Effectiveness of Exercise Loading on Bone Mineral Density and Quality of Life Among People Diagnosed with Osteoporosis, Osteopenia, and at Risk of Osteoporosis—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Data Collection Process
2.4. Assessment of Methodological Quality and Grading of Evidence
2.5. Assessment of Risk of Bias
2.6. Data Synthesis
3. Results
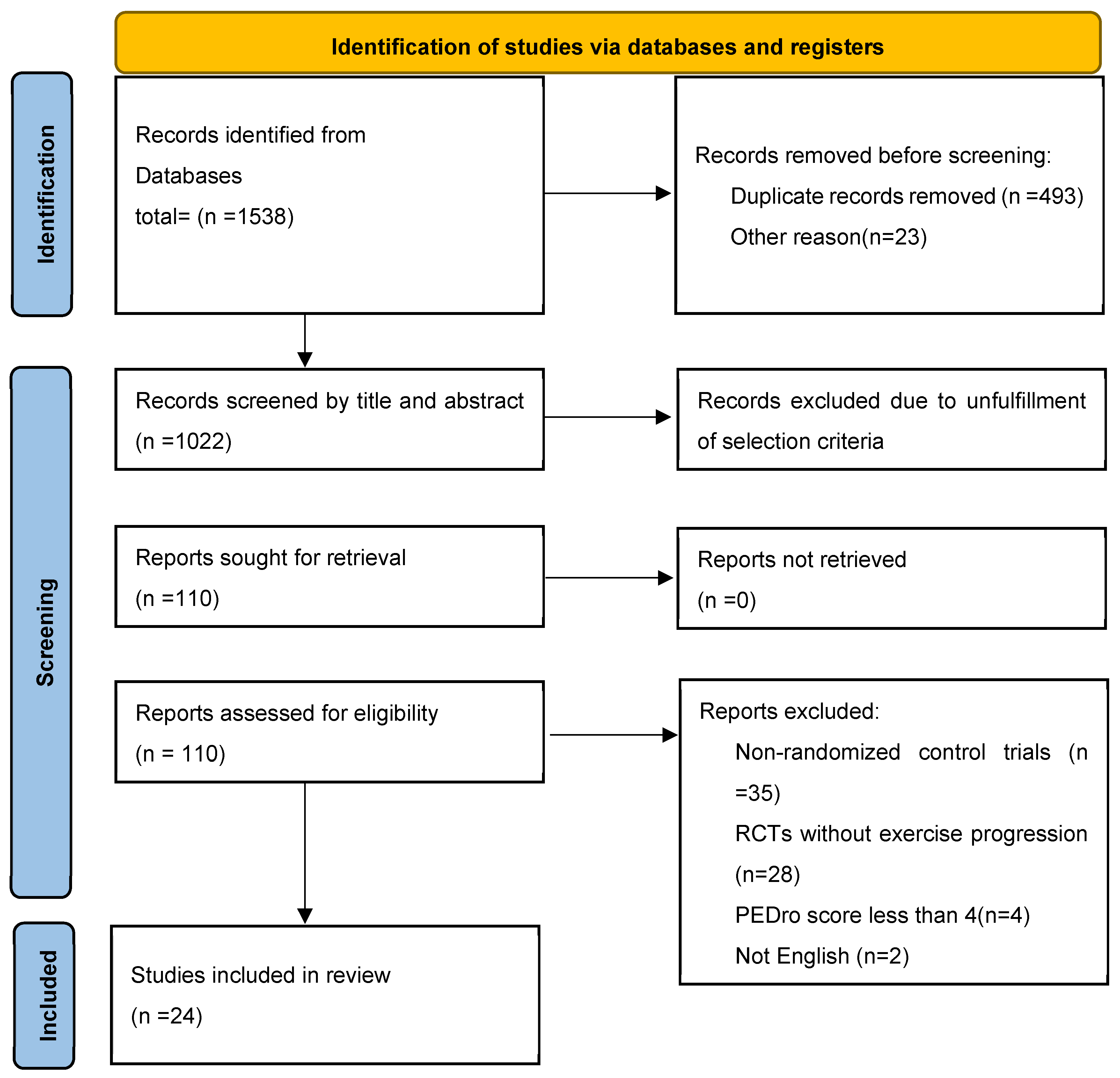
3.1. Search Outcomes
3.2. Characteristics of the Included Studies
3.3. Outcome Measures
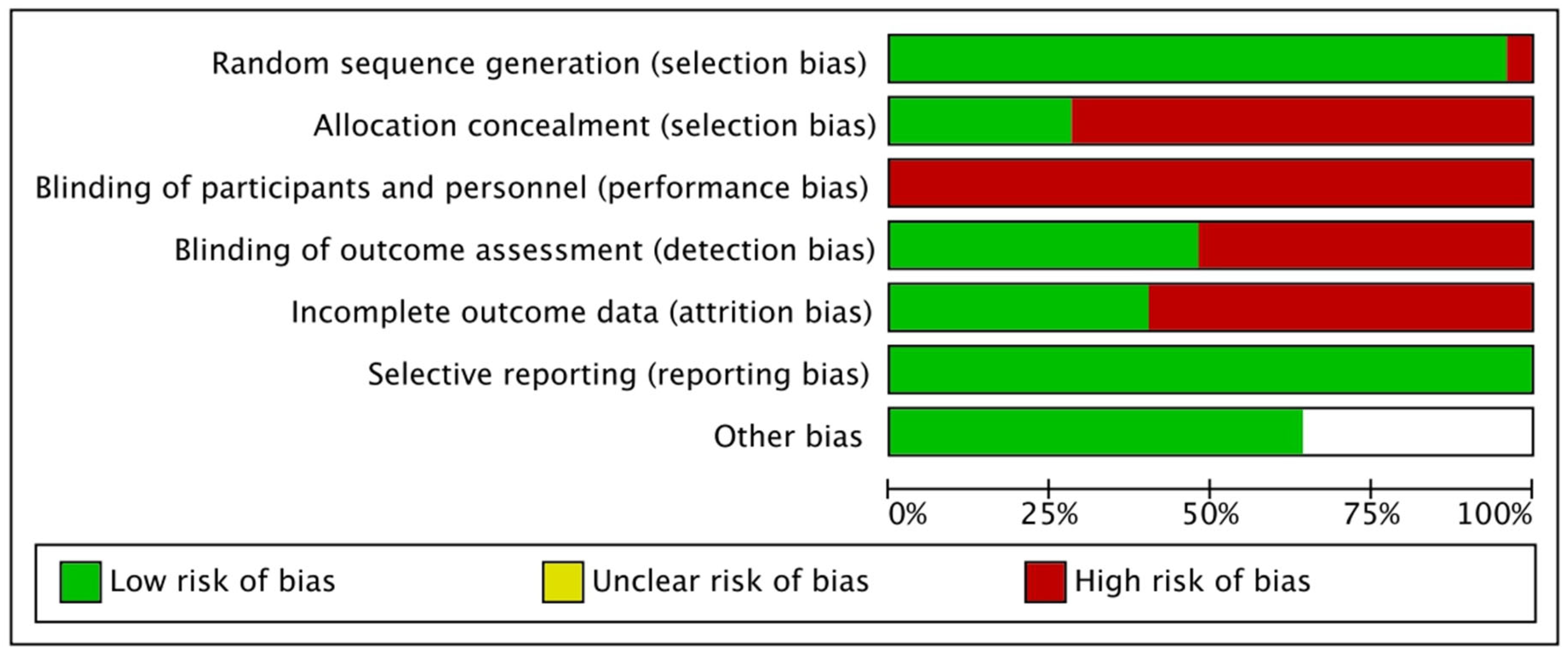
3.4. Assessment of Methodological Quality, Level of Evidence, and Bias
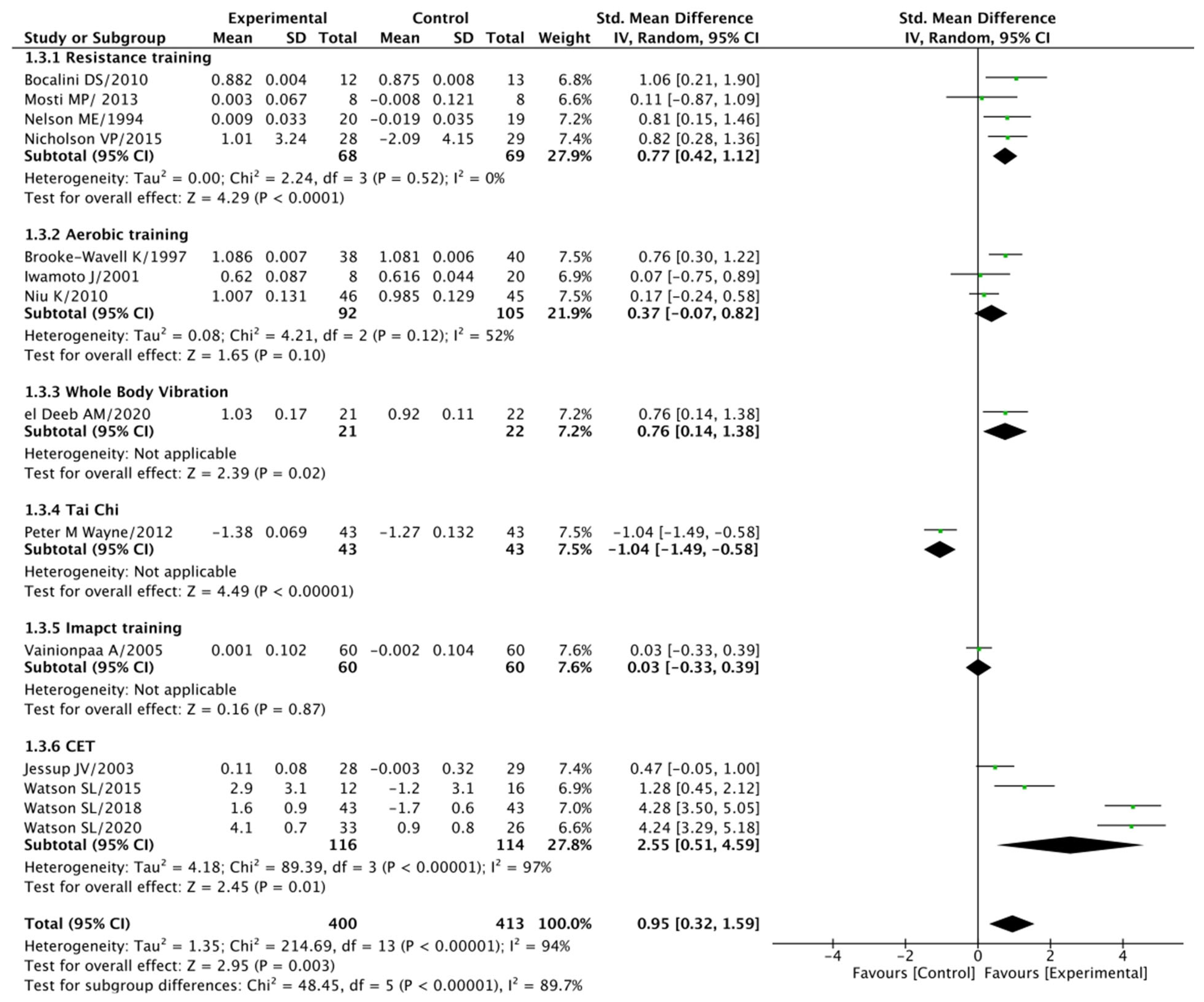
3.5. Quantitative Synthesis
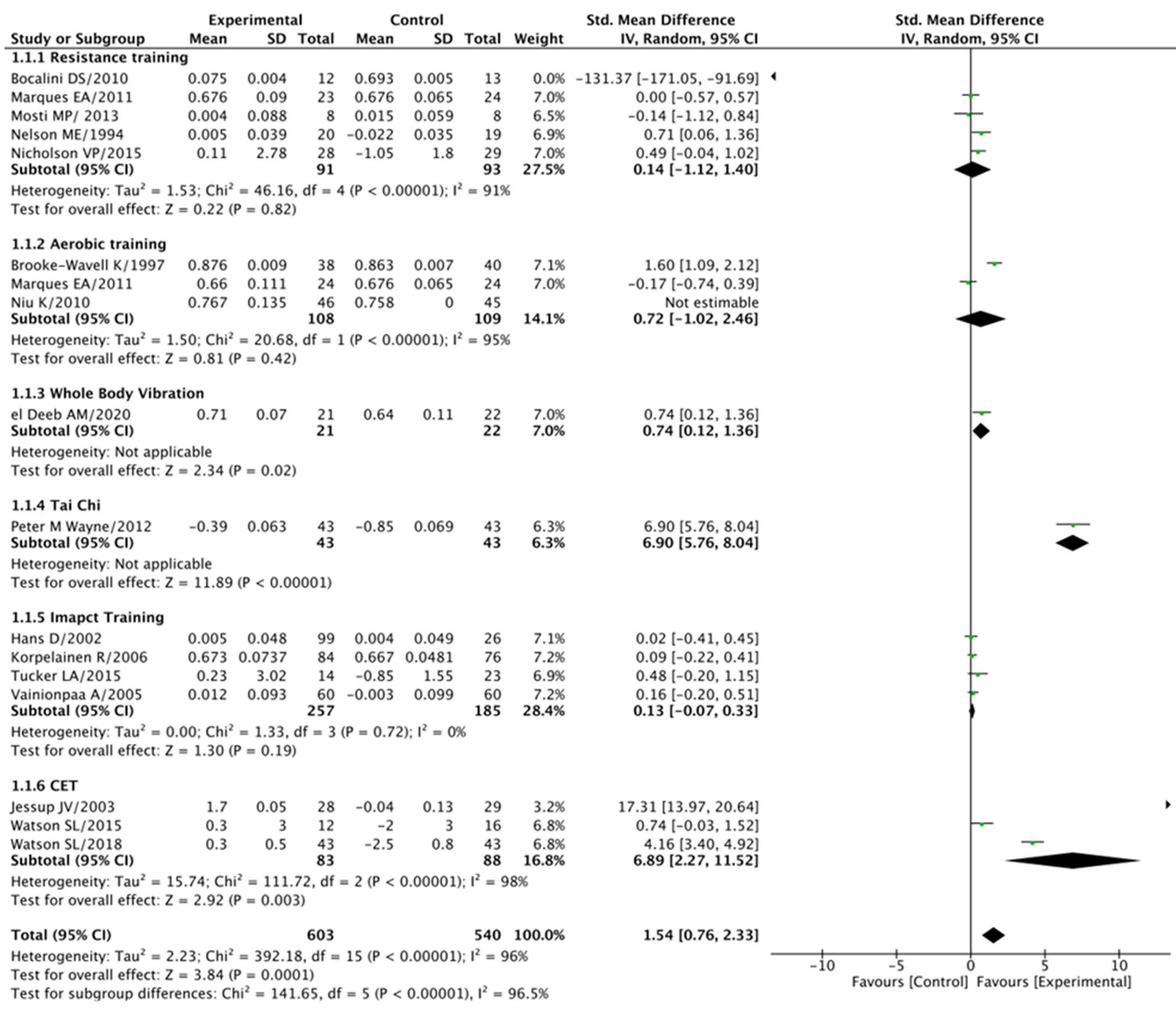
3.5.1. Femoral Neck (BMD) Analysis
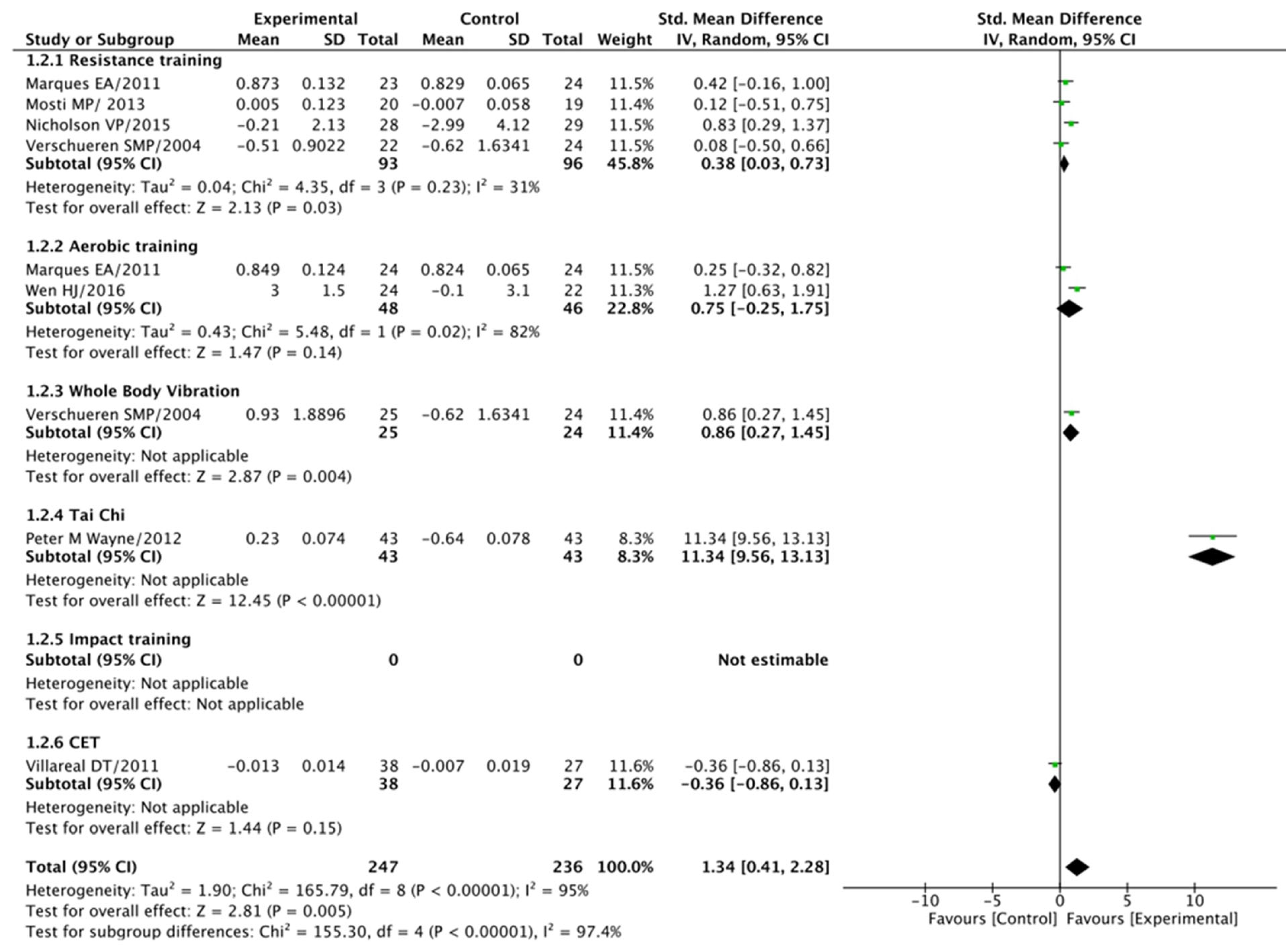
3.5.2. Total Hip (BMD) Analysis
3.5.3. Lumbar Spine (BMD) Analysis
4. Discussion
4.1. The Impact of Exercise Loading Training on BMD
4.2. Diverse Exercise Modalities in Exercise Loading
4.3. The Promise of Multimodal Exercise Loading Programs
4.4. Quality of Life Considerations
4.5. Methodological Quality and Risk of Bias
4.6. Addressing Sources of Bias
4.7. Limitations
4.8. Implications and Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anupama, D.; Norohna, J.A.; Acharya, K.K.; George, A. Effect of exercise on bone mineral density and quality of life among postmenopausal women with osteoporosis without fracture: A systematic review. Int. J. Orthop. Trauma Nurs. 2020, 39, 100796. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.; WHO Collaborating Centre for Metabolic Bone Diseases. Assessment of Osteoporosis at the Primary Health Care Level; University of Sheffield: Sheffield, UK, 2007. [Google Scholar]
- De Villiers, T.; Goldstein, S. Bone Health 2022: An Update; Taylor & Francis: Abingdon, UK, 2022; pp. 1–3. [Google Scholar]
- Balkhi, B.; Alghamdi, A.; Alqusair, S.; Alotaibi, B.; AlRuthia, Y.; Alsanawi, H.; Nasser, A.B.; Fouda, M.A. Estimated direct medical cost of osteoporosis in Saudi Arabia: A single-center retrospective cost analysis. Int. J. Environ. Res. Public Health 2021, 18, 9831. [Google Scholar] [CrossRef]
- El-Desouki, M.I. Osteoporosis in postmenopausal Saudi women using dual X-ray bone densitometry. Saudi Med. J. 1999, 20, 283–286. [Google Scholar]
- Sadat-Ali, M.; AlElq, A. Osteoporosis among male Saudi Arabs: A pilot study. Ann. Saudi Med. 2006, 26, 450–454. [Google Scholar] [CrossRef]
- Ji, M.-X.; Yu, Q. Primary osteoporosis in postmenopausal women. Chronic Dis. Transl. Med. 2015, 1, 9–13. [Google Scholar]
- Lerner, Z.F.; Shultz, S.P.; Board, W.J.; Kung, S.; Browning, R.C. Does adiposity affect muscle function during walking in children? J. Biomech. 2014, 47, 2975–2982. [Google Scholar] [CrossRef]
- Moldovan, F.; Moldovan, L. A Modeling Study for Hip Fracture Rates in Romania. J. Clin. Med. 2025, 14, 3162. [Google Scholar] [CrossRef]
- Williamson, S.; Landeiro, F.; McConnell, T.; Fulford-Smith, L.; Javaid, M.K.; Judge, A.; Leal, J. Costs of fragility hip fractures globally: A systematic review and meta-regression analysis. Osteoporos. Int. 2017, 28, 2791–2800. [Google Scholar] [CrossRef]
- Beck, B.R.; Daly, R.M.; Singh, M.A.F.; Taaffe, D.R. Exercise and Sports Science Australia (ESSA) position statement on exercise prescription for the prevention and management of osteoporosis. J. Sci. Med. Sport 2017, 20, 438–445. [Google Scholar] [CrossRef]
- Robling, A.G.; Hinant, F.M.; Burr, D.B.; Turner, C.H. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J. Bone Miner. Res. 2002, 17, 1545–1554. [Google Scholar] [CrossRef]
- Kohrt, W.M.; Bloomfield, S.A.; Little, K.D.; Nelson, M.E.; Yingling, V.R. Physical activity and bone health. Med. Sci. Sports Exerc. 2004, 36, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.G.; Furlini, G.; Zati, A.; Letizia Mauro, G. The effectiveness of physical exercise on bone density in osteoporotic patients. Biomed. Res. Int. 2018, 2018, 4840531. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lombardi, G.; Jiao, W.; Banfi, G. Effects of exercise on bone status in female subjects, from young girls to postmenopausal women: An overview of systematic reviews and meta-analyses. Sports Med. 2016, 46, 1165–1182. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Terashima, T.; Yamashita, T.; Hatanaka, Y.; Honda, A.; Umemura, Y. Effect of low-repetition jump training on bone mineral density in young women. J. Appl. Physiol. 2006, 100, 839–843. [Google Scholar] [CrossRef]
