Impact of Resistance Exercise and Nitrate Supplementation on Muscle Function and Clinical Outcomes After Knee Osteoarthritis Surgery in Middle-Aged Women with Sarcopenia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
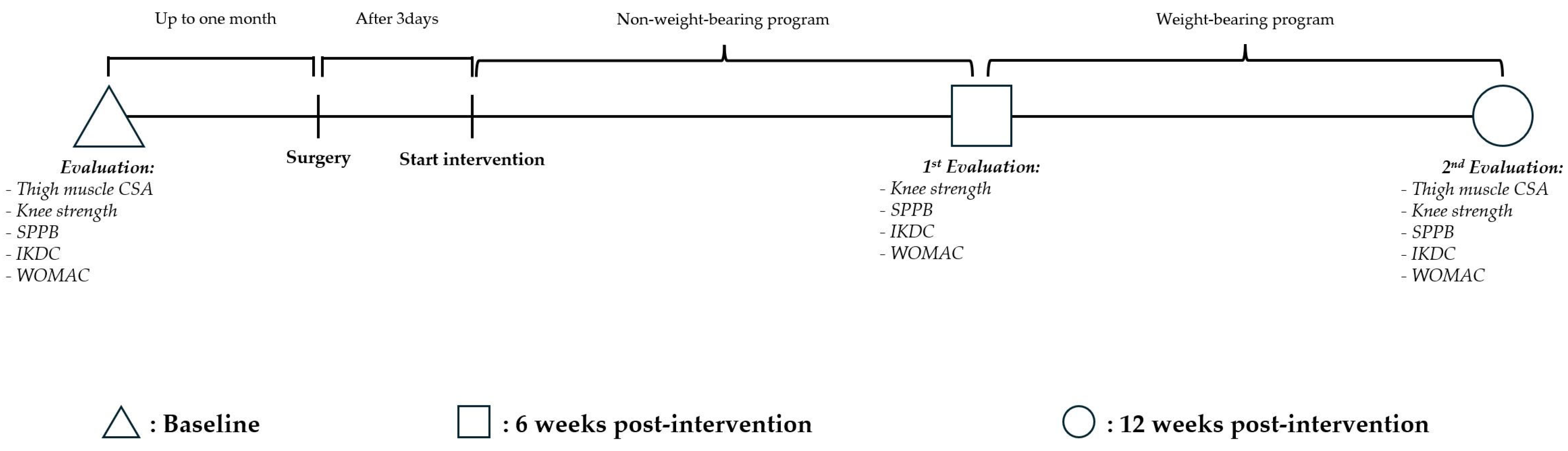
2.1. Study Design and Blinding
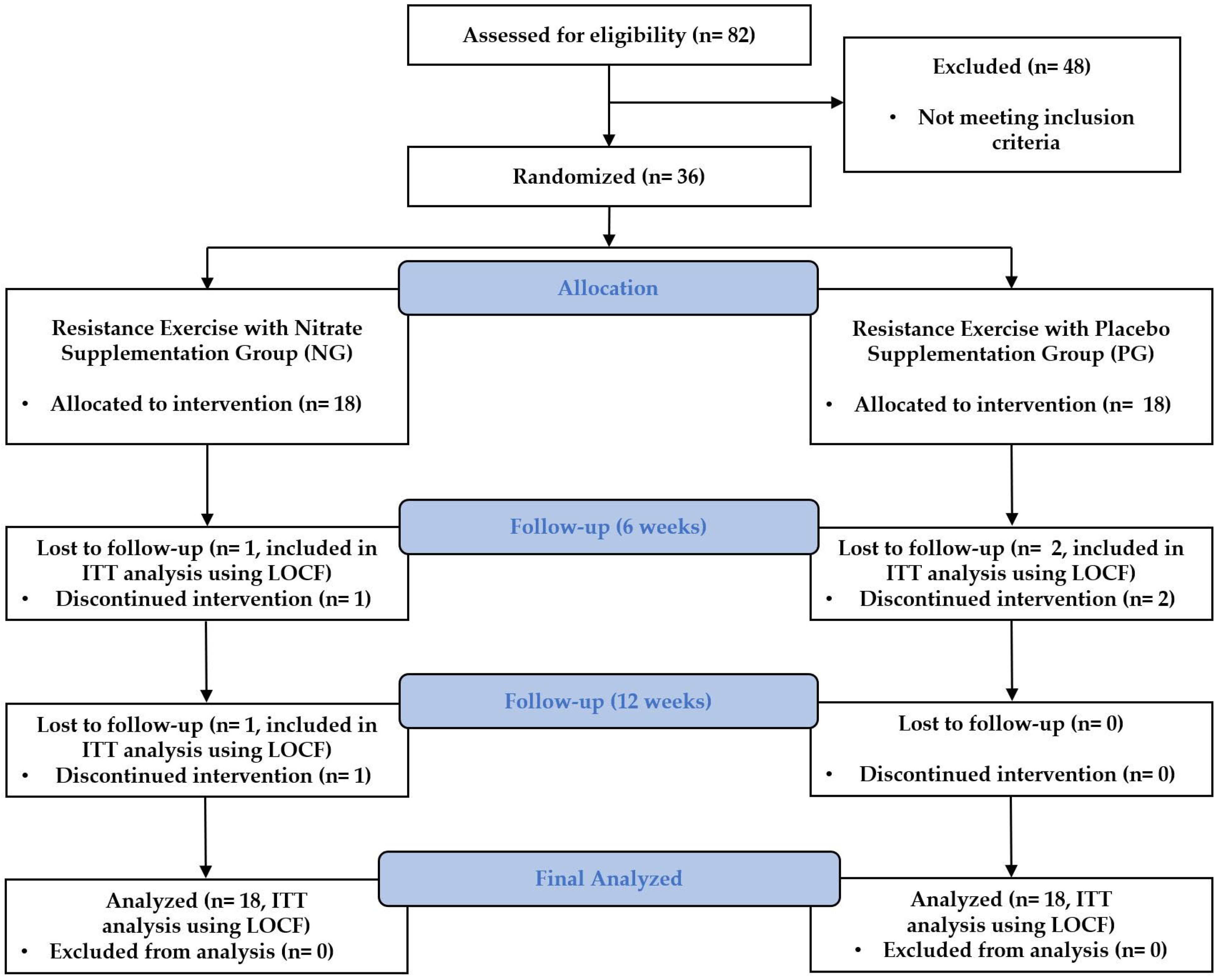
2.2. Participants
2.3. Intervention
2.3.1. Resistance Exercise Protocol
2.3.2. Nitrate Supplementation Details
2.4. Primary Outcome
2.4.1. Thigh Muscle Cross-Sectional Area
2.4.2. Knee Strength
2.5. Secondary Outcome
2.5.1. Sarcopenia-Related Outcomes
2.5.2. Osteoarthritis-Related Outcome
2.6. Statistical Analysis
3. Results
3.1. Primary Outcomes
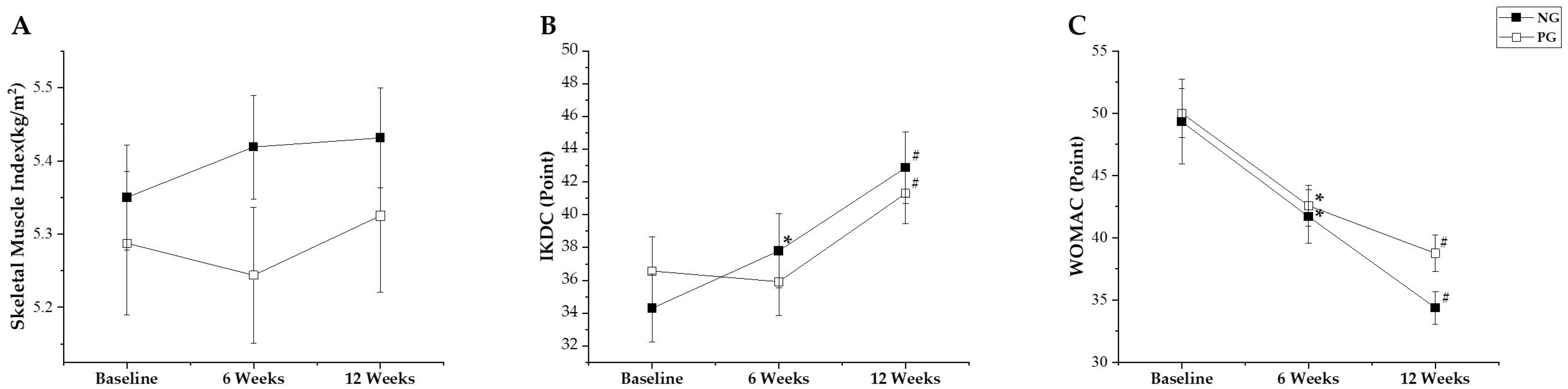
3.1.1. Thigh Muscle Cross-Sectional Area
3.1.2. Knee Strength
3.2. Secondary Outcomes
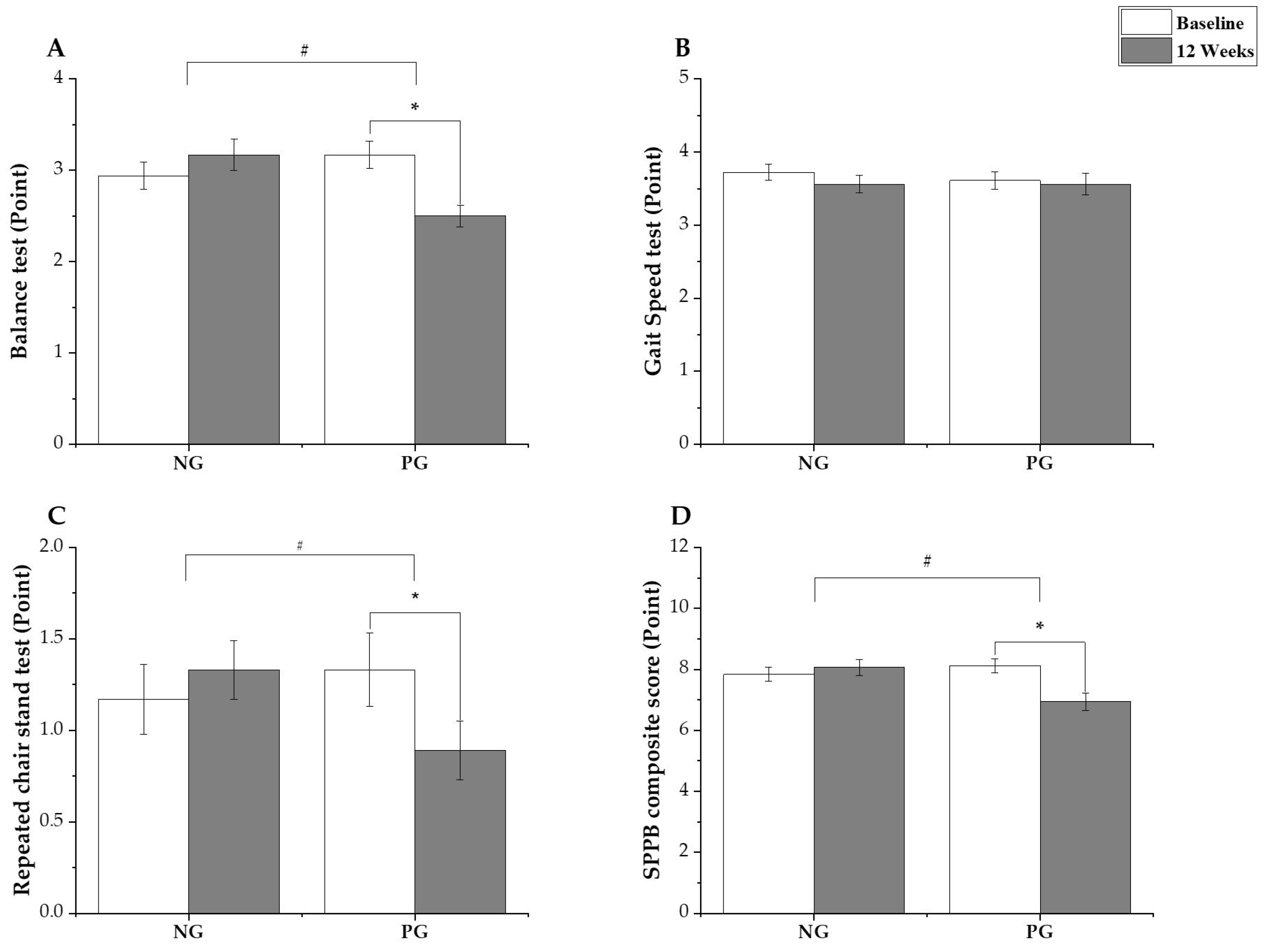
3.2.1. Sarcopenia-Related Outcome
3.2.2. Osteoarthritis-Related Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global Prevalence of sarcopenia and severe sarcopenia: S systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.H.; Han, K.D.; Hong, J.Y.; Park, J.H.; Bae, J.H.; Moon, Y.W.; Kim, J.G. Body composition Is more closely related to the development of knee osteoarthritis in women than men: A cross-sectional study using the fifth Korea National Health and Nutrition Examination Survey (KNHANES V-1, 2). Osteoarthr. Cartil. 2016, 24, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Han, H.; Jin, J.; Zhou, G.; Li, Z. Osteoarthritis and sarcopenia-related traits: The cross-sectional study from NHANES 2011–2014 and Mendelian Randomization study. J. Orthop. Surg. Res. 2023, 18, 502. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-S.; Hong, K.-T.; Kim, N.-M.; Jung, J.-Y.; Park, H.-S.; Lee, S.H.; Cho, Y.J.; Kim, S.J. Implantation of allogenic umbilical cord blood-derived mesenchymal stem cells improves knee osteoarthritis outcomes: Two-year follow-up. Regen. Ther. 2020, 14, 32–39. [Google Scholar] [CrossRef]
- Liao, C.-D.; Chen, H.-C.; Huang, S.-W.; Liou, T.-H. Impact of sarcopenia on rehabilitation outcomes after total knee replacement in older adults with knee osteoarthritis. Ther. Adv. Musculoskelet. 2021, 13, 1759720X21998508. [Google Scholar] [CrossRef]
