C-Reactive Protein in Peritoneal Fluid for Predicting Anastomotic Leakage After Colorectal Cancer Surgery: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Information Sources
2.2. Eligibility Criteria
2.3. Publication Screening and Data Extraction
2.4. Quality Assessment
2.5. Synthesis Methods
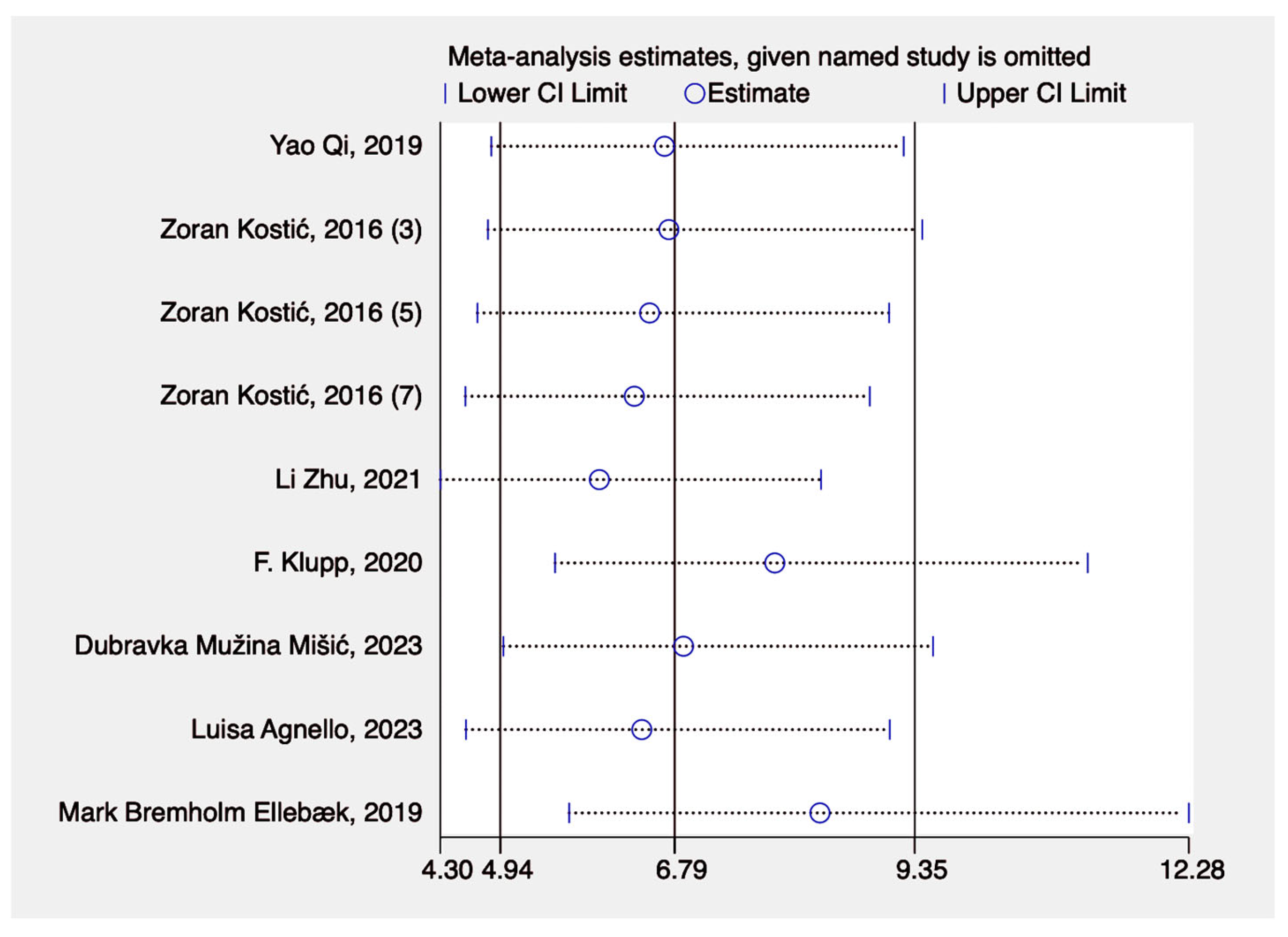
3. Results
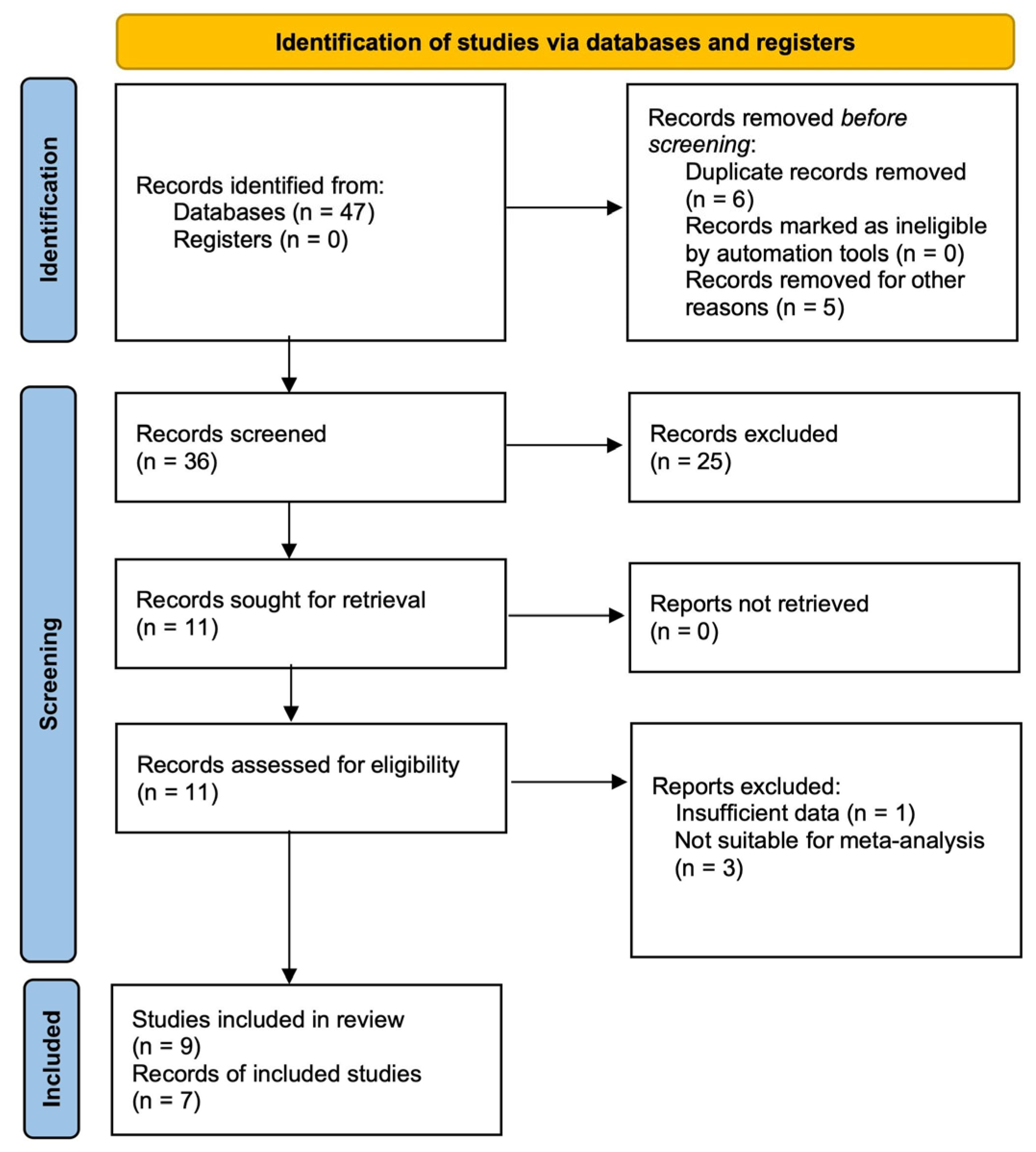
3.1. Study Selection
3.2. Study Characteristics
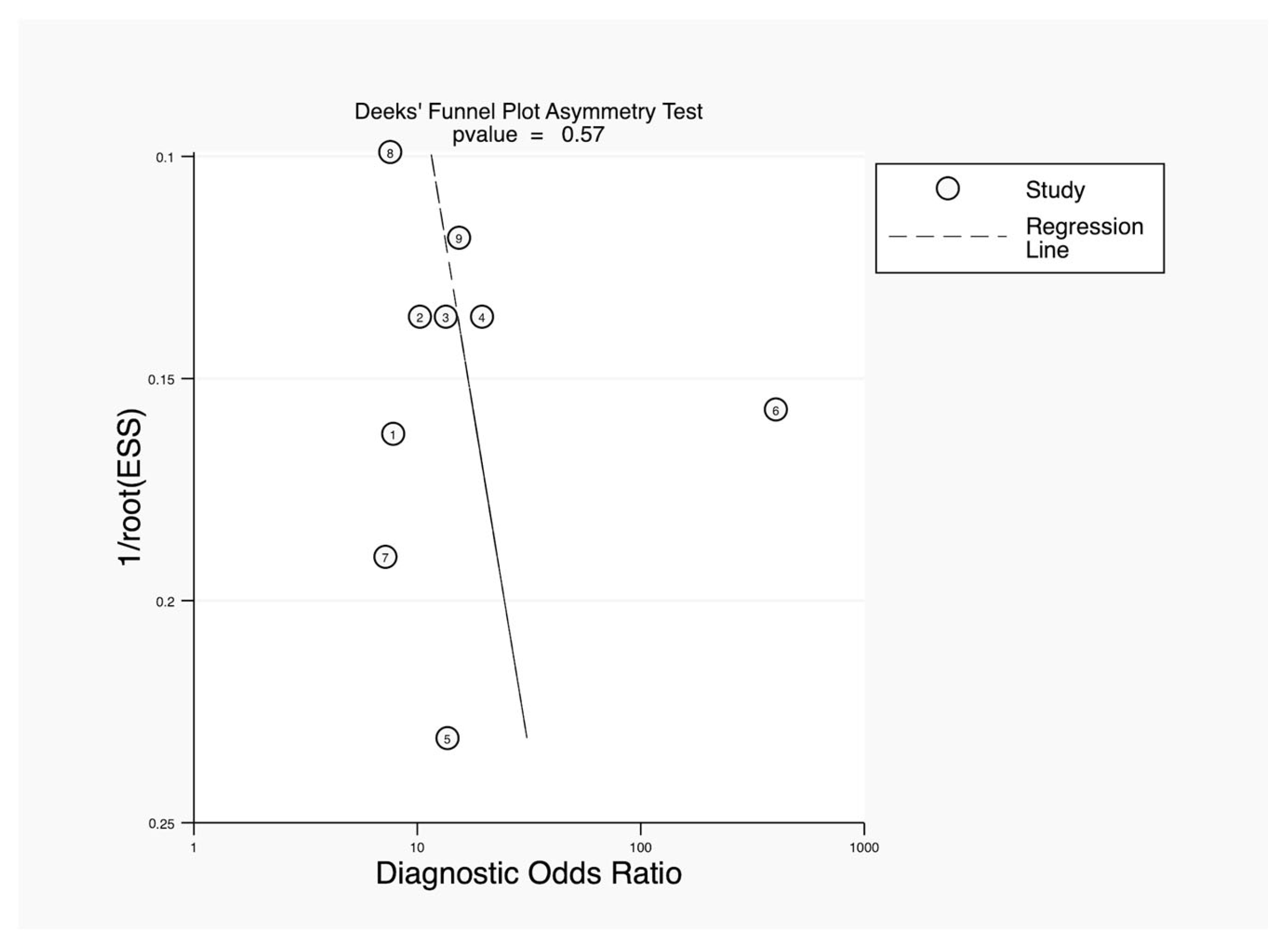
3.3. Results of Syntheses
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Center, M.M.; Jemal, A.; Ward, E. International trends in colorectal cancer incidence rates. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, P.; Clavien, P.A.; Hahnloser, D. Complications in colorectal surgery: Risk factors and preventive strategies. Patient Saf. Surg. 2010, 4, 5. [Google Scholar] [CrossRef]
- Ptok, H.; Marusch, F.; Meyer, F.; Schubert, D.; Gastinger, I.; Lippert, H.; Study Group Colon/Rectum, C. Impact of anastomotic leakage on oncological outcome after rectal cancer resection. Br. J. Surg. 2007, 94, 1548–1554. [Google Scholar] [CrossRef]
- Bruce, J.; Krukowski, Z.H.; Al-Khairy, G.; Russell, E.M.; Park, K.G. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br. J. Surg. 2001, 88, 1157–1168. [Google Scholar] [CrossRef]
- Hyman, N.; Manchester, T.L.; Osler, T.; Burns, B.; Cataldo, P.A. Anastomotic leaks after intestinal anastomosis: It’s later than you think. Ann. Surg. 2007, 245, 254–258. [Google Scholar] [CrossRef]