- Babatunde, O.; Forsyth, J.; Gidlow, C. A meta-analysis of brief high-impact exercises for enhancing bone health in premenopausal women. Osteoporos. Int. 2012, 23, 109–119. [Google Scholar] [CrossRef]
- Dr Bassey, E.; Rothwell, M.; Littlewood, J.; Pye, D. Pre- and postmenopausal women have different bone mineral density responses to the same high-impact exercise. J. Bone Miner. Res. 1998, 13, 1805–1813. [Google Scholar] [CrossRef]
- Kaptoge, S.; Jakes, R.W.; Dalzell, N.; Wareham, N.; Khaw, K.T.; Loveridge, N.; Beck, T.J.; Reeve, J. Effects of physical activity on evolution of proximal femur structure in a younger elderly population. Bone 2007, 40, 506–515. [Google Scholar] [CrossRef]
- Lerner, U. Bone remodeling in post-menopausal osteoporosis. J. Dent. Res. 2006, 85, 584–595. [Google Scholar] [CrossRef]
- Niu, K.; Ahola, R.; Guo, H.; Korpelainen, R.; Uchimaru, J.; Vainionpää, A.; Sato, K.; Sakai, A.; Salo, S.; Kishimoto, K.; et al. Effect of office-based brief high-impact exercise on bone mineral density in healthy premenopausal women: The Sendai Bone Health Concept Study. J. Bone Miner. Metab. 2010, 28, 568–577. [Google Scholar] [CrossRef]
- Wayne, P.M.; Kiel, D.P.; Buring, J.E.; Connors, E.M.; Bonato, P.; Yeh, G.Y.; Cohen, C.J.; Mancinelli, C.; Davis, R.B. Impact of Tai Chi exercise on multiple fracture-related risk factors in post-menopausal osteopenic women: A pilot pragmatic, randomized trial. BMC Complement. Altern. Med. 2012, 12, 1–12. [Google Scholar] [CrossRef]
- Iwamoto, J.; Takeda, T.; Ichimura, S. Effect of exercise training and detraining on bone mineral density in postmenopausal women with osteoporosis. J. Orthop. Sci. 2001, 6, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Brooke-Wavell, K.; Jones, P.; Hardman, A.; Tsuritani, I.; Yamada, Y. Commencing, continuing and stopping brisk walking: Effects on bone mineral density, quantitative ultrasound of bone and markers of bone metabolism in postmenopausal women. Osteoporos. Int. 2001, 12, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Korpelainen, R.; Keinänen-Kiukaanniemi, S.; Heikkinen, J.; Väänänen, K.; Korpelainen, J. Effect of impact exercise on bone mineral density in elderly women with low BMD: A population-based randomized controlled 30-month intervention. Osteoporos. Int. 2006, 17, 109–118. [Google Scholar] [CrossRef]
- Hans, D.; Genton, L.; Drezner, M.K.; Schott, A.M.; Pacifici, R.; Avioli, L.; Slosman, D.O.; Meunier, P.J. Monitored impact loading of the hip: Initial testing of a home-use device. Calcif. Tissue Int. 2002, 71, 112. [Google Scholar] [CrossRef]
- Verschueren, S.M.; Roelants, M.; Delecluse, C.; Swinnen, S.; Vanderschueren, D.; Boonen, S. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: A randomized controlled pilot study. J. Bone Miner. Res. 2004, 19, 352–359. [Google Scholar] [CrossRef]
- Nicholson, V.P.; McKean, M.R.; Slater, G.J.; Kerr, A.; Burkett, B.J. Low-load very high-repetition resistance training attenuates bone loss at the lumbar spine in active post-menopausal women. Calcif. Tissue Int. 2015, 96, 490–499. [Google Scholar] [CrossRef]
- Jessup, J.V.; Horne, C.; Vishen, R.; Wheeler, D. Effects of exercise on bone density, balance, and self-efficacy in older women. Biol. Res. Nurs. 2003, 4, 171–180. [Google Scholar] [CrossRef]
- Paolucci, T.; Morone, G.; Iosa, M.; Grasso, M.R.; Buzi, E.; Zangrando, F.; Paolucci, S.; Saraceni, V.M.; Fusco, A. Efficacy of group-adapted physical exercises in reducing back pain in women with postmenopausal osteoporosis. Aging Clin. Exp. Res. 2014, 26, 395–402. [Google Scholar] [CrossRef]
- Watson, S.; Weeks, B.; Weis, L.; Horan, S.; Beck, B. Heavy resistance training is safe and improves bone, function, and stature in postmenopausal women with low to very low bone mass: Novel early findings from the LIFTMOR trial. Osteoporos. Int. 2015, 26, 2889–2894. [Google Scholar] [CrossRef]
- Watson, S.L.; Weeks, B.K.; Weis, L.J.; Harding, A.T.; Horan, S.A.; Beck, B.R. High-intensity resistance and impact training improves bone mineral density and physical function in postmenopausal women with osteopenia and osteoporosis: The LIFTMOR randomized controlled trial. J. Bone Miner. Res. 2018, 33, 211–220. [Google Scholar] [CrossRef]
