A Narrative Review of Independent Treatment Methods for ED: Assessment of the Effectiveness of Diet, Supplements, Pharmacotherapy, and Physiotherapy
Abstract
1. Introduction
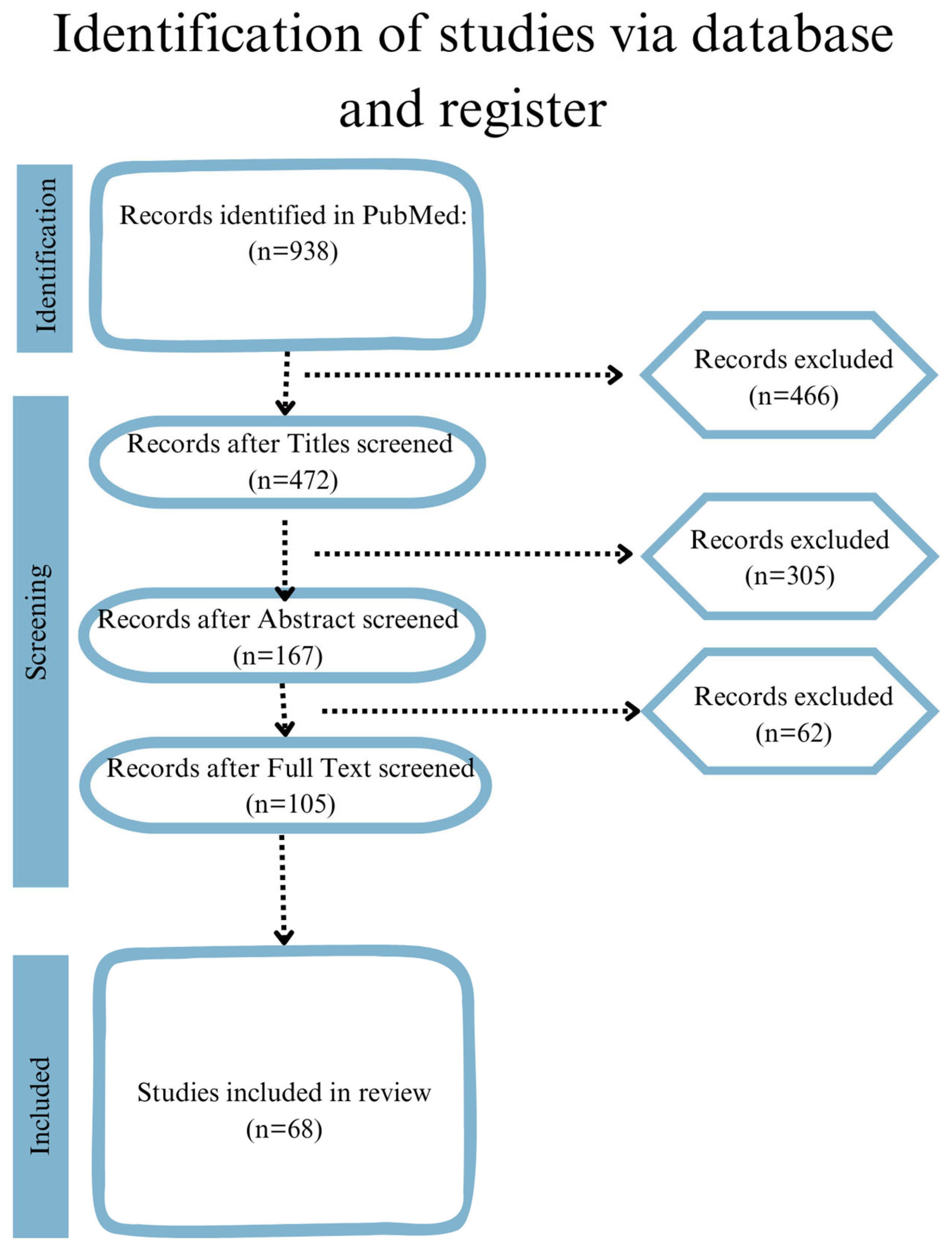
2. Materials and Methods
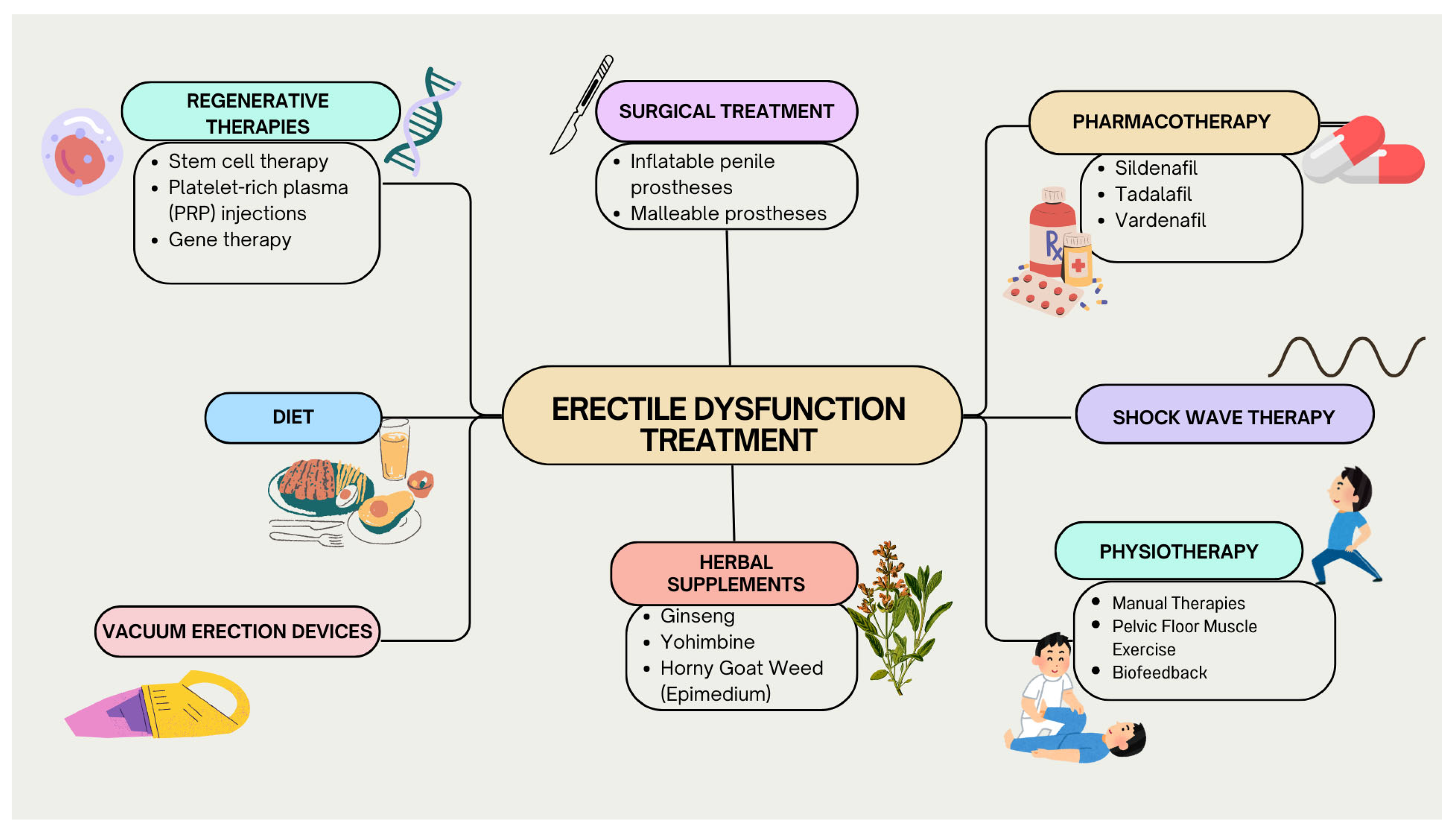
3. Discussion
3.1. Pharmacotherapy
3.2. Regenerative Therapies
3.3. Stem Cell Therapy
3.4. PRP Therapy
3.5. Gene Therapy
3.6. Challenges and Ethical Considerations
3.7. Shock Wave Therapy
3.8. Physiotherapy
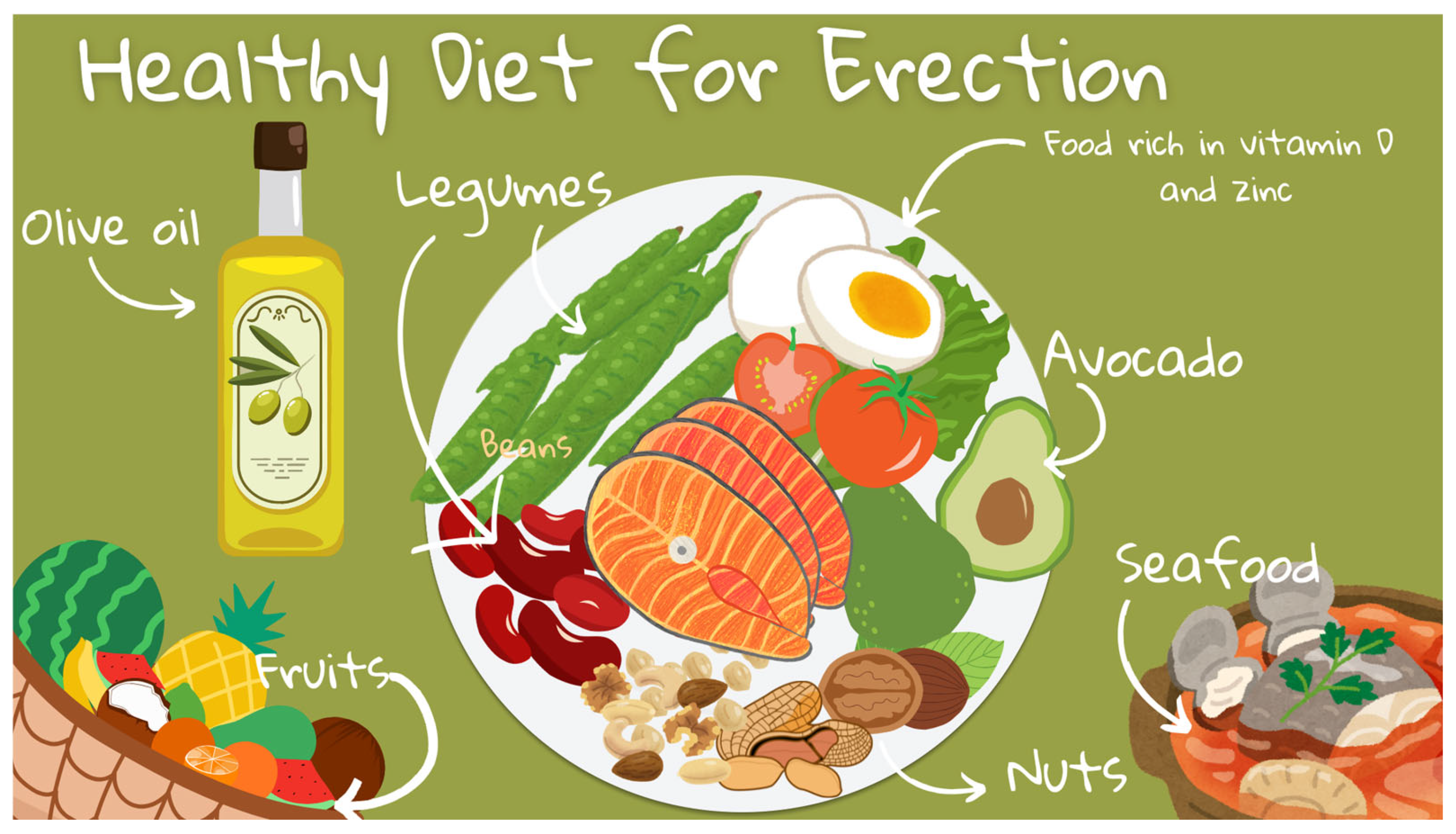
3.9. Impact of Dietary Patterns
The Mediterranean Diet
3.10. Herbal Supplements
3.11. Ginseng
3.12. Yohimbine
3.13. Horny Goat Weed (Epimedium)
3.14. Surgical Treatment
3.15. Vacuum Erection Devices
3.16. Psychological Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salonia, A.; Bettocchi, C.; Boeri, L.; Capogrosso, P.; Carvalho, J.; Cilesiz, N.C.; Cocci, A.; Corona, G.; Dimitropoulos, K.; Gül, M.; et al. European Association of Urology Guidelines on Sexual and Reproductive Health-2021 Update: Male Sexual Dysfunction. Eur. Urol. 2021, 80, 333–357. [Google Scholar] [PubMed]
- Forbes, C.M.; Flannigan, R.; Paduch, D.A. Perineal Ultrasound: A Review in the Context of Ejaculatory Dysfunction. Sex. Med. Rev. 2018, 6, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Naveen, N.R.; Anitha, P.; Goudanavar, P.S.; Rao, G.S.N.K.; Fattepur, S.; Rahman, M.M.; Shiroorkar, P.N.; Habeebuddin, M.; Meravanige, G.; et al. The Race to Replace PDE5i: Recent Advances and Interventions to Treat or Manage ED: Evidence from Patent Landscape (2016–2021). J. Clin. Med. 2022, 11, 3140. [Google Scholar] [CrossRef]
- de Souza, I.L.L.; Ferreira, E.D.S.; Vasconcelos, L.H.C.; Cavalcante, F.D.A.; Silva, B.A.D. ED: Key Role of Cavernous Smooth Muscle Cells. Front. Pharmacol. 2022, 13, 895044. [Google Scholar] [CrossRef]
- Laumann, E.O.; West, S.; Glasser, D.; Carson, C.; Rosen, R.; Kang, J.-H. Prevalence and Correlates of ED by Race and Ethnicity Among Men Aged 40 or Older in the United States: From the Male Attitudes Regarding Sexual Health Survey. J. Sex. Med. 2007, 4, 57–65. [Google Scholar] [CrossRef]
- Pozzi, E.; Capogrosso, P.; Fallara, G.; Boeri, L.; Belladelli, F.; Corsini, C.; Cignoli, D.; Bertini, A.; Lanzaro, F.; Raffo, M.; et al. (378) Still One Out of Five Men Presenting for ED are Young than 40 years of Age: Findings of a Real-life Cross-sectional Study over Last Decade. J. Sex. Med. 2023, 20 (Suppl. S1), qdad060-352. [Google Scholar] [CrossRef]
- Burnett, A.L.; Rojanasarot, S.; Amorosi, S.L. An Analysis of a Commercial Database on the Use of ED Treatments for Men with Employer-Sponsored Health Insurance. Urology 2021, 149, 140–145. [Google Scholar] [CrossRef]