- Chiarello, M.M.; Fransvea, P.; Cariati, M.; Adams, N.J.; Bianchi, V.; Brisinda, G. Anastomotic leakage in colorectal cancer surgery. Surg. Oncol. 2022, 40, 101708. [Google Scholar] [CrossRef]
- Choi, H.K.; Law, W.L.; Ho, J.W. Leakage after resection and intraperitoneal anastomosis for colorectal malignancy: Analysis of risk factors. Dis. Colon. Rectum 2006, 49, 1719–1725. [Google Scholar] [CrossRef]
- Tsalikidis, C.; Mitsala, A.; Mentonis, V.I.; Romanidis, K.; Pappas-Gogos, G.; Tsaroucha, A.K.; Pitiakoudis, M. Predictive Factors for Anastomotic Leakage Following Colorectal Cancer Surgery: Where Are We and Where Are We Going? Curr. Oncol. 2023, 30, 3111–3137. [Google Scholar] [CrossRef]
- Bakker, I.S.; Grossmann, I.; Henneman, D.; Havenga, K.; Wiggers, T. Risk factors for anastomotic leakage and leak-related mortality after colonic cancer surgery in a nationwide audit. Br. J. Surg. 2014, 101, 424–432; discussion 432. [Google Scholar] [CrossRef] [PubMed]
- McDermott, F.D.; Heeney, A.; Kelly, M.E.; Steele, R.J.; Carlson, G.L.; Winter, D.C. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br. J. Surg. 2015, 102, 462–479. [Google Scholar] [CrossRef] [PubMed]
- Kyrochristou, I.; Spartalis, E.; Anagnostopoulos, G.; Tsourouflis, G.; Dimitroulis, D.; Nikiteas, N.I. CRP in Drain Fluid as a Predictive Marker of Anastomotic Leak in Colorectal Surgery: A Systematic Review of the Literature. In Vivo 2023, 37, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Komen, N.; Slieker, J.; Willemsen, P.; Mannaerts, G.; Pattyn, P.; Karsten, T.; de Wilt, H.; van der Harst, E.; de