- Teixeira, L.E.P.P.; Silva, K.N.G.; Imoto, A.M.; Teixeira, T.J.P.; Kayo, A.H.; Montenegro-Rodrigues, R.; Peccin, M.S.; Trevisani, V.F.M. Progressive load training for the quadriceps muscle associated with proprioception exercises for the prevention of falls in postmenopausal women with osteoporosis: A randomized controlled trial. Osteoporos. Int. 2010, 21, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Huang, T.; Li, T.; Chong, P.; Ang, B. Effects of short-term step aerobics exercise on bone metabolism and functional fitness in postmenopausal women with low bone mass. Osteoporos. Int. 2017, 28, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.E.; Fiatarone, M.A.; Morganti, C.M.; Trice, I.; Greenberg, R.A.; Evans, W.J. Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures: A randomized controlled trial. JAMA 1994, 272, 1909–1914. [Google Scholar] [CrossRef]
- Bocalini, D.S.; Serra, A.J.; Dos Santos, L. Moderate resistive training maintains bone mineral density and improves functional fitness in postmenopausal women. J. Aging Res. 2010, 2010, 760818. [Google Scholar] [CrossRef]
- Karinkanta, S.; Heinonen, A.; Sievänen, H.; Uusi-Rasi, K.; Pasanen, M.; Ojala, K.; Fogelholm, M.; Kannus, P. A multi-component exercise regimen to prevent functional decline and bone fragility in home-dwelling elderly women: Randomized, controlled trial. Osteoporos. Int. 2007, 18, 453–462. [Google Scholar] [CrossRef]
- Villareal, D.T.; Chode, S.; Parimi, N.; Sinacore, D.R.; Hilton, T.; Armamento-Villareal, R.; Napoli, N.; Qualls, C.; Shah, K. Weight loss, exercise, or both and physical function in obese older adults. New Engl. J. Med. 2011, 364, 1218–1229. [Google Scholar] [CrossRef]
- Mosti, M.P.; Kaehler, N.; Stunes, A.K.; Hoff, J.; Syversen, U. Maximal strength training in postmenopausal women with osteoporosis or osteopenia. J. Strength Cond. Res. 2013, 27, 2879–2886. [Google Scholar] [CrossRef]
- Marques, E.A.; Wanderley, F.; Machado, L.; Sousa, F.; Viana, J.L.; Moreira-Goncalves, D.; Moreira, P.; Mota, J.; Carvalho, J. Effects of resistance and aerobic exercise on physical function, bone mineral density, OPG and RANKL in older women. Exp. Gerontol. 2011, 46, 524–532. [Google Scholar] [CrossRef]
- Harding, A.T.; Weeks, B.K.; Lambert, C.; Watson, S.L.; Weis, L.J.; Beck, B.R. A comparison of bone-targeted exercise strategies to reduce fracture risk in middle-aged and older men with osteopenia and osteoporosis: LIFTMOR-M semi-randomized controlled trial. J. Bone Miner. Res. 2020, 35, 1404–1414. [Google Scholar] [CrossRef]
- Tucker, L.A.; Strong, J.E.; LeCheminant, J.D.; Bailey, B.W. Effect of two jumping programs on hip bone mineral density in premenopausal women: A randomized controlled trial. Am. J. Health Promot. 2015, 29, 158–164. [Google Scholar] [CrossRef]
- Vainionpää, A.; Korpelainen, R.; Leppäluoto, J.; Jämsä, T. Effects of high-impact exercise on bone mineral density: A randomized controlled trial in premenopausal women. Osteoporos. Int. 2005, 16, 191–197. [Google Scholar] [CrossRef] [PubMed]
- ElDeeb, A.M.; Abdel-Aziem, A.A. Effect of whole-body vibration exercise on power profile and bone mineral density in postmenopausal women with osteoporosis: A randomized controlled trial. J. Manip. Physiol. Ther. 2020, 43, 384–393. [Google Scholar] [CrossRef] [PubMed]
| No | Databases | Search Terms |
|---|---|---|
| 1 | PEDro | (Osteoporosis OR postmenopausal OR premenopausal OR osteopenia OR Elderly) AND (exercise OR high intensity OR aerobic OR impact training OR high impact or resistance or jumping or weight-bearing exercise) AND (bone mineral density OR bone mass or bone metabolism) AND (quality of life) AND RCT or randomized controlled trial |
| 2 | Web of Science | (Osteoporosis OR postmenopausal OR premenopausal OR osteopenia OR Elderly) AND (exercise OR high intensity OR aerobic OR impact training OR high impact or resistance or jumping or weight bearing exercise) AND (bone mineral density OR bone mass or bone metabolism) AND (quality of life) |
| 3 | PubMed | Osteoporosis, postmenopausal, premenopausal, osteopenia, or Elderly, in combination with exercise, high intensity, aerobic, impact training, high impact, resistance, jumping, or weight-bearing exercise, as well as bone mineral density, bone mass, or bone metabolism. Additionally, we considered the term quality of life in conjunction with clinical trials, encompassing “clinical trial” [Publication Type], “clinical trials as topic” [MeSH Terms], or “clinical trial” [All Fields]. |