- van Vugt, J.L.A.; Braam, H.J.; van Oudheusden, T.R.; Vestering, A.; Bollen, T.L.; Wiezer, M.J.; de Hingh, I.H.J.T.; van Ramshorst, B.; Boerma, D. Skeletal muscle depletion Is associated with severe postoperative complications in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal cancer. Ann. Surg. Oncol. 2015, 22, 3625–3631. [Google Scholar] [CrossRef]
- Dreyer, H.C. Tourniquet use during knee replacement surgery may contribute to muscle atrophy in older adults. Exerc. Sport. Sci. Rev. 2016, 44, 61–70. [Google Scholar] [CrossRef]
- Rice, D.A.; McNair, P.J. Quadriceps arthrogenic muscle inhibition: Neural mechanisms and treatment perspectives. In Proceedings of the Seminars in Arthritis and Rheumatism; Elsevier: Amsterdam, The Netherlands, 2010; Volume 40, pp. 250–266. [Google Scholar] [CrossRef]
- Chen, N.; He, X.; Feng, Y.; Ainsworth, B.E.; Liu, Y. Effects of resistance training in healthy older people with sarcopenia: A systematic review and meta-analysis of randomized controlled trials. Eur. Rev. Aging Phys. Act. 2021, 18, 23. [Google Scholar] [CrossRef]
- Moon, Y.-W.; Kim, H.-J.; Ahn, H.-S.; Lee, D.-H. Serial changes of quadriceps and hamstring muscle strength following total knee arthroplasty: A meta-analysis. PLoS ONE 2016, 11, e0148193. [Google Scholar] [CrossRef]
- Hardy, E.J.; Deane, C.S.; Lund, J.N.; Phillips, B.E. Loss of muscle mass in the immediate post-operative period is associated with inadequate dietary protein and energy intake. Eur. J. Clin. Nutr. 2023, 77, 503–505. [Google Scholar] [CrossRef]
- Bescós, R.; Sureda, A.; Tur, J.A.; Pons, A. The effect of nitric-oxide-related supplements on human performance. Sports Med. 2012, 42, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Coggan, A.R.; Leibowitz, J.L.; Kadkhodayan, A.; Thomas, D.P.; Ramamurthy, S.; Spearie, C.A.; Waller, S.; Farmer, M.; Peterson, L.R. Effect of acute dietary nitrate intake on maximal knee extensor speed and power in healthy men and women. Nitric Oxide 2015, 48, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Córdova-Martínez, A.; Caballero-García, A.; Bello, H.J.; Pons-Biescas, A.; Noriega, D.C.; Roche, E. L-Arginine and beetroot extract supplementation in the prevention of sarcopenia. Pharmaceuticals 2022, 15, 290. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.-W.; Jung, S.-W.; Kim, S.-W.; Lee, J.-M.; Jung, H.C.; Song, J.-K. Effects of 16 weeks of resistance training on muscle quality and muscle growth factors in older adult women with sarcopenia: A randomized controlled trial. Int. J. Environ. Res. Public Health 2021, 18, 6762. [Google Scholar] [CrossRef]