- Song, W.H.; Park, J.; Yoo, S.; Oh, S.; Cho, S.Y.; Cho, M.C.; Jeong, H.; Son, H. Changes in the Prevalence and Risk Factors of ED during a Decade: The Korean Internet Sexuality Survey (KISS), a 10-Year-Interval Web-Based Survey. World J. Men’s Health 2019, 37, 199. [Google Scholar] [CrossRef]
- Abeway, S.; Dagne, K.; Zegeye, T. ED and Correlates Among Diabetic Men at Dessie Referral Hospital: North Central Ethiopia, 2020. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 4201–4208. [Google Scholar] [CrossRef]
- Yang, S.C.; Weinberger, J.M.; Shahinyan, R.H.; Shahinyan, G.K.; Mills, J.N.; Eleswarapu, S.V. Regenerative therapies for ED: The influence of direct-to-consumer marketing on patient interest. Transl. Androl. Urol. 2023, 12, 586–593. [Google Scholar] [CrossRef]
- Boettcher, M.; Nowotny, B.; Krausche, R.; Becker, C. Evaluation of the Influence of Sildenafil on the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Vericiguat in Healthy Adults. Clin. Pharmacokinet. 2023, 62, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, A.; Gupta, M. PDE5 Inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Wang, C.M.; Wu, B.R.; Xiang, P.; Xiao, J.; Hu, X.C. Management of male ED: From the past to the future. Front. Endocrinol. 2023, 14, 1148834. [Google Scholar]
- Chung, D.Y.; Ryu, J.K.; Yin, G.N. Regenerative therapies as a potential treatment of ED. Investig. Clin. Urol. 2023, 64, 312–324. [Google Scholar] [PubMed]
- Russo, G.I.; Broggi, G.; Cocci, A.; Capogrosso, P.; Falcone, M.; Sokolakis, I.; Gül, M.; Caltabiano, R.; Di Mauro, M. Relationship between Dietary Patterns with Benign Prostatic Hyperplasia and ED: A Collaborative Review. Nutrients 2021, 13, 4148. [Google Scholar] [PubMed]
- Romano, L.; Zagari, R.M.; Arcaniolo, D.; Crocetto, F.; Spirito, L.; Sciorio, C.; Gravina, A.G.; Dajti, E.; Barone, B.; La Rocca, R.; et al. Sexual dysfunction in gastroenterological patients: Do gastroenterologists care enough? A nationwide survey from the Italian Society of Gastroenterology (SIGE). Dig. Liver Dis. 2022, 54, 1494–1501. [Google Scholar]
- Cantone, E.; Massanova, M.; Crocetto, F.; Barone, B.; Esposito, F.; Arcaniolo, D.; Corlianò, F.; Romano, L.; Motta, G.; Celia, A. The relationship between obstructive sleep apnoea and ED: An underdiagnosed link? A prospective cross-sectional study. Andrologia 2022, 54, e14504. [Google Scholar]
- Yuan, J.; Zhang, R.; Yang, Z.; Lee, J.; Liu, Y.; Tian, J.; Qin, X.; Ren, Z.; Ding, H.; Chen, Q.; et al. Comparative Effectiveness and Safety of Oral Phosphodiesterase Type 5 Inhibitors for ED: A Systematic Review and Network Meta-analysis. Eur. Urol. 2013, 63, 902–912. [Google Scholar]