| 4 | Scopus | TITLE-ABS-KEY Osteoporosis OR postmenopausal OR premenopausal OR osteopenia OR Elderly) AND (exercise OR high intensity OR aerobic OR impact training OR high impact or resistance or jumping or weight bearing exercise) AND (bone mineral density OR bone mass or bone metabolism) |
| 5 | EBSCO | Osteoporosis OR postmenopausal OR premenopausal OR osteopenia OR Elderly) AND (exercise OR high intensity OR aerobic OR impact training OR high impact or resistance or jumping or weight bearing exercise) AND (bone mineral density OR bone mass or bone metabolism) AND (“clinical trial) |
| 6 | MEDLINE | |
| 7 | CINAHL | |
| 8 | Science Direct |
| S. No | Author/Year | Age (No of Participants) | Participants | Intervention | Exercise Prescription | Outcome Measures | Assessment Intervals | Adverse Effects/Compliance | Site/Equipment Used for BMD Measures | Inference | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EG | CG | ||||||||||
| 1 | Karinkanta et al., 2007 [37] | E: 72.9 ± 2.2 (38) C1: 72.7 ± 2.5 (37) C2: 72.9 ± 2.3 (37) C3: 72.0 ± 2.1 (37) | EW | EG: CET (RT + BT + IT) | CG1: RT CG2: BT + IT CG3: To continue baseline physical activity. | CG1: Intensity-50–60% progressed to 75–80% of 1 RM. Sets: 2 progressed to 3 Repetitions: 10–15 progressed to 8–10 CG2: Gradual escalation in complexity of movements, number of steps, impacts, and jumps. |
|
| Falls, ligament injury, fracture, minor knee injuries, partial rupture of muscles and overuse syndrome/In total, 67% of compliance with highest to C1 followed by E and C2 groups | FN, DT, TS, DS, RS/Dual-energy X-ray absorptiometry | Combination of balance, resistance, and impact training improved strength, balance, and BMD. |
| 2 | Wayne et al., 2012 [22] | E: 58.8 ± 5.6 (43) C: 60.4 ± 5.3 (43) | PMPW | EG: Tai chi + Usual care | CG: Usual care | CG: Daily consumption of calcium and vitamin D, along with regular exercise as recommended by a healthcare provider. EG: Usual care + 30 min of tai chi exercises, 2 sessions per week for 1st month Later progressed to 3 sessions per week from 2nd to 9th month |
|
| Minor musculoskeletal related injuries were reported in 9 participants with E (7) and C (2)/NR | FN, TH, SP/Dual-energy X-ray absorptiometry | Tai chi along with usual care showed better improvements in BMD, QOL, and sway parameters when compared to usual care alone. |
| 3 | Iwamoto et al., 2001 [23] | E: 65.3 ± 4.7 (8) C1: 64.3 ± 3.0 (7) C2: 64.9 ± 5.7 (20) | PMPW | EG: AT + Gymnasium | CG1: DT + Gymnasium CG2: No exercise | Experimental Group (EG): Participants were motivated to enhance their daily step count through brisk walking for a duration of 2 years, combined with two sets of gymnastic training sessions (including exercises like SLR, squatting, abdominal, and back strengthening exercises) for 2 years. Control Group 1 (CG1): Participants were encouraged to increase their daily step count by brisk walking for 1 year, along with two sets of gymnastic training sessions (comprising exercises such as SLR, squatting, abdominal, and back strengthening exercises) conducted 5 days per week for a duration of 2 years. Control Group 2 (CG2): Participants did not engage in any specific exercise regimen. All participants received calcium lactate and vitamin D supplementation for a period of 2 years. |
|
| Not reported | LS/Dual-energy X-ray absorptiometry | Sustained exercise training is necessary to preserve the bone mass acquired through exercise training. |
| 4 | Brooke-Wavell et al., 2001 [24] | E: 64.9 ± 3.0 (38) C: 64.2 ± 3.1 (40) | PMPW | EG: AT | CG: Swimming routine physical activity | EG: Progressively increased the duration from 120 min to 280 min. Frequency: 20–50 min per day for 12 months CG: 20 min of swimming per session 2 sessions per week for 12 months. |
|
| Two women reported minor walking-related foot injuries | Lumbar spine (LS), Femoral neck (FN), and calcaneum assessments were conducted utilizing Dual-Energy X-ray Absorptiometry (DXA). | Walking mitigated bone loss, particularly in the calcaneus, and may have had a positive impact on bone density in the lumbar spine. Additionally, it improved functional capacity and thwarted the increase in body mass that was noted in the control group. |
| 5 | Korpelainen et al., 2006 [25] | E: 72.9 ± 1.1 (84) C: 72.8 ± 1.2 (76) | EW | EG: IT | CG: Continue with regular activities | EG: The supervised and home exercise regimens were regularly adjusted every two months to ensure continued progress and variety. Participants engaged in 1 h of supervised impact exercises along with a 20 min home exercise program for a duration of 30 months. |
