Effectiveness of Combined Cognitive Stimulation and Physical Activity Interventions on Activities of Daily Living, Cognitive Function, and Physical Function in Older People with Mild Cognitive Impairment: A Systematic Review with Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Search Process and Databases
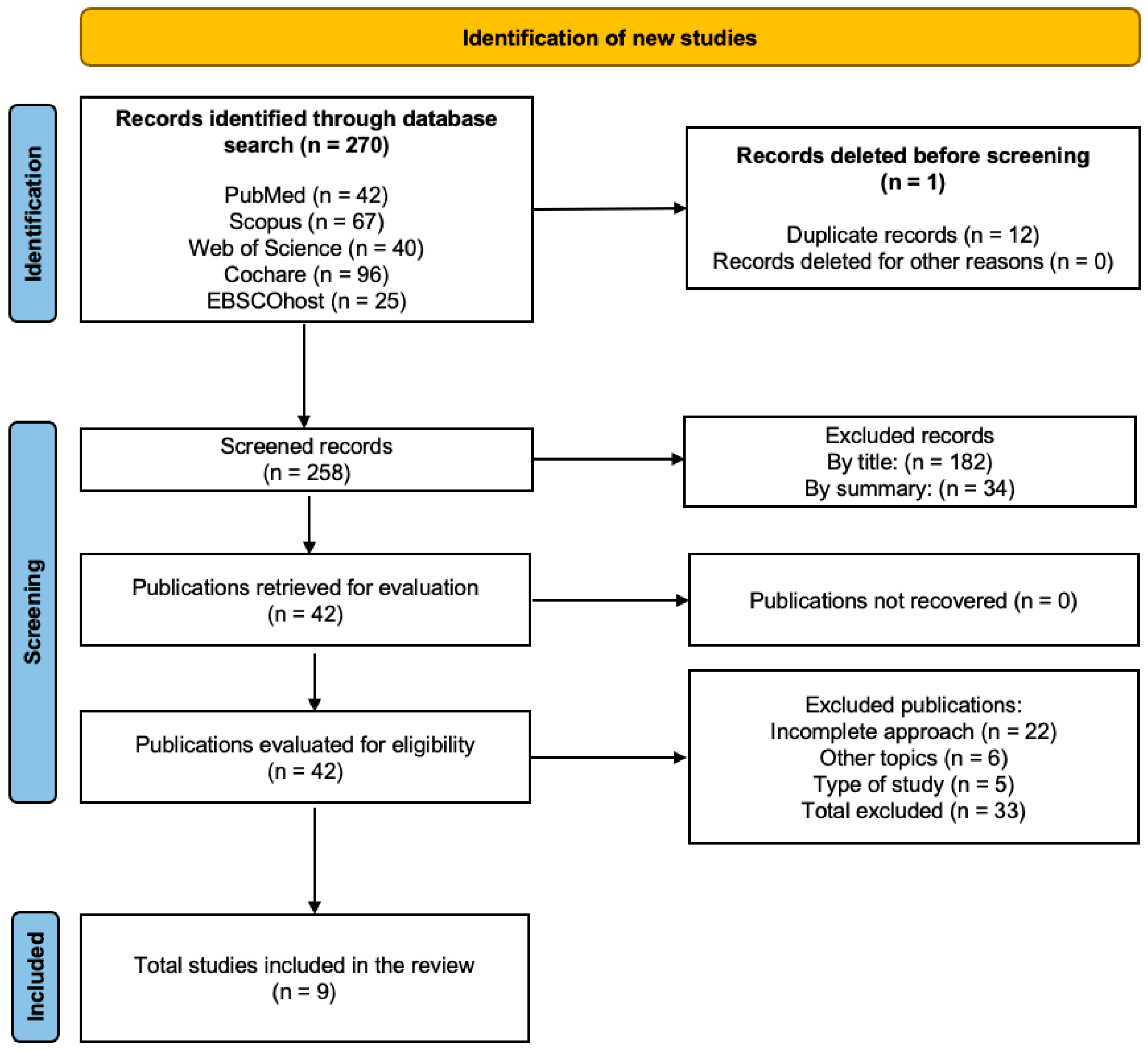
2.4. Study Selection and Data Collection Process
2.5. Methodological Quality Assessment
2.6. Data Collection Process
2.7. Risk of Bias Assessment
2.8. Measures for Meta-Analysis
2.9. Certainty of Evidence
3. Results
3.1. Methodological Quality
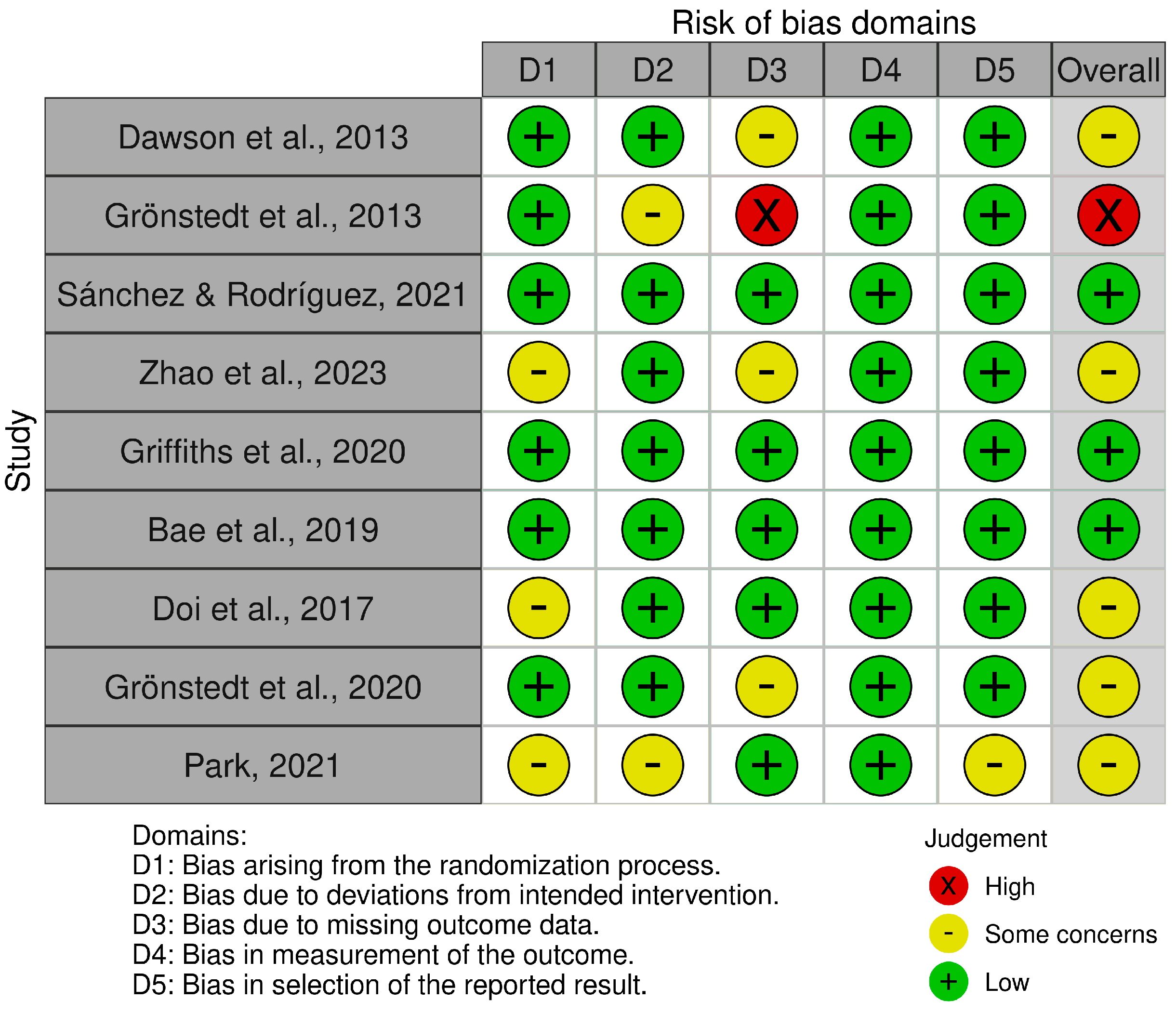
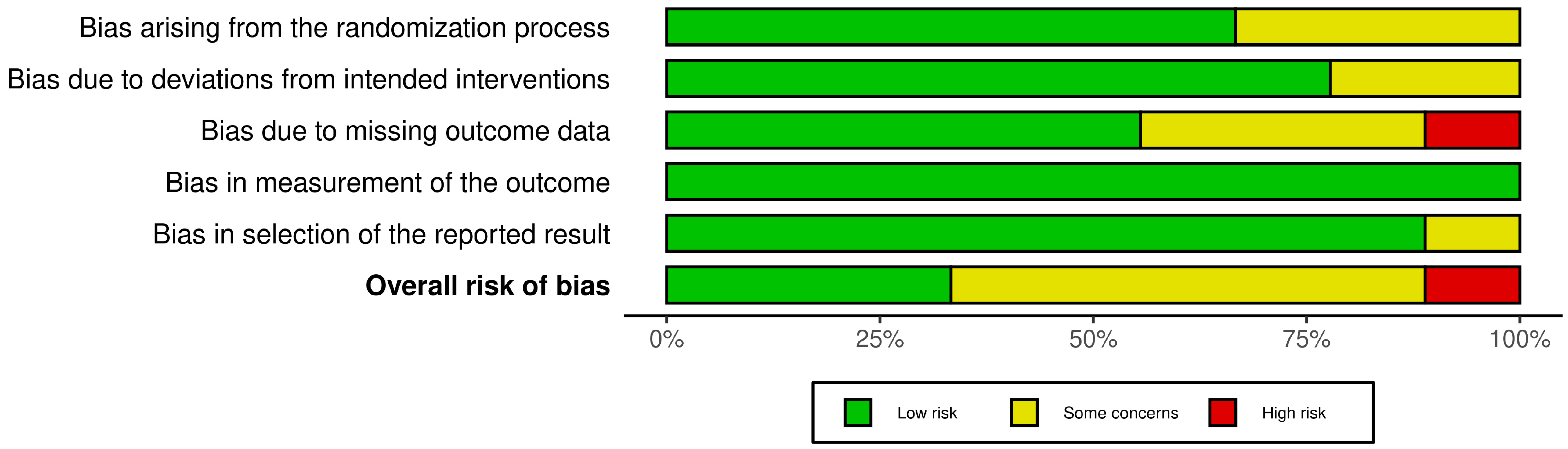
3.2. Risk of Bias
3.3. Characteristics of the Studies
| Study | Country or Multicenter | Study Design | Sample | Groups (n) | Mean Age (Years) | Type of Intervention and Control Group | Training Volume | Training Intensity | Cognitive Function (Assessment) | Physical Function (Assessments) | ADL (Assessments) | Main Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weeks | Frequency (Sessions /Week) | Session Duration (Minutes) | ||||||||||||
| Dawson et al. [30] | CA | RCT | Healthy older adults with cognitive complaints | EG: 10 CG: 9 | EG: 74.10 (8.77), 90% female. CG: 73.67 (5.43), 78% female | EG: education about self-management and occupation-based meta-cognitive strategy training CG: education brain health and cognitively stimulating exercises | 8 | 2 | 60 | Moderate | General Self-Efficacy Scale, D-KEFS Tower Test, verbal fluency | Stanford Chronic Disease Questionnaire | COPM | Both groups: ↔ ADL (COPM) (p = 0.54) EG: ↑ Word fluency (p = 0.01) ↑ Untrained everyday life problems (p = 0.03) CG: ↓ Communication with physicians (p = 0.02) ↓ Physical activity (p = 0.02) |