- Ala, M.; Mohammad Jafari, R.; Dehpour, A.R. Sildenafil beyond ED and pulmonary arterial hypertension: Thinking about new indications. Fundam. Clin. Pharmacol. 2021, 35, 235–259. [Google Scholar]
- Krzastek, S.C.; Bopp, J.; Smith, R.P.; Kovac, J.R. Recent advances in the understanding and management of ED. F1000Research 2019, 8, 102. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, X.; Shi, L.; Gao, Z. Efficacy and safety of phosphodiesterase type 5 (PDE5) inhibitors in treating ED after bilateral nerve-sparing radical prostatectomy. Andrologia 2016, 48, 20–28. [Google Scholar] [CrossRef]
- Jiang, H.; Lin, H.; Li, F.; Dai, Y.; Zhang, X.; Jiang, T.; Deng, J. Efficacy and Safety of Avanafil in Chinese Subjects with ED: A Multi-Center, Randomized, Double-Blinded, Placebo-Controlled Phase III Clinical Trial. Sex. Med. 2021, 9, 100337. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.J.; Hung, S.-Y.; Lee, T.-H.; Jiann, B.-P. A real-world pilot study assessing treatment satisfaction with avanafil in patients with ED. Sex. Med. 2024, 12, qfae001. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Kwon, T.G.; Kwak, C.; Sung, G.T.; Kim, S.D.; Cho, J.S.; Kim, H.J.; Ahn, H.; Jeon, S.S. Efficacy and Safety of Udenafil Once Daily in Patients with ED after Bilateral Nerve-Sparing Robot-Assisted Laparoscopic Radical Prostatectomy: A Randomized, Double-Blind, Placebo-Controlled Study. World J. Men’s Health 2023, 41, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Tema, G.; De Nunzio, C. Efficacy and safety of udenafil for the treatment of ED after total mesorectal excision of rectal cancer: A randomized, double-blind, placebo-controlled trial. Surgery 2015, 157, 64–71. [Google Scholar] [CrossRef]
- Perez-Aizpurua, X.; Garranzo-Ibarrola, M.; Simón-Rodríguez, C.; García-Cardoso, J.V.; Chávez-Roa, C.; López-Martín, L.; Tufet i Jaumot, J.J.; Alonso-Román, J.; Maqueda-Arellano, J.; Gómez-Jordana, B.; et al. Stem Cell Therapy for ED: A Step towards a Future Treatment. Life 2023, 13, 502. [Google Scholar] [CrossRef]
- Siregar, S.; Novesar, A.R.; Mustafa, A. Application of Stem Cell in Human ED—A Systematic Review. Res. Rep. Urol. 2022, 14, 379–388. [Google Scholar]
- Protogerou, V.; Michalopoulos, E.; Mallis, P.; Gontika, I.; Dimou, Z.; Liakouras, C.; Stavropoulos-Giokas, C.; Kostakopoulos, N.; Chrisofos, M.; Deliveliotis, C. Administration of Adipose Derived Mesenchymal Stem Cells and Platelet Lysate in ED: A Single Center Pilot Study. Bioengineering 2019, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Argiolas, A.; Argiolas, F.M.; Argiolas, G.; Melis, M.R. ED: Treatments, Advances and New Therapeutic Strategies. Brain Sci. 2023, 13, 802. [Google Scholar] [CrossRef]
- Andersson, K.E.; Christ, G.J. Gene Therapy in ED: Dead or Alive? J. Sex. Med. 2020, 17, 1587–1589. [Google Scholar] [CrossRef]