|
| Three female participants reported minor injuries. In the experimental group, six individuals experienced fractures, while in the control group, 16 individuals had fractures. The compliance rate for supervised sessions ranged from 73% to 78% | TF, TR, and FN/Peripheral DXA (Osteometer DTX 200, Osteometer Meditech, Roedovre, Denmark.) | Impact exercise did not significantly influence (BMD), but it did have a positive impact on bone mineral content (BMC) at the trochanter. This exercise regimen may help reduce the risk of fractures related to falls in elderly women with low bone mass. |
| 6 | Hans et al., 2002 [26] | E: 67.6 ± 5.2 (99) C1: 66.3 ± 7.6 (32) C2: 66.0 ± 4.8 (26) | PMPW | EG: IT | CG1: Heel drops without impact. CG2: Continue with regular activities. | In the experimental group (EG), the exercise parameters were as follows:
|
|
| Slight backache without any serious adverse events/91% and 65% of compliance at 18 months and 24 months, respectively | FN, IT, TR, Ward’s tr/Hologic QDR 1000 or 2000 densitometers | Maintaining hip BMD could potentially be achieved with a short, secure, supervised impact loading program conducted at home. |
| 7 | Verschueren et al., 2004 [27] | E: 64.6 ± 3.3 (25) C1: 63.90 ± 3.8 (22) C2: 64.2 ± 3.1 (24) | PMPW | EG: WBV | CG1: Resistance training. CG2: Continue with regular activities. | Experimental Group (EG) Protocol:
|
|
| Not reported | Measurement of trabecular thickness (TH) and total bone density (TB) through Dual-Energy X-ray Absorptiometry (DXA) utilizing the QDR-4500A apparatus (Hologic, Waltham, MA, USA). | Whole Body Vibration (WBV) training appears to be a viable and efficacious approach for altering established risk factors associated with falls and fractures in elderly women, underscoring the necessity for additional human research in this area. |
| 8 | Nicholson et al., 2015 [28] | E: 66.0 ± 4.1 (28) C: 65.6± 4.7 (29) | PMPW | EG: RT | CG: Continue with regular activities. | EG: 50 min of 2 sessions per week for 6 months Progression: Systematic increase in the load |
|
| One participant complaint of exacerbation of knee pain/89% compliance | Lumbar Spine (LS), Femoral Neck (FN), Total Hip (TH), Total Radius (TR), and Total Body (TB) assessments were conducted using Pencil Beam Dual-Energy X-ray Absorptiometry (DXA) technology, specifically the Lunar DPX Pro system from GE Healthcare based in the U.K. | Low-load, high-repetition resistance training has proven effective in mitigating declines in lumbar spine (BMD) when compared to control groups in healthy and physically active women aged over 55 years. However, this type of training did not exhibit a significant impact on BMD in the hip and total body, nor did it influence measures of fat mass and fat-free soft tissue mass. |
| 9 | Jessup et al., 2003 [29] | E: 69.1 ± 2.8 (28) C: 69.4 ± 4.2 (29) | PMPW | EG: CET (RT + BT + AT) | CG: No exercise | EG: For instance: Duration: 60–90 min per session, Frequency: Three sessions per week for a total of 32 weeks. Progression: RT: 8–10 repetitions with 50% of 1 RM progressed to 75% of 1 RM. BT + AT: Started with no weights progressed to 10% of body weight with increase in complexity of exercise. |
|
| No adverse effects were reported/Not reported | Femoral neck and Lumbar spine measurements were obtained using the Norland Excel DEXA scan system manufactured by Norland Medical Systems in White Plains, New York. | The experimental group (EG) experienced substantial enhancements in femoral neck bone density and balance, coupled with notable weight loss. Self-efficacy levels remained unchanged in both groups. |
| 10 | Paolucci et al., 2014 [30] | E: 65.6 ±5.8 (40) C: 65.6 ±5.3 (20) | PMPW | EG: Supervised CET (RT + AT + BT) | CG: Unsupervised MM | Both the Experimental Group (EG) and Control Group (CG) engaged in low-intensity exercise sessions lasting 60 min each. These sessions occurred three times per week and spanned a total of 10 sessions. The exercise programs included a progression in exercise intensity over time. |
|
| The attendance rates for the Experimental Group (EG) sessions were not reported. However, it is noteworthy that a high attendance rate was observed, with 93% of participants attending all sessions. | - | Supervised multimodal exercises have demonstrated their effectiveness in reducing back pain and enhancing functional status and quality of life among women coping with postmenopausal osteoporosis. Importantly, these positive outcomes have been sustained for a period of six months. |