| Grönstedt et al. [31] | Multicenter | RCT | Subjects diagnosed with MCI | EG: 170 CG: 152 | EG: 85, 71% female CG: 87.74, 76% female | EG: individual physical training and group activities such as outdoor walks and personal care, clothing and nutrition GC: standard care (without a specific focus on physical rehabilitation) | 10 | 3 | 30 | Moderate | MMSE | Berg Balance Scale, timed CST, Short Falls Efficacy Scale | FIM | Both groups: ↔ ADL (FIM) (p = 0.293) EG: ↑ Balance (p = 0.001) ↑ Physical activity (p = 0.038) ↑ Transfers (p = 0.024) CG: ↓ ADL (p = 0.012) ↓ Balance (p = 0.004) ↓ Deterioration in transfers (p = 0.023) |
| Sánchez & Rodríguez [32] | SP | RCT | Older adults with MCI | EG: 137 CG: 130 | EG: 73.89, 83.90% female CG: 72.99, 83.10% female | EG: Everyday Cognition Training Program CG: conventional cognitive training program | 10 | 2 | 50 | Moderate | ERFC | NR | ECB | EG: ↑ Cognitive performance (ERFC) between 1-PRE and 8-POST (p < 0.001) ↑ ECB between 1-PRE and 8-POST (p < 0.001) CG: ↓ Cognitive performance (ERFC) between 1-PRE and 8-POST (p < 0.001) ↓ ECB between 1-PRE and 8-POST (p < 0.001) |
| Zhao et al. [33] | USA | RCT | Older sedentary adults with T2DM and cognitive impairment | EG: 32 CG: 40 | EG: 66.1, 50% female GC: 65.9, 50% female | EG: combined aerobic and resistance exercise program CG: sedentary healthy older adults with no specific intervention | 10 | 3 | 30 | Moderate | Mini-Cog, TMT | NR | NR | Cognitive Performance: ↔ Mini-Cog (p = 0.0005) ↔ TMT-A (p = 0.006) ↔ TMT-B (p < 0.001) EG: ↑ Mini-Cog scores (p = 0.005) ↑ TMT-A (p = 0.006) ↑ TMT-B (p < 0.001) CG: ↓ Mini-Cog performance (p = 0.005) ↓ TMT-A (p = 0.006) ↓ Deterioration in TMT-B (p < 0.001) Physical Fitness Outcomes: Both Groups: ↔ 6-MWT (p = 0.293) EG: ↑ 6-MWT distance (p < 0.01) |