- Noyes, F.R. Noyes’ Knee Disorders: Surgery, Rehabilitation, Clinical Outcomes E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016; ISBN 978-0-323-42855-2. [Google Scholar]
- Alvares, T.S.; de Oliveira, G.V.; Volino-Souza, M.; Conte-Junior, C.A.; Murias, J.M. Effect of dietary nitrate ingestion on muscular performance: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2022, 62, 5284–5306. [Google Scholar] [CrossRef]
- Lixandrão, M.E.; Ugrinowitsch, C.; Laurentino, G.; Libardi, C.A.; Aihara, A.Y.; Cardoso, F.N.; Tricoli, V.; Roschel, H. Effects of exercise intensity and occlusion pressure after 12 weeks of resistance training with blood-flow restriction. Eur. J. Appl. Physiol. 2015, 115, 2471–2480. [Google Scholar] [CrossRef]
- Bade, M.J.; Kohrt, W.M.; Stevens-Lapsley, J.E. Outcomes before and after total knee arthroplasty compared to healthy adults. J. Orthop. Sports Phys. Ther. 2010, 40, 559–567. [Google Scholar] [CrossRef]
- Gómez, J.F.; Curcio, C.-L.; Alvarado, B.; Zunzunegui, M.V.; Guralnik, J. Validity and reliability of the short physical performance battery (SPPB): A Pilot Study on Mobility in the Colombian Andes. Colomb. Medica 2013, 44, 165–171. [Google Scholar] [CrossRef]
- Kim, J.G.; Ha, J.K.; Lee, J.Y.; Seo, S.S.; Choi, C.H.; Lee, M.C. Translation and validation of the Korean version of the international knee documentation committee subjective knee form. Knee Surg. Relat. Res. 2013, 25, 106. [Google Scholar] [CrossRef][Green Version]
- Alghadir, A.; Anwer, S.; Iqbal, Z.A.; Alsanawi, H.A. Cross-Cultural adaptation, reliability and validity of the arabic version of the reduced western ontario and mcmaster universities osteoarthritis index in patients with knee osteoarthritis. Disabil. Rehabil. 2016, 38, 689–694. [Google Scholar] [CrossRef]