| 11 | Watson et al., 2018 [32] | E: 65 ± 5 (43) C: 65 ± 5 (43) | PMPW | EG: CET (RT + IT) | CG: RT | EG and CG: Duration: 30 min per session, Frequency: 2 times per week for 8 months Progression: EG: RT: Intensity progressed from 50–70% to 80–85% of 1 RM and IT: jumping with flexed lower limb position to stiff leg landing. C: Progressively adding weights up to 3 kg |
|
| One participant had mild low back pain in the experimental group/92 ± 11% for the experimental group and 85± 24%, for the control group. | LS, and FN/Dual-energy X-ray absorptiometry | Short-term, supervised multimodal exercise programs have shown the potential to improve BMD and physical performance in postmenopausal women who have low to very low bone mass. |
| 12 | Watson et al., 2015 [31] | E: 65.3 ± 3.9 (12) C: 66.7 ± 5.4 (16) | PMPW | EG: CET (RT + IT) | CG: RT | EG and CG: Duration: 30 min per session, Frequency: 2 times per week for 8 months Progression: E: RT: Intensity progressed from 50–70% to 80–85% of 1 RM and IT: jumping with flexed lower limb position to stiff leg landing C: Progressively adding weights up to 3 kg |
|
| No adverse events have been reported/87.2 ± 3.9% for the experimental group and 92.7 ± 3.8%, for the control group. | LS, and FN/Dual-energy X-ray absorptiometry | Brief supervised multimodal exercise is considered a safe and effective exercise therapy for postmenopausal women who have low to very low bone mass. |
| 13 | Harding et al., 2020 [41] | E: 64.9 ± 8.6 (33) C: 67.4 ± 6.3 (26) | Middle age and older men | EG: CET (RT + IT) | CG: Continue with regular activities. | EG and CG: Duration: 30 min per session, Frequency: 2 times per week for 8 months Progression: EG: RT: Intensity progressed from 50–70% to 80–85% of 1 RM with RPE ≥16 on the 6-to-20-point Borg scale and IT: jumping with flexed lower limb position to stiff leg landing |
|
| Five mild musculoskeletal discomfort occurred and muscle soreness/77.8% 16.6% for experimental group and 78.5% 14.8% for the control group. | LS, FN, TH and TR/Dual-energy X-ray absorptiometry | Multimodal exercise improved BMD, function and facture risk when compared to control group. |
| 14 | Teixeira et al., 2010 [33] | E: 63.1± 4.5 (33) C: 62.7 ± 4.8 (26) | PMPW | EG: CET (RT + PT + +BT+ DT) | CG: DT | Experimental Group (EG) Protocol:
|
|
| Five and six participants reported falls after treatment sessions in experimental and control groups, respectively/82 ± 5.83% of compliance rate. | - | The combination of progressive strength training for the quadriceps and proprioceptive training has proven to be effective in preventing falls. This approach enhances muscle power and both static and dynamic balance, and accelerates motor response times, ultimately leading to improved performance in daily activities. |
| 15 | Villareal et al., 2011 [38] | E: 70± 4 (38) C1: 70 ± 4 (26) C2: 70 ± 4 (26) C3: 69 ± 4 (27) | EW | EG: CET (RT + AT + Diet) | CG1: RT+ AT CG2: Diet CG3: No diet and exercises | Both the Experimental Group (EG) and Control Group 1 (CG1) engaged in exercise sessions with the following parameters:
Aerobic Training (AT):
|
|
| During the study, one participant in the research cohort experienced an ankle fracture. The compliance rates for the interventions were as follows:
| TH/Dual-energy X-ray absorptiometry | The combination of weight loss and exercise has been found to yield greater improvements in physical function when compared to either intervention alone. |
| 16 | Wen et al., 2017 [34] | E: 57.5 ± 3.5 (24) C: 58.8 ± 3.2 (22) | PMPW | EG: AT | CG: No exercises | Experimental Group (EG) Protocol:
|
|
| No adverse events occurred/96.7 ± 0.9% compliance towards the experimental group. | TH and TB/Dual-energy X-ray absorptiometer | Short-term step aerobic exercise showed significant improvements on bone metabolism and general health but not on BMD. |
| 17 | Niu et al., 2010 [21] | E: 38.1 ± 1.2 (46) C: 39.7 ± 1.2 (45) | PRMPW | EG: AT | CG: SE | EG and CG: Duration:16 min, Frequency: Three times per week for 12 months. Intensity: 5 X10 vertical and versatile jumps in experimental group. Progression: EG: Progressed up to 50 jumps by 3 months; 6 months onwards, jumping from 10 cm step. |
|