| Griffiths et al. [34] | TH | RCT | Older adult and people with MCI | EG: 35 CG: 35 | EG: 65.14, 74% female CG: 67.23, 72% female | EG: combined physical movement activity and multifaceted cognitive training CG: waitlist control (standard care) | 12 | 2 | NR | Moderate | TMT, DSF, DSB, DSS | 10 m walking, grip strength, timed CST | NR | EG: ↑ Attention (TMT-A) (p = 0.023) ↑ Executive function (BD) (p = 0.029) ↑ LVF (p = 0.001) ↑ CVF (p = 0.004) ↑ WLL imm (p = 0.023) ↑ WLL delayed (p = 0.036) CG: ↔ Stable performance in attention (TMT-A) (p = 0.293) ↔ No significant changes in executive function (BD) (p = 0.036) ↔ Deterioration in verbal fluency (LVF) (p = 0.036) ↔ Deterioration in WLL imm (p = 0.012) |
| Bae et al. [35] | Multicenter | RCT | Adults diagnosed with MCI | EG: 41 CG: 42 | EG: 76.4, 61% female CG: 75.5, 43.9% female | EG: multicomponent intervention (community activity program) CG: health education classes | 24 | 2 | 90 | Moderate to vigorous | NCGG-FAT (memory, attention, executive function, processing speed), MMSE, Word Recall Test, TMT | Grip strength, 10 m walking speed, timed CST, MVPA, step count | NR | EG: ↑ TMT-A (p = 0.001) ↑ Working memory scores (p = 0.010) ↑ MVPA (p = 0.048) ↑ Step count (p = 0.059) CG: ↔ Walking speed (p = 0.099) ↔ Grip strength (p = 0.136) ↔ MMSE (p = 0.434) EG: ↑ TMT-A (p = 0.001) ↑ Working memory scores (p = 0.010) ↑ MVPA (p = 0.048) ↑ Step count (p = 0.059) CG: ↔ Walking speed (p = 0.099) ↔ Grip strength (p = 0.136) ↔ MMSE score (p = 0.434) |
| Doi et al. [36] | JP | RCT | Subjects diagnosed with MCI | EG: 109 CG: 67 | EG: 75.7, 50.7% female 76.0% 58.2% female | EG: cognitive leisure activities (dance or music) CG: health education | 40 | 1 | 60 | Low | TMT-A, TMT-B, MMSE | NR | NR | EG: ↑ MMSE scores compared to CG (dance: p = 0.026, music: p = 0.008) ↔ TMT-A and TMT-B scores compared to the CG |