- Song, J.-S.; Hong, K.-T.; Kim, N.-M.; Park, H.-S.; Choi, N.-H. human umbilical cord blood-derived mesenchymal stem cell implantation for osteoarthritis of the knee. Arch. Orthop. Trauma. Surg. 2020, 140, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Vitale, J.A.; Messina, C.; Albano, D.; Fascio, E.; Galbusera, F.; Corbetta, S.; Sconfienza, L.M.; Banfi, G. Appendicular muscle mass, thigh intermuscular fat infiltration, and risk of fall in postmenopausal osteoporotic elder women. Gerontology 2021, 67, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Coggan, A.R.; Hoffman, R.L.; Gray, D.A.; Moorthi, R.N.; Thomas, D.P.; Leibowitz, J.L.; Thies, D.; Peterson, L.R. A single dose of dietary nitrate increases maximal knee extensor angular velocity and power in healthy older men and women. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.; Lewis, J.R.; Blekkenhorst, L.C.; Bondonno, C.P.; Devine, A.; Zhu, K.; Peeling, P.; Prince, R.L.; Hodgson, J.M. Dietary nitrate intake is associated with muscle function in older women. J. Cachexia Sarcopenia Muscle 2019, 10, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.K.; Hirai, D.M.; Copp, S.W.; Holdsworth, C.T.; Allen, J.D.; Jones, A.M.; Musch, T.I.; Poole, D.C. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J. Physiol. 2013, 591, 547–557. [Google Scholar] [CrossRef]
- Hernández, A.; Schiffer, T.A.; Ivarsson, N.; Cheng, A.J.; Bruton, J.D.; Lundberg, J.O.; Weitzberg, E.; Westerblad, H. Dietary nitrate increases tetanic [ca2+ ]i and contractile force in mouse fast-twitch muscle. J. Physiol. 2012, 590, 3575–3583. [Google Scholar] [CrossRef]
- Campos, H.O.; Drummond, L.R.; Rodrigues, Q.T.; Machado, F.S.; Pires, W.; Wanner, S.P.; Coimbra, C.C. Nitrate supplementation improves physical performance specifically in non-athletes during prolonged open-ended tests: A systematic review and meta-analysis. Br. J. Nutr. 2018, 119, 636–657. [Google Scholar] [CrossRef]
- Vårvik, F.T.; Bjørnsen, T.; Gonzalez, A.M. Acute effect of citrulline malate on repetition performance during strength training: A systematic review and meta-analysis. Int. J. Sport. Nutr. Exerc. Metab. 2021, 31, 350–358. [Google Scholar] [CrossRef]
- Fry, A.C. The role of resistance exercise intensity on muscle fibre adaptations. Sports Med. 2004, 34, 663–679. [Google Scholar] [CrossRef]
- Rojano-Ortega, D.; Peña Amaro, J.; Berral-Aguilar, A.J.; Berral-de la Rosa, F.J. Effects of beetroot supplementation on recovery after exercise-induced muscle damage: A systematic review. Sports Health 2022, 14, 556–565. [Google Scholar] [CrossRef]