| No adverse events occurred/2.4 (0.8–3.2) times per week for both groups. | LS, PF, FN, IT, TF, Ward’s triangle/Dual-energy X-ray absorptiometer | The experimental group demonstrated statistically significant changes in Femoral Neck Bone Mineral Density (BMD) when compared to the control group. |
| 18 | Tucker et al., 2015 [42] | E1: 41.09 ± 4,3(23) E2: 39.79 ± 4.7(14) C: 37.65± 6.4 (23) | PRMPW | EG1: IT (10 jumps) EG2: IT (20 jumps) | CG: SE | Participants were instructed to perform either 10 or 20 jumps during each session, with a total of 2 sessions per day. This regimen was followed for 6 days per week over a span of 16 weeks. |
|
| Adverse events not reported/73% compliance | TH/Dual-energy X-ray absorptiometry | Hip BMD improved in both jumping groups compared to control group. |
| 19 | Vainionpää et al., 2005 [43] | E: 38.1± 1.7 (60) C: 38.5 ± 1.6 (60) | PRMPW | EG: IT | CG: No exercise | Experimental Group (EG) Protocol:
|
|
| No adverse events occurred/Not reported | FN, TR, IT, TF, Ward’s triangle, LS, RA, UL, DR, CL/Dual-energy X-ray absorptiometry | High-impact exercises have been shown to lead to improvements in BMD, specifically in the lumbar spine and upper femur among premenopausal women. |
| 20 | Nelson et al., 1994 [35] | E: 61.1 ± 3.7 (20) C: 57.3 ± 6.3 (19) | PMPW | EG: RT | CG: Continue with regular activities. | EG: Duration: 45 min, Frequency: 2 days per week for 54-week, Intensity: 50% and 60% of the baseline 1 RM with 16 on the Borg scale Progressed to 80% of 1 RM. |
| Seven participants in the exercise group complained of mild musculoskeletal pain. One woman suffered an ankle sprain and two others suffered wrist fractures due to falls in the control group/87.5 ± 1.8% compliance to the experimental group. | LS, FN/Dual-energy X-ray absorptiometry | High-intensity strength training retains BMD and improves muscle mass, strength, and balance. | |
| 21 | Mosti et al., 2013 [39] | E: 61.9 ± 5.0 (8) C: 66.7 ± 7.4 (8) | EW | EG: RT | CG: Continue with osteopenia exercise guidelines. | Experimental Group (EG) followed a regimen consisting of three sessions per week over a 12-week period. The intensity level involved four sets, each comprising 3–5 repetitions, with a resistance set at 85–90% of their initial 1 RM (one-repetition maximum). If participants successfully completed 5 repetitions, the training load was elevated by 2.5 kg to ensure progression. |
|
| No adverse events occurred/87% compliance to exercise program. | LS, FN, and TH/Dual X-ray Absorptiometry | Maximum strength training program improves BMD. |
| 22 | Bocalini et al., 2010 [36] | E: 66 ± 9 (12) C: 64 ± 8 (13) | PMPW | EG: RT | CG: No exercises | The Experimental Group (EG) engaged in 60-min sessions, three times a week, over a 24-week period. The initial intensity was set at 40% of their 1 RM (one-repetition maximum). Progression involved performing three sets of 10–12 repetitions for the specific exercise at an intensity level of 60–70% of their 1 RM. |
|
| No adverse events occurred/Not reported. | LS, FN,/Dual X-ray Absorptiometry | RT suppresses the decline in BMD and simultaneously improves the functional fitness of postmenopausal women. |
| 23 | ElDeeb and Abdel-Aziem, 2020 [44] | E: 55.09 ± 4.19 (21) C: 57.29 ± 4.44 (22) | PMPW | EG: WBV | CG: CG | Duration: 5–10 min, Frequency: 2 sessions per week for 24 weeks Intensity: Frequency 20 Hz, amplitude ranged from 2.5 to 5 mm, 5 min, holding the position for 30 s, rest period 45 s with 3 repetitions. Progression: Progression in frequency up to 35 Hz, amplitude, position holding time up to 60 s, rest period reduced to 5 s and repetitions up to 9 by 6 months. |
|
| LS, FN, Ward’s triangle, and GT/Dual X-ray Absorptiometry | Whole body vibration improved muscle work and BMD. | |
| 24 | Marques et al., 2011 [40] | E1: 67.3 ± 5.2 (23) E2: 70.3 ± 5.5 (24) C: 67.9 ± 5.9 (24) | EW | EG1: RT EG2:AT | CG: No exercises | EG1: 60 min per session, 3 sessions per week or 32 weeks. Progression: 50–60% of 1 RM, 2 sets of 10–15 repetitions progressed to 75–80% of 1 RM, 2 sets 6–8 repetitions. EG2: 60 min per session, 3 sessions per week or 32 weeks Progression: Exercise intensity at initial weeks was 50–60% of heart reserve later progressed to 65% to 80% of heart rate reserve. |
|
| No adverse events related to exercise or assessments were reported during the study. The compliance rate for resistance exercise (RE) sessions was 78.4%, with a range from 61.6% to 95.9%. For aerobic exercise (AE) training, the mean compliance rate was 77.7%, with a range from 64.2% to 96.8%. | FN, TR, IT, and TH/Dual X-ray Absorptiometry | |