| Grönstedt et al. [37] | Multicenter | RCT | Older adults with MCI | EG: 35 CG: 35 | EG: 85.9, 62% female CG: 85.9, 58% female | EG: sit-to-Stand exercises in conjunction with the ADLs combined with protein-rich oral supplementation CG: standard care (without a specific focus on physical rehabilitation) | 12 | 7 | Variable (integrated into daily activities) | MMSE | 10 m walking speed, grip strength, timed CST | FIM | Both groups ↔ 30sCST (p = 0.325) ↔ ADL (FIM) (p = 0.55) ↔ Quality of Life (EQ5D-5L) (p = 0.59) EG: ↑ Nutritional status (p = 0.007) ↑ Fat-free mass (p = 0.007) CG: ↓ Balance (Berg Balance Scale) (p = 0.10) ↓ Walking speed (p = 0.10) | |
| Park [38] | KR | RCT | Older adults with MCI | EG: 18, CG: 18 | EG: 74.00, 50% female; CG: 74.00, 50%female | EG: cognitive–physical dual-task training; CG: single cognitive training focused on executive function | 8 | 2 | 40 | Moderate | TMT-B | NR | K-IADL | Both groups: ↔ K-IADL (p > 0.05) EG: ↑ TMT-B performance (p < 0.001) ↓ PFC activity during TMT-B (p < 0.001) CG: ↑ TMT-B performance (p < 0.001) ↓ PFC activity during TMT-B (p < 0.001) |
3.4. Sample Characteristics
3.5. Dosages and Interventions Performed
3.6. Activities of Daily Living
3.7. Cognitive Function
3.8. Physical Function
3.9. Certainty of Evidence
3.10. Adverse Effects and Adherence
4. Discussion
4.1. Activities of Daily Living
4.2. Cognitive Function
4.3. Physical Function
4.4. Strengths and Limitations
4.5. Practical Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Dementia. Available online: https://www.who.int/es/news-room/fact-sheets/detail/dementia (accessed on 4 July 2023).
