve 384 (63%) Abnormal MPT: CABG 32 (9.5%), valve 211 (62%) Normal MPT: CABG 26 (9.7%), valve 173 (64%) |
|
| Study | Method of Assessment | Patients with Dysphagia, n (%) | Type of Dysphagia | Prognostic Factors of Dysphagia | Conclusions |
|---|---|---|---|---|---|
| Hogue CW Jr et al. [14] | Barium swallow cineradiography | 34 out of 869 (4%) | Oropharyngeal |
|
|
| Rousou JA et al. [15] | Barium swallow cineradiography | 23 out of 838 (2.7%) | Oropharyngeal |
|
|
| Barker J et al. [5] | A speech–language pathologist conducted a full swallowing assessment at the bedside in addition to a more objective videofluoroscopic assessment if required. | 130 out of 254 (51%) | Oropharyngeal |
|
|
| Grimm JC et al. [16] | Videofluoroscopic swallow study (VFSS) | 115 out of 1314 (8.8%) | Oropharyngeal |
|
|
| Daly E et al. [17] | Speech–language pathologist by bedside assessment and/or instrumental assessment (FEES or VFSS) | 33 out of 190 (17.4%) | Oropharyngeal |
|
|
| Nguyen S et al. [4] | FEES performed by inpatient speech and language pathologist (SLP) | 56 out of 354 (16%) | Oropharyngeal |
|
|
| Miles A et al. [19] |
| 64 out of 106 (60%) patients were classified as severe or moderate–severe dysphagia | Oropharyngeal |
|
|
| Black RJ et al. [21] | Standard bedside assessment (BSA) and/or an objective swallowing assessment via videofluoroscopy or FEES | 58 out of 66 (88%) who were referred to SP | Oropharyngeal |
|
|
| Plowman EK et al. [6] | FEES performed by a trained speech–language pathologist | 95 out of 182 (52%) clinically significant pharyngeal residue | Oropharyngeal |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kourek, C.; Labropoulou, V.; Michou, E.; Dimopoulos, S. Incidence and Risk Factors of Dysphagia After Cardiac Surgery: A Scoping Review. J. Clin. Med. 2025, 14, 4279. https://doi.org/10.3390/jcm14124279
Kourek C, Labropoulou V, Michou E, Dimopoulos S. Incidence and Risk Factors of Dysphagia After Cardiac Surgery: A Scoping Review. Journal of Clinical Medicine. 2025; 14(12):4279. https://doi.org/10.3390/jcm14124279
Chicago/Turabian StyleKourek, Christos, Vania Labropoulou, Emilia Michou, and Stavros Dimopoulos. 2025. "Incidence and Risk Factors of Dysphagia After Cardiac Surgery: A Scoping Review" Journal of Clinical Medicine 14, no. 12: 4279. https://doi.org/10.3390/jcm14124279
APA StyleKourek, C., Labropoulou, V., Michou, E., & Dimopoulos, S. (2025). Incidence and Risk Factors of Dysphagia After Cardiac Surgery: A Scoping Review. Journal of Clinical Medicine, 14(12), 4279. https://doi.org/10.3390/jcm14124279