| S. No. | Author/References | Eligibility Criteria | Random Allocation | Concealed Allocation | Baseline Comparability | Blinding of Participants | Blinding of Therapist | Blinding of Assessor | Adequate Follow Up (>85%) | Intention to Treat Analysis | Between Group Comparison | Point Estimates and Variability | PEDro Score (10) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Karinkanta et al., 2007 [37] | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | 7 |
| 2 | Wayne et al., 2012 [22] | No | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 7 |
| 3 | Iwamoto et al., 2001 [23] | No | Yes | No | Yes | No | No | No | No | No | Yes | Yes | 4 |
| 4 | Brooke-Wavell et al., 2001 [24] | Yes | Yes | No | Yes | No | No | Yes | Yes | No | Yes | Yes | 6 |
| 5 | Korpelainen et al., 2006 [25] | Yes | Yes | No | Yes | No | No | Yes | No | Yes | Yes | Yes | 6 |
| 6 | Hans et al., 2002 [26] | No | Yes | Yes | Yes | No | No | No | No | No | Yes | Yes | 5 |
| 7 | Verschueren et al., 2004 [27] | No | Yes | No | Yes | No | No | Yes | No | No | Yes | Yes | 5 |
| 8 | Nicholson et al., 2015 [28] | Yes | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 7 |
| 9 | Jessup et al., 2003 [29] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 8 |
| 10 | Paolucci et al., 2014 [30] | Yes | Yes | No | Yes | Yes | No | No | No | No | Yes | Yes | 5 |
| 11 | Watson et al., 2015 [31] | Yes | Yes | No | Yes | Yes | No | No | No | No | Yes | Yes | 5 |
| 12 | Watson et al., 2018 [32] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 8 |
| 13 | Harding et al., 2020 [41] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 9 |
| 14 | Teixeira et al., 2010 [33] | Yes | Yes | Yes | Yes | No | No | Yes | No | No | Yes | Yes | 6 |
| 15 | Villareal et al., 2011 [38] | Yes | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 7 |
| 16 | Wen et al., 2017 [34] | Yes | Yes | No | Yes | No | No | No | Yes | No | Yes | Yes | 5 |
| 17 | Niu et al., 2010 [21] | Yes | Yes | No | Yes | No | No | No | No | No | Yes | Yes | 4 |
| 18 | Tucker et al., 2015 [42] | Yes | Yes | No | Yes | No | No | No | No | No | Yes | Yes | 4 |
| 19 | Vainionpää et al., 2005 [43] | Yes | Yes | No | Yes | No | No | No | No | No | Yes | Yes | 4 |
| 20 | Nelson et al., 1994 [35] | Yes | Yes | No | No | No | No | No | Yes | Yes | Yes | Yes | 5 |
| 21 | Mosti et al., 2013 [39] | Yes | Yes | No | Yes | No | No | No | No | No | Yes | Yes | 4 |
| 22 | Bocalini et al., 2010 [36] | Yes | Yes | No | Yes | No | Yes | No | No | No | Yes | Yes | 5 |
| 23 | ElDeeb and Abdel-Aziem, 2020 [44] | No | Yes | Yes | Yes | No | No | No | Yes | No | Yes | Yes | 6 |
| 24 | Marques et al., 2011 [40] | Yes | Yes | Yes | Yes | No | No | Yes | No | Yes | Yes | Yes | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnasser, S.M.; Babakair, R.A.; Al Mukhlid, A.F.; Al hassan, S.S.S.; Nuhmani, S.; Muaidi, Q. Effectiveness of Exercise Loading on Bone Mineral Density and Quality of Life Among People Diagnosed with Osteoporosis, Osteopenia, and at Risk of Osteoporosis—A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 4109. https://doi.org/10.3390/jcm14124109
Alnasser SM, Babakair RA, Al Mukhlid AF, Al hassan SSS, Nuhmani S, Muaidi Q. Effectiveness of Exercise Loading on Bone Mineral Density and Quality of Life Among People Diagnosed with Osteoporosis, Osteopenia, and at Risk of Osteoporosis—A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(12):4109. https://doi.org/10.3390/jcm14124109
Chicago/Turabian StyleAlnasser, Saeed Mufleh, Reem Abdullah Babakair, Amal Fahad Al Mukhlid, Salihah Saleh Saeed Al hassan, Shibili Nuhmani, and Qassim Muaidi. 2025. "Effectiveness of Exercise Loading on Bone Mineral Density and Quality of Life Among People Diagnosed with Osteoporosis, Osteopenia, and at Risk of Osteoporosis—A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 12: 4109. https://doi.org/10.3390/jcm14124109
APA StyleAlnasser, S. M., Babakair, R. A., Al Mukhlid, A. F., Al hassan, S. S. S., Nuhmani, S., & Muaidi, Q. (2025). Effectiveness of Exercise Loading on Bone Mineral Density and Quality of Life Among People Diagnosed with Osteoporosis, Osteopenia, and at Risk of Osteoporosis—A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(12), 4109. https://doi.org/10.3390/jcm14124109