- Jones, L.; Bailey, S.J.; Rowland, S.N.; Alsharif, N.; Shannon, O.M.; Clifford, T. The effect of nitrate-rich beetroot juice on markers of exercise-induced muscle damage: A systematic review and meta-analysis of human intervention trials. J. Diet. Suppl. 2022, 19, 749–771. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Gray, S.R.; Pell, J.P.; Celis-Morales, C.; Ho, F.K. Biomarkers profile of people with sarcopenia: A cross-sectional analysis from UK biobank. J. Am. Med. Dir. Assoc. 2020, 21, e1–e2017. [Google Scholar] [CrossRef] [PubMed]
- Esen, O.; Faisal, A.; Zambolin, F.; Bailey, S.J.; Callaghan, M.J. Effect of nitrate supplementation on skeletal muscle motor unit activity during isometric blood flow restriction exercise. Eur. J. Appl. Physiol. 2022, 122, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Lee, S.-U.; Lim, J.-Y.; Chung, S.G.; Lee, S.J.; Lee, S.Y. Low skeletal muscle mass and radiographic osteoarthritis in knee, hip, and lumbar spine: A cross-sectional study. Aging Clin. Exp. Res. 2019, 31, 1557–1562. [Google Scholar] [CrossRef]
- Rinaldo, L.; Caligari, M.; Acquati, C.; Nicolazzi, S.; Paracchini, G.; Sardano, D.; Giordano, A.; Marcassa, C.; Corrà, U. Functional capacity assessment and minimal clinically important difference in post-acute cardiac patients: The role of short physical performance battery. Eur. J. Prev. Cardiol. 2022, 29, 1008–1014. [Google Scholar] [CrossRef]
- Guralnik, J.; Bandeen-Roche, K.; Bhasin, S.A.R.; Eremenco, S.; Landi, F.; Muscedere, J.; Perera, S.; Reginster, J.-Y.; Woodhouse, L.; Vellas, B.; et al. Clinically meaningful change for physical performance: Perspectives of the ICFSR task force. J. Frailty Aging 2020, 9, 9–13. [Google Scholar] [CrossRef]
- Brech, G.C.; Alonso, A.C.; Luna, N.M.S.; Greve, J.M. Correlation of postural balance and knee muscle strength in the sit-to-stand test among women with and without postmenopausal osteoporosis. Osteoporos. Int. 2013, 24, 2007–2013. [Google Scholar] [CrossRef]
- Takacs, J.; Carpenter, M.G.; Garland, S.J.; Hunt, M.A. Factors associated with dynamic balance in people with knee osteoarthritis. Arch. Phys. Med. Rehabil. 2015, 96, 1873–1879. [Google Scholar] [CrossRef]
- The IOF-ESCEO Sarcopenia Working Group; Beaudart, C.; Dawson, A.; Shaw, S.C.; Harvey, N.C.; Kanis, J.A.; Binkley, N.; Reginster, J.Y.; Chapurlat, R.; Chan, D.C.; et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: Systematic review. Osteoporos. Int. 2017, 28, 1817–1833. [Google Scholar] [CrossRef]