- Forbes, C.C.; Swan, F.; Greenley, S.L.; Lind, M.; Johnson, M.J. Physical activity and nutrition interventions for older adults with cancer: A systematic review. J. Cancer Surviv. 2020, 14, 689–711. [Google Scholar] [CrossRef]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sports Med. 2018, 52, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kramer, A.F. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [PubMed]
- Szuhany, K.L.; Bugatti, M.; Otto, M.W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res. 2015, 60, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Gates, N.; Singh, M.A.F.; Sachdev, P.S.; Valenzuela, M. The effect of exercise training on cognitive function in older adults with mild cognitive impairment: A meta-analysis of randomized controlled trials. Am. J. Geriatr. Psychiatry 2019, 21, 1086–1097. [Google Scholar] [CrossRef]
- Yi, Q.; Liu, Z.; Zhong, F.; Selvanayagam, V.S.; Cheong, J.P.G. Cognitive and physical impact of combined exercise and cognitive intervention in older adults with mild cognitive impairment: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0308466. [Google Scholar] [CrossRef]
- Bahar-Fuchs, A.; Martyr, A.; Goh, A.M.; Sabates, J.; Clare, L. Cognitive training for people with mild to moderate dementia. Cochrane Database Syst. Rev. 2019, 3, CD013069. [Google Scholar] [CrossRef]
- Gheysen, F.; Poppe, L.; De Smet, A.; Swinnen, S.P.; Cardon, G.; De Bourdeaudhuij, I.; Chastin, S. Physical activity to improve cognition in older adults: Can physical activity programs enriched with cognitive challenges enhance the effects? A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Chang, W.P.; Chang, M.C. Occupational therapy interventions to improve activities of daily living for community-dwelling older adults: A systematic review. Am. J. Occup. Ther. 2018, 72, 7204190060p1–7204190060p11. [Google Scholar] [CrossRef] [PubMed]
- Galassi, F.; Merizzi, A.; D’Amen, B.; Santini, S. Creativity and art therapies to promote healthy aging: A scoping review. Front. Psychol. 2022, 13, 906191. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Gao, Q.; Huo, L.; Yang, J. Impaired activity of daily living status of older adults and its influencing factors: A cross-sectional study. Int. J. Environ. Res. Public Health 2022, 19, 15607. [Google Scholar] [CrossRef]
- Fien, S.; Linton, C.; Mitchell, J.S.; Wadsworth, D.P.; Szabo, H.; Askew, C.D.; Schaumberg, M.A. Characteristics of community-based exercise programs for community-dwelling older adults in rural/regional areas: A scoping review. Aging Clin. Exp. Res. 2022, 34, 1511–1528. [Google Scholar] [CrossRef] [PubMed]
- Bermejo Ferrer, E.; López Aristica, M.A.; Santana Isaac, J.; Macías Lima, A.; Rodríguez Oropesa, Y.; González Toledo, E. Physical, functional and cognitive stimulation in older adults, based on recreational activity. Conrado 2021, 17, 120–135. [Google Scholar]
- León Rodríguez, J.; Ureña, A.; Bonnemaison, V. Design of a physical-cognitive exercise program for older people. J. Sport Health Res. 2014, 6, 269–282. [Google Scholar]
- Ballarín-Naya, L.; Malo, S.; Moreno-Franco, B. Efecto de intervenciones basadas en ejercicio físico y dieta sobre la evolución de deterioro cognitivo leve a demencia en sujetos mayores de 45 años. Rev. Esp. Salud Publica 2021, 95, e202102032. [Google Scholar] [PubMed]
- Dominguez-Rodriguez, A.; Gonzalez-Colaço Harmand, M.; Baez-Ferrer, N.; Abreu-Gonzalez, P. Going beyond the mortality: The forgotten quality of life in the very elderly. J. Am. Med. Dir. Assoc. 2018, 19, 554–555. [Google Scholar] [CrossRef]
- Suzuki, T.; Shimada, H.; Makizako, H.; Doi, T.; Yoshida, D.; Ito, K.; Kato, T. A randomized controlled trial of multicomponent exercise in older adults with mild cognitive impairment. PLoS ONE 2013, 8, e61483. [Google Scholar] [CrossRef] [PubMed]
- Karssemeijer, E.G.; Bossers, W.J.; Aaronson, J.A.; Sanders, L.M.; Kessels, R.P.; Olde Rikkert, M.G. The effect of an integrated physical and cognitive training on cognitive function in older adults with mild cognitive impairment: A meta-analysis. Aging Res. Rev. 2017, 40, 75–83. [Google Scholar] [CrossRef] [PubMed]
- De Coninck, L.; Bekkering, G.E.; Bouckaert, L.; Declercq, A.; Graff, M.J.L.; Aertgeerts, B. Home- and community-based occupational therapy improves functioning in frail older people: A systematic review. J. Am. Geriatr. Soc. 2017, 65, 1863–1869. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Chapter 8: Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Chichester, UK, 2023; version 6.4. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Alonso-Fernández, S. PRISMA 2020 statement: An updated guideline for the publication of systematic reviews. Rev. Esp. Cardiol. 2021, 74, 790–799. [Google Scholar] [CrossRef]
- Manterola, C.; Zavando, D.; GRUPO MINCIR. How to interpret the “Levels of Evidence” in different clinical scenarios. Chil. J. Surg. 2009, 61, 582–595. [Google Scholar] [CrossRef]
- Verhagen, A.P.; de Vet, H.C.; de Bie, R.A.; Kessels, A.G.; Boers, M.; Bouter, L.M.; Knipschild, P.G. The Delphi list: A criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J. Clin. Epidemiol. 1998, 51, 1235–1241. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration, John Wiley & Sons: Chichester, UK, 2008; version 5.0.0; Available online: https://training.cochrane.org/handbook (accessed on 16 November 2024).