- Hassanin, A.M.; Abdel-Hamid, A.Z. Cavernous smooth muscles: Innovative potential therapies are promising for an unrevealed clinical diagnosis. Int. Urol. Nephrol. 2020, 52, 205–217. [Google Scholar] [CrossRef]
- Jiang, N.; Wu, C.; Zhou, X.; Zhai, G.; Wu, J. Cavernous Nerve Injury Resulted ED and Regeneration. J. Immunol. Res. 2021, 2021, 5353785. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.F.; Wang, S.H.; Xu, S. [Low-intensity extracorporeal shock wave therapy for ED: An update]. Zhonghua Nan Ke Xue 2023, 29, 364–368. [Google Scholar] [PubMed]
- Bocchino, A.C.; Pezzoli, M.; Martínez-Salamanca, J.I.; Russo, G.I.; Giudice, A.L.; Cocci, A. Low-intensity extracorporeal shock wave therapy for ED: Myths and realities. Investig. Clin. Urol. 2023, 64, 118–125. [Google Scholar] [CrossRef]
- Sokolakis, I.; Dimitriadis, F.; Teo, P.; Hatzichristodoulou, G.; Hatzichristou, D.; Giuliano, F. The Basic Science Behind Low-Intensity Extracorporeal Shockwave Therapy for ED: A Systematic Scoping Review of Pre-Clinical Studies. J. Sex. Med. 2019, 16, 168–194. [Google Scholar] [CrossRef] [PubMed]
- Myers, C.; Smith, M. Pelvic floor muscle training improves ED and premature ejaculation: A systematic review. Physiotherapy 2019, 105, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Gonzalez, J.; Goldstein, I. The Role of Pelvic Floor Muscles in Male Sexual Dysfunction and Pelvic Pain. Sex. Med. Rev. 2016, 4, 53–62. [Google Scholar] [CrossRef]
- Yaacov, D.; Nelinger, G.; Kalichman, L. The Effect of Pelvic Floor Rehabilitation on Males with Sexual Dysfunction: A Narrative Review. Sex. Med. Rev. 2022, 10, 162–167. [Google Scholar] [CrossRef]
- Pischedda, A.; Fusco, F.; Curreli, A.; Grimaldi, G.; Farina, F.P. Pelvic floor and sexual male dysfunction. Arch. Ital. Urol. Androl. 2013, 85, 1–7. [Google Scholar] [CrossRef]
- Pan, L.H.; Lin, M.-H.; Pang, S.-T.; Wang, J.; Shih, W.-M. Improvement of Urinary Incontinence, Life Impact, and Depression and Anxiety with Modified Pelvic Floor Muscle Training After Radical Prostatectomy. Am. J. Men’s Health 2019, 13, 1557988319851618. [Google Scholar] [CrossRef]
- Gbiri, C.A.O.; Akumabor, J.C. Effectiveness of Physiotherapy Interventions in the Management Male Sexual Dysfunction: A Systematic Review. Int. J. Sex. Health 2023, 35, 52–66. [Google Scholar] [CrossRef]
- Park, J.J.; Doo, S.W.; Kwon, A.; Kim, D.K.; Yang, W.J.; Song, Y.S.; Shim, S.R.; Kim, J.H. Effects of Sexual Rehabilitation on Sexual Dysfunction in Patients with Cardiovascular Disease: A Systematic Review and Meta-Analysis. World J. Men’s Health 2023, 41, 330–341. [Google Scholar]