- McKendry, J.; Currier, B.S.; Lim, C.; Mcleod, J.C.; Thomas, A.C.; Phillips, S.M. Nutritional supplements to support resistance exercise in countering the sarcopenia of aging. Nutrients 2020, 12, 2057. [Google Scholar] [CrossRef]
- Martien, S.; Delecluse, C.; Boen, F.; Seghers, J.; Pelssers, J.; Van Hoecke, A.-S.; Van Roie, E. Is knee extension strength a better predictor of functional performance than handgrip strength among older adults in three different settings? Arch. Gerontol. Geriatr. 2015, 60, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Casaña, J.; Calatayud, J.; Silvestre, A.; Sánchez-Frutos, J.; Andersen, L.L.; Jakobsen, M.D.; Ezzatvar, Y.; Alakhdar, Y. Knee Extensor muscle strength is more important than postural balance for stair-climbing ability in elderly patients with severe knee osteoarthritis. Int. J. Environ. Res. Public Health 2021, 18, 3637. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Ackermann, J.; Mestriner, A.B.; Merkely, G.; Gomoll, A.H. The minimal clinically important difference and substantial clinical benefit in the patient-reported outcome measures of patients undergoing osteochondral allograft transplantation in the knee. Cartilage 2021, 12, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Koh, I.J.; Choi, K.Y.; Sung, Y.G.; Park, D.C.; Lee, H.J.; In, Y. The minimal clinically important difference (MCID) for the womac and factors related to achievement of the MCID after medial opening wedge high tibial osteotomy for knee osteoarthritis. Am. J. Sports Med. 2021, 49, 2406–2415. [Google Scholar] [CrossRef]
| Phase | Classification | Exercise | Cadence | Intensity (OMNI Scale) | Repetition |
|---|---|---|---|---|---|
| NWB Phase (0–6 weeks) | Warm-up | UBC | - | - | 5 min |
| Stretching | - | - | 10 min | ||
| Strengthening | Q/H setting | - | - | 10 s × 10 reps | |
| Four-way SLR | - | - | 10 s × 10 reps | ||
| Knee extension with Thera-band (Yellow/red) | Moderate | 4–6 | 12 reps × 5 sets (by 3 weeks) 8 reps × 4 sets (by 4–6 weeks) | ||
| Hamstring curl with Thera-band (Yellow/red) | Moderate | 4–6 | |||
| Additional exercise | Ankle dorsi/plantar flexion with Thera-band (Yellow/red) | Moderate | 4–6 | 12 reps × 5 sets | |
| Hip ab/adduction | |||||