- Davey, J.; Turner, R.M.; Clarke, M.J.; Higgins, J.P.T. Characteristics of meta-analyses and their component studies in the Cochrane Database of Systematic Reviews: A cross-sectional, descriptive analysis. BMC Med. Res. Methodol. 2011, 11, 160. [Google Scholar] [CrossRef]
- Morris, S.B.; DeShon, R.P. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol. Methods 2002, 7, 105–125. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Xie, C.X.; Machado, G.C. Clinimetrics: Grading of Recommendations, Assessment, Development and Evaluation (GRADE). J. Physiother. 2021, 67, 66. [Google Scholar] [CrossRef]
- Dawson, D.; Richardson, J.; Troyer, A.; Binns, M.; Clark, A.; Polatajko, H.; Winocur, G.; Hunt, A.; Bar, Y. An occupation-based strategy training approach to managing age-related executive changes: A pilot randomized controlled trial. Clin. Rehabil. 2014, 28, 118–127. [Google Scholar] [CrossRef]
- Grönstedt, H.; Frändin, K.; Bergland, A.; Helbostad, J.L.; Granbo, R.; Puggaard, L.; Andresen, M.; Hellström, K. Effects of individually tailored physical and daily activities in nursing home residents on activities of daily living, physical performance and physical activity level: A randomized controlled trial. Gerontology 2013, 59, 220–229. [Google Scholar] [CrossRef]
- Sánchez, C.S.; Rodríguez, E.J.F. The effectiveness of a training program in everyday cognition in healthy older adults: A randomized controlled trial. BMC Geriatr. 2021, 21, 79. [Google Scholar] [CrossRef]
- Zhao, F.; Tomita, M.; Dutta, A. Operational modal analysis of near-infrared spectroscopy measure of 2-month exercise intervention effects in sedentary older adults with diabetes and cognitive impairment. Brain Sci. 2023, 13, 1099. [Google Scholar] [CrossRef]
- Griffiths, J.; Thaikruea, L.; Wongpakaran, N.; Munkhetvit, P.; Kittisares, A.; Varnado, P. Effects of combined physical movement activity and multifaceted cognitive training in older people with mild neurocognitive disorder in a rural community: A randomized control trial. Dement. Geriatr. Cogn. Disord. 2020, 49, 194–201. [Google Scholar] [CrossRef]
- Bae, S.; Lee, S.; Lee, S.; Jung, S.; Makino, K.; Harada, K.; Shinkai, Y.; Chiba, I.; Shimada, H. The effect of a multicomponent intervention to promote community activity on cognitive function in older adults with mild cognitive impairment: A randomized controlled trial. Complement. Ther. Med. 2019, 42, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Verghese, J.; Makizako, H.; Tsutsumimoto, K.; Hotta, R.; Nakakubo, S.; Suzuki, T.; Shimada, H. Effects of cognitive leisure activity on cognition in mild cognitive impairment: Results of a randomized controlled trial. J. Am. Med. Dir. Assoc. 2017, 18, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Grönstedt, H.; Vikström, S.; Cederholm, T.; Franzén, E.; Luiking, Y.C.; Seiger, Å.; Wimo, A.; Faxén-Irving, G.; Boström, A.M. Effect of sit-to-stand exercises combined with protein-rich oral supplementation in older persons: The older person’s exercise and nutrition study. J. Am. Med. Dir. Assoc. 2020, 21, 1229–1237. [Google Scholar] [CrossRef]
- Park, J.H. Effects of cognitive-physical dual-task training on executive function and activity in the prefrontal cortex of older adults with mild cognitive impairment. Brain Neurorehabil. 2021, 14, e23. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimized digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Liu, C.J.; Xu, H.; Keith, N.R.; Clark, D.O. Promoting ADL independence in vulnerable, community-dwelling older adults: A pilot RCT comparing 3-Step Workout for Life versus resistance exercise. Clin. Interv. Aging 2017, 12, 1141–1149. [Google Scholar] [CrossRef]
- Liu-Ambrose, T.; Barha, C.K.; Best, J.R. Physical activity for brain health in older adults. Appl. Physiol. Nutr. Metab. 2018, 43, 1105–1112. [Google Scholar] [CrossRef]
- Jardim, N.Y.V.; Bento-Torres, N.V.O.; Costa, V.O.; Carvalho, J.P.R.; Pontes, H.T.S.; Tomás, A.M.; Sosthenes, M.C.K.; Erickson, K.I.; Bento-Torres, J.; Diniz, C.W.P. Dual-task exercise to improve cognition and functional capacity of healthy older adults. Front. Aging Neurosci. 2021, 13, 589299. [Google Scholar] [CrossRef]
- Yang, C.; Moore, A.; Mpofu, E.; Dorstyn, D.; Li, Q.; Yin, C. Effectiveness of combined cognitive and physical interventions to enhance functioning in older adults with mild cognitive impairment: A systematic review of randomized controlled trials. Gerontologist 2020, 60, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.Y.; Chen, I.H.; Lin, Y.J.; Chen, Y.; Hsu, W.C. Effects of virtual reality-based physical and cognitive training on executive function and dual-task gait performance in older adults with mild cognitive impairment: A randomized control trial. Front. Aging Neurosci. 2019, 11, 162. [Google Scholar] [CrossRef]