- Kannan, P.; Winser, S.J.; Ho, L.C.; Hei, L.C.; Kin, L.C.; E Agnieszka, G.; Jeffrey, L.H. Effectiveness of physiotherapy interventions for improving erectile function and climacturia in men after prostatectomy: A systematic review and meta-analysis of randomized controlled trials. Clin. Rehabil. 2019, 33, 1298–1309. [Google Scholar] [PubMed]
- Kim, J.K.; Lee, Y.J.; Kim, H.; Song, S.H.; Jeong, S.J.; Byun, S.-S. A prospectively collected observational study of pelvic floor muscle strength and erectile function using a novel personalized extracorporeal perineometer. Sci. Rep. 2021, 11, 18389. [Google Scholar] [CrossRef]
- Chen, G.; Yang, L.; Suliya, Y.; Dai, Y.; Qu, R. Effects of low-intensity pulsed ultrasound and biofeedback electric stimulation on the patient with ED. Continence 2023, 7, 101035. [Google Scholar]
- Ruan, Z.; Xie, X.; Yu, H.; Liu, R.; Jing, W.; Lu, T. Association between dietary inflammation and ED among US adults: A cross-sectional analysis of the National Health and Nutrition Examination Survey 2001–2004. Front. Nutr. 2022, 9, 930272. [Google Scholar]
- ElJalby, M.; Thomas, D.; Elterman, D.; Chughtai, B. The effect of diet on BPH, LUTS and ED. World J. Urol. 2019, 37, 1001–1005. [Google Scholar] [PubMed]
- Mostafaei, H.; Mori, K.; Hajebrahimi, S.; Abufaraj, M.; Karakiewicz, P.I.; Shariat, S.F. Association of ED and cardiovascular disease: An umbrella review of systematic reviews and meta-analyses. BJU Int. 2021, 128, 3–11. [Google Scholar] [PubMed]
- Orimoloye, O.A.; Feldman, D.I.; Blaha, M.J. ED links to cardiovascular disease—Defining the clinical value. Trends Cardiovasc. Med. 2019, 29, 458–465. [Google Scholar] [CrossRef]
- Pozzi, E.; Capogrosso, P.; Boeri, L.; Belladelli, F.; Baudo, A.; Schifano, N.; Abbate, C.; Dehò, F.; Montorsi, F.; Salonia, A. Longitudinal Risk of Developing Cardiovascular Diseases in Patients with ED-Which Patients Deserve More Attention? J. Sex. Med. 2020, 17, 1489–1494. [Google Scholar] [CrossRef]
- Ostfeld, R.J.; Allen, K.E.; Aspry, K.; Brandt, E.J.; Spitz, A.; Liberman, J.; Belardo, D.; O’Keefe, J.H.; Aggarwal, M.; Miller, M.; et al. Vasculogenic ED: The Impact of Diet and Lifestyle. Am. J. Med. 2021, 134, 310–316. [Google Scholar] [CrossRef]
- da Silva Schmitt, C.; da Costa, C.M.; Souto, J.C.S.; Chiogna, L.M.; Santos, Z.E.d.A.; Rhoden, E.L.; Neto, B.S. The effects of a low carbohydrate diet on erectile function and serum testosterone levels in hypogonadal men with metabolic syndrome: A randomized clinical trial. BMC Endocr. Disord. 2023, 23, 30. [Google Scholar] [CrossRef]
- Angelis, A.; Chrysohoou, C.; Tzorovili, E.; Laina, A.; Xydis, P.; Terzis, I.; Ioakeimidis, N.; Aznaouridis, K.; Vlachopoulos, C.; Tsioufis, K. The Mediterranean Diet Benefit on Cardiovascular Hemodynamics and Erectile Function in Chronic Heart Failure Male Patients by Decoding Central and Peripheral Vessel Rheology. Nutrients 2020, 13, 108. [Google Scholar] [CrossRef]