| Cool-down | Cool-down | 5 min | |||
| FWB Phase (6–12 weeks) | Warm-up | Stationary bike | - | - | 5 min |
| Stretching | - | - | 10 min | ||
| Strengthening | Leg extension with machine | Moderate to Slow | 4–6 | 12 reps × 5 sets (by 9 weeks) 8 reps × 4 sets (by 9–12 weeks) | |
| Hamstring curl with machine | Moderate to Slow | 4–6 | |||
| Leg press | Moderate to Slow | 4–6 | |||
| Squat | Moderate to Slow | 4–6 | |||
| Lunge | Moderate to Slow | 4–6 | |||
| Additional exercise | Balance and proprioceptive exercises | - | - | 10 min | |
| Cool-down | Cool-down | 5 min |
| NG (n = 18) | PG (n = 18) | Δ (95% CI) | p Value | |
|---|---|---|---|---|
| Age (years) | 59.44 ± 3.24 | 59.33 ± 4.00 | 0.11 (−2.35 to 2.58) | 0.928 † |
| Height (cm) | 157.93 ± 4.04 | 156.71 ± 4.27 | 1.22 (−1.64 to 4.08) | 0.391 † |
| Weight (kg) | 59.57 ± 5.18 | 60.02 ± 4.97 | −0.45 (−3.95 to 3.04) | 0.795 † |
| BMI (kg/m2) | 23.92 ± 2.32 | 24.45 ± 1.95 | −0.53 (−2.02 to 0.95) | 0.468 † |
| KL grade 2 (n) | 4 (22.2%) | 5 (27.8%) | 0.700 § | |
| KL grade 3 (n) | 14 (77.8%) | 13 (72.2%) | ||
| Right knee (n) | 9 (50.0%) | 8 (44.4%) | 0.738 § | |
| Left knee (n) | 9 (50.0%) | 10 (55.6%) | ||
| CSA (cm2) | 76.96 ± 5.38 | 75.25 ± 3.90 | 1.72 (−1.47 to 4.90) | 0.281 † |
| Knee Extension MVIC (%) | 71.52 ± 21.62 | 61.42 ± 18.45 | 10.09 (−3.52 to 23.71) | 0.084 ‡ |
| Knee Flexion MVIC (%) | 35.17 ± 16.27 | 31.53 ± 12.02 | 3.63 (−6.06 to 13.32) | 0.451 † |
| SMI (kg/m2) | 5.35 ± 0.27 | 5.29 ± 0.37 | 0.06 (−0.16 to 0.28) | 0.602 † |
| HGS (kg/f) | 14.72 ± 2.00 | 14.87 ± 1.69 | −0.14 (−1.40 to 1.11) | 0.816 † |
| IKDC (point) | 34.94 ± 8.40 | 36.28 ± 7.82 | −1.35 (−6.85 to 4.15) | 0.621 † |
| WOMAC (point) | 49.31 ± 12.85 | 50.07 ± 7.55 | −0.76 (−7.90 to 6.38) | 0.930 † |
| Balance test (point) | 2.94 ± 0.64 | 3.17 ± 0.62 | −0.22 (−0.65 to 0.20) | 0.292 ‡ |
| Gait Speed test (point) | 3.72 ± 0.46 | 3.61 ± 0.50 | 0.11 (−0.22 to 0.44) | 0.486 ‡ |
| Chair Standing test (point) | 1.17 ± 0.79 | 1.33 ± 0.84 | −0.17 (−0.72 to 0.38) | 0.575 ‡ |
| SPPB composite (point) | 7.83 ± 0.99 | 8.11 ± 0.96 | −0.28 (−0.94 to 0.38) | 0.443 ‡ |
| Baseline | 6 Weeks | 12 Weeks | Mean Change (95% CI) † | Mean Change (95% CI) ‡ | Mean Difference (95% CI) § | Mean Difference (95% CI) †† | |
|---|---|---|---|---|---|---|---|
| Thigh Muscle Cross-Sectional Area (cm2) | |||||||