Rijke, Y.B.; Murawska, M.; et al. Acute phase proteins in drain fluid: A new screening tool for colorectal anastomotic leakage? The APPEAL study: Analysis of parameters predictive for evident anastomotic leakage. Am. J. Surg. 2014, 208, 317–323. [Google Scholar] [CrossRef]
- Marnell, L.; Mold, C.; Du Clos, T.W. C-reactive protein: Ligands, receptors and role in inflammation. Clin. Immunol. 2005, 117, 104–111. [Google Scholar] [CrossRef]
- Volanakis, J.E. Human C-reactive protein: Expression, structure, and function. Mol. Immunol. 2001, 38, 189–197. [Google Scholar] [CrossRef]
- Black, S.; Kushner, I.; Samols, D. C-reactive Protein. J. Biol. Chem. 2004, 279, 48487–48490. [Google Scholar] [CrossRef]
- Gray, M.; Marland, J.R.K.; Murray, A.F.; Argyle, D.J.; Potter, M.A. Predictive and Diagnostic Biomarkers of Anastomotic Leakage: A Precision Medicine Approach for Colorectal Cancer Patients. J. Pers. Med. 2021, 11, 471. [Google Scholar] [CrossRef]
- Wright, E.C.; Connolly, P.; Vella, M.; Moug, S. Peritoneal fluid biomarkers in the detection of colorectal anastomotic leaks: A systematic review. Int. J. Color. Dis. 2017, 32, 935–945. [Google Scholar] [CrossRef]
- Buchs, N.C.; Gervaz, P.; Secic, M.; Bucher, P.; Mugnier-Konrad, B.; Morel, P. Incidence, consequences, and risk factors for anastomotic dehiscence after colorectal surgery: A prospective monocentric study. Int. J. Color. Dis. 2008, 23, 265–270. [Google Scholar] [CrossRef]
- Daams, F.; Luyer, M.; Lange, J.F. Colorectal anastomotic leakage: Aspects of prevention, detection and treatment. World J. Gastroenterol. 2013, 19, 2293–2297. [Google Scholar] [CrossRef] [PubMed]