- Huynh, L.M.; Liang, K.; Osman, M.M.; El-Khatib, F.M.; Dianatnejad, S.; Towe, M.; Roberts, N.H.; Yafi, F.A. Organic Diet and Intermittent Fasting are Associated with Improved Erectile Function. Urology 2020, 144, 147–151. [Google Scholar] [CrossRef]
- Sultan, M.I.; Ibrahim, S.A.; Youssef, R.F. Impact of a Mediterranean diet on prevention and management of urologic diseases. BMC Urol. 2024, 24, 48. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Lee, M.S.; Kim, T.H.; Alraek, T.; Zaslawski, C.; Kim, J.W.; Moon, D.G. Ginseng for ED. Cochrane Database Syst. Rev. 2021, 4, CD012654. [Google Scholar] [PubMed]
- Rebez, G.; Capogrosso, P.; Boeri, L.; Rizzo, M.; Miacola, C.; Cai, T.; Palumbo, F.; Ortensi, I.; Ceruti, C.; Lauretti, S.; et al. Effectiveness of Ginseng, Rutin and Moringa for the Treatment of ED: A Systematic Review. Uro 2022, 2, 65. [Google Scholar] [CrossRef]
- Leisegang, K.; Finelli, R. Alternative medicine and herbal remedies in the treatment of ED: A systematic review. Arab. J. Urol. 2021, 19, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, J.; Ma, D.; Zhao, Z.; Yan, B.; Wang, F. The role of red ginseng in men’s reproductive health: A literature review. Basic Clin. Androl. 2023, 33, 27. [Google Scholar] [CrossRef]
- Ghamari, K.; Kashani, L.; Jafarinia, M.; Najafabadi, B.T.; Shokraee, K.; Esalatmanesh, S.; Akhondzadeh, S. Vitamin E and ginseng supplementation to enhance female sexual function: A randomized, double-blind, placebo-controlled, clinical trial. Women Health 2020, 60, 1164–1173. [Google Scholar] [CrossRef]
- Jabir, N.R.; Firoz, C.K.; Zughaibi, T.A.; Alsaadi, M.A.; Abuzenadah, A.M.; Al-Asmari, A.I.; Alsaieedi, A.; Ahmed, B.A.; Ramu, A.K.; Tabrez, S. A literature perspective on the pharmacological applications of yohimbine. Ann. Med. 2022, 54, 2861–2875. [Google Scholar] [CrossRef]
- Pyke, R.E. Sexual Performance Anxiety. Sex. Med. Rev. 2020, 8, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cui, Y.; Zhou, Z.; Zhao, H.; Zhang, Y. Analysis of pharmacological mechanisms of Yinyanghuo as treatment of ED with network pharmacology-based strategy. Andrologia 2021, 53, e13943. [Google Scholar] [CrossRef]
- Song, W.; Yuan, Y.; Tan, X.; Gu, Y.; Zeng, J.; Song, W.; Guan, R. Icariside II induces rapid phosphorylation of endothelial nitric oxide synthase via multiple signaling pathways. PeerJ 2022, 10, e14192. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wu, Q.; Li, L.; Gong, J.; Huo, R.; Cui, W. Icariside II: Anticancer Potential and Molecular Targets in Solid Cancers. Front. Pharmacol. 2021, 12, 663776. [Google Scholar] [CrossRef]