- Mlinac, M.E.; Feng, M.C. Assessment of activities of daily living, self-care, and independence. Arch. Clin. Neuropsychol. 2016, 31, 506–516. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, X.; Li, B.; Cai, Y.; Zhang, S.; Wan, Q.; Yu, F. Comparative efficacy of various exercise interventions on cognitive function in patients with mild cognitive impairment or dementia: A systematic review and network meta-analysis. J. Sport Health Sci. 2022, 11, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Yin, H.; Wang, S.; Shang, B.; Meng, X.; Yan, M.; Li, G.; Chu, J.; Chen, L. The effect of combined cognitive intervention and physical exercise on cognitive function in older adults with mild cognitive impairment: A meta-analysis of randomized controlled trials. Aging Clin. Exp. Res. 2022, 34, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Biazus-Sehn, L.F.; Schuch, F.B.; Firth, J.; Stigger, F.S. Effects of physical exercise on cognitive function of older adults with mild cognitive impairment: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2020, 89, 104048. [Google Scholar] [CrossRef]
- Salzman, T.; Sarquis-Adamson, Y.; Son, S.; Montero-Odasso, M.; Fraser, S. Associations of multidomain interventions with improvements in cognition in mild cognitive impairment: A systematic review and meta-analysis. JAMA Netw. Open 2022, 5, e226744. [Google Scholar] [CrossRef] [PubMed]
- Henskens, M.; Nauta, I.M.; van Eekeren, M.C.A.; Scherder, E.J.A. Effects of physical activity in nursing home residents with dementia: A randomized controlled trial. Dement. Geriatr. Cogn. Disord. 2018, 46, 60–80. [Google Scholar] [CrossRef] [PubMed]
- Law, L.L.; Barnett, F.; Yau, M.K.; Gray, M.A. Effects of combined cognitive and exercise interventions on cognition in older adults with and without cognitive impairment: A systematic review. Ageing Res. Rev. 2014, 15, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Castellote-Caballero, Y.; Carcelén Fraile, M.D.C.; Aibar-Almazán, A.; Afanador-Restrepo, D.F.; González-Martín, A.M. Effect of combined physical-cognitive training on the functional and cognitive capacity of older people with mild cognitive impairment: A randomized controlled trial. BMC Med. 2024, 22, 281. [Google Scholar] [CrossRef]
- Nascimento, C.M.; Pereira, J.R.; de Andrade, L.P.; Garuffi, M.; Talib, L.L.; Forlenza, O.V.; Cancela, J.M.; Cominetti, M.R.; Stella, F. Physical exercise in MCI elderly promotes reduction of pro-inflammatory cytokines and improvements on cognition and BDNF peripheral levels. Curr. Alzheimer Res. 2014, 11, 799–805. [Google Scholar] [CrossRef]

| Category | Inclusion | Exclusion |
|---|---|---|
| Population | Studies were included if they involved populations with a mean age of 60 years or older, with a diagnosis of MCI | Studies with populations whose main pathology was unrelated to MCI (i.e., chronic diseases, physical deterioration, or social problems). |
| Intervention | Studies involving OT interventions or programs combined with cognitive stimulation and physical activity | Studies whose focus is on interventions unrelated to OT interventions or cognitive stimulation and physical activity |
| Comparator | Interventions with active or inactive control groups | Studies lacking control groups or having only inactive control groups |
| Outcome | At least one assessment of ADL, cognitive function, or physical function. | Studies without baseline data and/or follow-ups |
| Study design | Randomized controlled trials, with pre- and post-assessment | Non-randomized, cross-sectional, retrospective, and prospective controlled studies |
| Level of evidence | 1a | 1b, 2a, 2b, 3a, 3b, 4, and 5 |
| Certainty of Evidence | Nº of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nº of Studies | Study Design | Risk Assessment | Inconsistency | Indirect Evidence | Vagueness | Other Considerations | [Conventional Therapy Plus Virtual Reality] | [Conventional Therapy] | Relative (95% CI) | Absolute (95% CI) | ||
| An occupation-based strategy training approach to managing age-related executive changes: a pilot randomized controlled trial | ||||||||||||
| 1 | RCT | Serious | It is not serious | It is not serious | It is not serious | None | 10/19 (52.6%) | 9/19 (47.4%) | Not estimable | +++ Moderate | IMPORTANT | |
| Effects of Individually Tailored Physical and Daily Activities in Nursing Home Residents on Activities of Daily Living, Physical Performance and Physical Activity Level: A Randomized Controlled Trial | ||||||||||||