| NG (n = 18) | 76.96 ± 5.38 | - | 75.57 ± 6.5 | - | −1.39 (−2.94 to 0.15) | - | 3.92 (0.12 to 7.71) |
| PG (n = 18) | 75.25 ± 3.90 | - | 71.65 ± 4.53 | - | −3.59 * (−5.14 to −2.05) | ||
| Knee Extension Maximal Voluntary Isometric Contraction (%) | |||||||
| NG (n = 18) | 71.52 ± 21.62 | 74.85 ± 18.15 | 92.24 ± 19.88 | 3.31 (−4.17 to 10.8) | 22.88 * (15.42 to 30.33) | 18.98 * (9.01 to 28.96) | 16.76 * (5.46 to 28.07) |
| PG (n = 18) | 61.42 ± 18.45 | 55.87 ± 10.20 | 75.48 ± 12.72 | −6.25 (−1.24 to 13.74) | 15.81 * (8.36 to 23.27) | ||
| Knee Flexion Maximal Voluntary Isometric Contraction (%) | |||||||
| NG (n = 18) | 35.17 ± 16.27 | 45.67 ± 16.12 | 47.00 ± 11.95 | 10.50 * (5.96 to 15.04) | 11.83 * (6.84 to 16.82) | 11.24 (1.87 to 20.62) | 7.91 (0.38 to 15.44) |
| PG (n = 18) | 31.53 ± 12.02 | 34.42 ± 11.11 | 39.09 ± 10.22 | 2.89 (−1.65 to 7.43) | 7.56 * (2.56 to 12.55) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-S.; Yoon, J.-H.; Oh, J.-K. Impact of Resistance Exercise and Nitrate Supplementation on Muscle Function and Clinical Outcomes After Knee Osteoarthritis Surgery in Middle-Aged Women with Sarcopenia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Clin. Med. 2025, 14, 615. https://doi.org/10.3390/jcm14020615
Park H-S, Yoon J-H, Oh J-K. Impact of Resistance Exercise and Nitrate Supplementation on Muscle Function and Clinical Outcomes After Knee Osteoarthritis Surgery in Middle-Aged Women with Sarcopenia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Journal of Clinical Medicine. 2025; 14(2):615. https://doi.org/10.3390/jcm14020615
Chicago/Turabian StylePark, Han-Soo, Jin-Ho Yoon, and Jae-Keun Oh. 2025. "Impact of Resistance Exercise and Nitrate Supplementation on Muscle Function and Clinical Outcomes After Knee Osteoarthritis Surgery in Middle-Aged Women with Sarcopenia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial" Journal of Clinical Medicine 14, no. 2: 615. https://doi.org/10.3390/jcm14020615
APA StylePark, H.-S., Yoon, J.-H., & Oh, J.-K. (2025). Impact of Resistance Exercise and Nitrate Supplementation on Muscle Function and Clinical Outcomes After Knee Osteoarthritis Surgery in Middle-Aged Women with Sarcopenia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Journal of Clinical Medicine, 14(2), 615. https://doi.org/10.3390/jcm14020615