- Di Lorenzo, G.; Verde, A.; Scafuri, L.; Costabile, F.; Caputo, V.; Di Trolio, R.; Strianese, O.; Montanaro, V.; Crocetto, F.; Del Giudice, F.; et al. The Impact of Flavonoid Supplementation on Serum Oxidative Stress Levels Measured via D-ROMs Test in the General Population: The PREVES-FLAVON Retrospective Observational Study. Nutrients 2024, 16, 3302. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Franz, M.; Rimm, E.B. Dietary flavonoid intake and incidence of ED. Am. J. Clin. Nutr. 2016, 103, 534–541. [Google Scholar] [CrossRef]
- Mykoniatis, I.; Grammatikopoulou, M.G.; Bouras, E.; Karampasi, E.; Tsionga, A.; Kogias, A.; Vakalopoulos, I.; Haidich, A.-B.; Chourdakis, M. Sexual Dysfunction Among Young Men: Overview of Dietary Components Associated with ED. J. Sex. Med. 2018, 15, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Cocera, R.; Torremade, J.; Suarez, J.F.; Fernandez-Concha, J.; Vigues, F. Comparative analysis of penile implants in patients with vasculogenic ED versus postradical prostatectomy ED. Int. J. Impot. Res. 2020, 32, 606–610. [Google Scholar] [CrossRef]
- Alamri, H.; Alsharyoufi, M.; Alkhathami, F.; Alsayed, R.; Alzaidy, A.; Albejawi, A.; Allam, A.; Alqubali, S. Malleable versus inflatable penile prosthesis: Systematic review. Int. J. Med. Dev. Ctries. 2023, 7, 1549–1555. [Google Scholar] [CrossRef]
- Menard, J.; Tremeaux, J.-C.; Faix, A.; Pierrevelcin, J.; Staerman, F. Erectile function and sexual satisfaction before and after penile prosthesis implantation in radical prostatectomy patients: A comparison with patients with vasculogenic ED. J. Sex. Med. 2011, 8, 3479–3486. [Google Scholar] [CrossRef]
- Manfredi, C.; Fortier, É.; Faix, A.; Martínez-Salamanca, J.I. Penile Implant Surgery Satisfaction Assessment. J. Sex. Med. 2021, 18, 868–874. [Google Scholar]
- Singh, A.; Cooper, C.A.; Hou, S.W.; Raheem, O.A. A Systematic Review of Partner Satisfaction After Penile Prosthesis with Special Emphasis on LGBTQ + Populations. Curr. Urol. Rep. 2023, 24, 105–115. [Google Scholar]
- Pazir, Y.; Yanaral, F.; Caglar, U.; Ortac, M.; Sarilar, O.; Ozgor, F. Evaluation of Satisfaction and Outcomes of Patients Who Underwent Two-Piece Inflatable Penile Prosthesis Implantation. Cureus 2022, 14, e26097. [Google Scholar] [PubMed]
- Ragheb, A.; Osmonov, D.; Abdallah, M.; Elmarakbi, A.; Massoud, A.; Emadeldin, M.; Abdelbary, A. (472) The AM-EDITS: A New Tool for Evaluating the Arab Patient and Partner Satisfaction Following Penile Prosthesis Implantation. J. Sex. Med. 2023, 20 (Suppl. S1), qdad060-444. [Google Scholar]