- MPage, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar]
- Yao, Q.; He, L.; Liu, X.; Zhou, J.; Lv, W. Predictive value of PCT and CRP in peritoneal drainage fluid for postoperative anastomotic leakage after laparoscopic Dixon radical resection of rectal cancer (腹腔镜Dixon直肠癌术后动态监测腹腔引流液中降钙素原、 CRP水平对吻合口瘘的预测价值). J. New Med. 2019, 50, 850–854. [Google Scholar] [CrossRef]
- Li, Z.; Liao, X.; Lv, H.; Xiang, D.; Hou, M. Clinical value of serum and abdominal drainage fluid CRP levels in predicting anastomotic leakage following laparoscopic surgery of colorectal cancer (血清CRP、 腹腔引流液CRP在预测腹腔镜结直肠癌术后吻合口瘘的临床价值). J. Guangzhou Med. Univ. 2021, 49, 77–82. [Google Scholar] [CrossRef]
- Klupp, F.; Schuler, S.; Kahlert, C.; Halama, N.; Franz, C.; Mayer, P.; Schmidt, T.; Ulrich, A. Evaluation of the inflammatory markers CCL8, CXCL5, and LIF in patients with anastomotic leakage after colorectal cancer surgery. Int. J. Color. Dis. 2020, 35, 1221–1230. [Google Scholar] [CrossRef]
- Muzina Misic, D.; Zovak, M.; Kopljar, M.; Cicek, S.; Bilic, Z. Comparison of C-Reactive Protein Levels in Serum and Peritoneal Fluid in Early Diagnosis of Anastomotic Leakage after Colorectal Surgery. Acta Clin. Croat. 2023, 62, 11–18. [Google Scholar] [CrossRef]
- Ellebaek, M.B.; Rahr, H.B.; Boye, S.; Fristrup, C.; Qvist, N. Detection of early anastomotic leakage by intraperitoneal microdialysis after low anterior resection for rectal cancer: A prospective cohort study. Color. Dis. 2019, 21, 1387–1396. [Google Scholar] [CrossRef]