| 1 | RCT | Very serious | It is not serious | It is not serious | It is not serious | None | 170/322 (52.8%) | 152/322 (47.5%) | Not estimable | ++ Low | IMPORTANT | |
| The Effectiveness of a Training Program in Everyday Cognition in Healthy Older Adults: A Randomized Controlled Trial | ||||||||||||
| 1 | RCT | It is not serious | It is not serious | It is not serious | It is not serious | None | 132/267 (51.3%) | 130/267 (48.7%) | Not estimable | ++++ High | IMPORTANT | |
| Operational Modal Analysis of Near-Infrared Spectroscopy Measure of 2-Month Exercise Intervention Effects in Sedentary Older Adults with and Cognitive Impairment | ||||||||||||
| 1 | RCT | Serious | It is not serious | It is not serious | It is not serious | None | 32/72 (44.4%) | 40/72 (55.6%) | Not estimable | +++ Moderate | IMPORTANT | |
| Effects of Combined Physical Movement Activity and Multifaceted Cognitive Training in Older People with Mild Neurocognitive Disorder in a Rural Community: A Randomized Control Trial | ||||||||||||
| 1 | RCT | It is not serious | It is not serious | It is not serious | It is not serious | None | 35/70 (50%) | 35/70 (50%) | Not estimable | ++++ High | IMPORTANT | |
| The Effect of a Multicomponent Intervention to Promote Community Activity on Cognitive Function in Older Adults with MCI: A randomized controlled trial | ||||||||||||
| 1 | RCT | It is not serious | It is not serious | It is not serious | It is not serious | None | 41/83 (49.4%) | 42/83 (50.6.%) | Not estimable | ++++ High | IMPORTANT | |
| Effects of Cognitive Leisure Activity on Cognition in Mild Cognitive Impairment: Results of a Randomized Controlled Trial | ||||||||||||
| 1 | RCT | Serious | It is not serious | It is not serious | It is not serious | None | 109/176 (61.9%) | 67/176 (38.1%) | Not estimable | +++ Moderate | IMPORTANT | |
| Effects of 6-Month Combined Physical Exercise and Cognitive Training on Neuropsychological and Neurophysiological Function in Older Adults with Subjective Cognitive Decline: A Randomized Controlled Trial | ||||||||||||
| 1 | RCT | Serious | It is not serious | It is not serious | It is not serious | None | 35/70 (50.0%) | 35/70 (50.0%) | Not estimable | +++ Moderate | IMPORTANT | |
| Effects of Cognitive–Physical Dual-Task Training on Executive Function and Activity in the Prefrontal Cortex of Older Adults with Mild Cognitive Impairment | ||||||||||||
| 1 | RCT | Serious | It is not serious | It is not serious | It is not serious | None | 18/36 (50.0%) | 18/36 (50.0%) | Not estimable | +++ Moderate | IMPORTANT | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vásquez-Carrasco, E.; Gómez, C.S.; Valdés-Badilla, P.; Hernandez-Martinez, J.; Villagrán-Silva, F.; Aravena-Sagardia, P.; Sandoval, C.; Miralles, P.M. Effectiveness of Combined Cognitive Stimulation and Physical Activity Interventions on Activities of Daily Living, Cognitive Function, and Physical Function in Older People with Mild Cognitive Impairment: A Systematic Review with Meta-Analysis. J. Clin. Med. 2025, 14, 2261. https://doi.org/10.3390/jcm14072261
Vásquez-Carrasco E, Gómez CS, Valdés-Badilla P, Hernandez-Martinez J, Villagrán-Silva F, Aravena-Sagardia P, Sandoval C, Miralles PM. Effectiveness of Combined Cognitive Stimulation and Physical Activity Interventions on Activities of Daily Living, Cognitive Function, and Physical Function in Older People with Mild Cognitive Impairment: A Systematic Review with Meta-Analysis. Journal of Clinical Medicine. 2025; 14(7):2261. https://doi.org/10.3390/jcm14072261
Chicago/Turabian StyleVásquez-Carrasco, Edgar, Celia Sánchez Gómez, Pablo Valdés-Badilla, Jordan Hernandez-Martinez, Francisca Villagrán-Silva, Pablo Aravena-Sagardia, Cristian Sandoval, and Pedro Moruno Miralles. 2025. "Effectiveness of Combined Cognitive Stimulation and Physical Activity Interventions on Activities of Daily Living, Cognitive Function, and Physical Function in Older People with Mild Cognitive Impairment: A Systematic Review with Meta-Analysis" Journal of Clinical Medicine 14, no. 7: 2261. https://doi.org/10.3390/jcm14072261
APA StyleVásquez-Carrasco, E., Gómez, C. S., Valdés-Badilla, P., Hernandez-Martinez, J., Villagrán-Silva, F., Aravena-Sagardia, P., Sandoval, C., & Miralles, P. M. (2025). Effectiveness of Combined Cognitive Stimulation and Physical Activity Interventions on Activities of Daily Living, Cognitive Function, and Physical Function in Older People with Mild Cognitive Impairment: A Systematic Review with Meta-Analysis. Journal of Clinical Medicine, 14(7), 2261. https://doi.org/10.3390/jcm14072261