- Jones, P.; Barba, H.S.; I Johnson, M.; Soomro, N.; Robson, W.; Ferguson, J.; Aning, J.J. ED after robotic radical prostatectomy: Real-life impact of vacuum erection device clinic. J. Clin. Urol. 2021, 14, 325–331. [Google Scholar] [CrossRef]
- Canguven, O.; Talib, R.A.; Campbell, J.; De Young, L.; El Ansari, W.; Al-Ansari, A. Is the daily use of vacuum erection device for a month before penile prosthesis implantation beneficial? a randomized controlled trial. Andrology 2017, 5, 103–106. [Google Scholar] [PubMed]
- Khan, S.; Amjad, A.; Rowland, D. Potential for Long-Term Benefit of CBT as an Adjunct Treatment for Men with ED. J. Sex. Med. 2019, 16, 300–306. [Google Scholar]
- Bilal, A.; Abbasi, N.U.H. Development of an indigenous manual of cognitive behavior sex therapy for young men. J. Fam. Med. Prim. Care 2022, 11, 4127–4130. [Google Scholar] [CrossRef]
- Han, M.; Wang, X.; Yang, H.; Wang, X.; Zhu, H.; Song, M. Efficacy of online CBT for nonorganic ED in reproductive-age males during the COVID-19 pandemic: A randomized wait list-controlled trial. J. Sex. Med. 2023, 20, 1325–1332. [Google Scholar] [CrossRef]
- Dewitte, M.; Bettocchi, C.; Carvalho, J.; Corona, G.; Flink, I.; Limoncin, E.; Pascoal, P.; Reisman, Y.; Van Lankveld, J. A Psychosocial Approach to ED: Position Statements from the European Society of Sexual Medicine (ESSM). Sex. Med. 2021, 9, 100434. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonarska, M.; Adasik, D.; Szymczyk, S.; Łocik, G.; Bumbul-Mazurek, E.; Marianowski, P.; Ludwin, A. A Narrative Review of Independent Treatment Methods for ED: Assessment of the Effectiveness of Diet, Supplements, Pharmacotherapy, and Physiotherapy. J. Clin. Med. 2025, 14, 2386. https://doi.org/10.3390/jcm14072386
Bonarska M, Adasik D, Szymczyk S, Łocik G, Bumbul-Mazurek E, Marianowski P, Ludwin A. A Narrative Review of Independent Treatment Methods for ED: Assessment of the Effectiveness of Diet, Supplements, Pharmacotherapy, and Physiotherapy. Journal of Clinical Medicine. 2025; 14(7):2386. https://doi.org/10.3390/jcm14072386
Chicago/Turabian StyleBonarska, Marta, Damian Adasik, Simone Szymczyk, Gabriela Łocik, Elżbieta Bumbul-Mazurek, Piotr Marianowski, and Artur Ludwin. 2025. "A Narrative Review of Independent Treatment Methods for ED: Assessment of the Effectiveness of Diet, Supplements, Pharmacotherapy, and Physiotherapy" Journal of Clinical Medicine 14, no. 7: 2386. https://doi.org/10.3390/jcm14072386
APA StyleBonarska, M., Adasik, D., Szymczyk, S., Łocik, G., Bumbul-Mazurek, E., Marianowski, P., & Ludwin, A. (2025). A Narrative Review of Independent Treatment Methods for ED: Assessment of the Effectiveness of Diet, Supplements, Pharmacotherapy, and Physiotherapy. Journal of Clinical Medicine, 14(7), 2386. https://doi.org/10.3390/jcm14072386