- Agnello, L.; Buscemi, S.; Di Buono, G.; Vidali, M.; Lo Sasso, B.; Agrusa, A.; Ciaccio, M. Drainage fluid LDH and neutrophil to lymphocyte ratio as biomarkers for early detecting anastomotic leakage in patients undergoing colorectal surgery. Clin. Chem. Lab. Med. 2024, 62, 967–978. [Google Scholar] [CrossRef]
- Kostic, Z.; Slavkovic, D.; Mijuskovic, Z.; Panisic, M.; Ignjatovic, M. C-reactive protein in drainage fluid as a predictor of anastomotic leakage after elective colorectal resection. Vojnosanit. Pregl. 2016, 73, 228–233. [Google Scholar] [CrossRef]
- Chi, P.; Huang, S. Anastomotic leakage after rectal cancer surgery: Classification and management (直肠癌术后吻合口漏的分类和治疗策略). Chin. J. Gastointest. Surg. 2018, 21, 365–371. [Google Scholar] [CrossRef]
- Singh, P.P.; Zeng, I.S.; Srinivasa, S.; Lemanu, D.P.; Connolly, A.B.; Hill, A.G. Systematic review and meta-analysis of use of serum C-reactive protein levels to predict anastomotic leak after colorectal surgery. Br. J. Surg. 2014, 101, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.H.; Noh, G.T.; Chung, S.S.; Kim, K.H.; Lee, R.A. Validity of C-Reactive Protein as a Surrogate Marker for Infectious Complications After Surgery for Colorectal Cancer. Surg. Infect. 2023, 24, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wu, Z.; Wu, X.; Shan, F.; Zhang, Y.; Ying, X.; Li, Z.; Ji, J. Early diagnosis of anastomotic leakage after colorectal cancer surgery using an inflammatory factors-based score system. BJS Open 2022, 6, zrac069. [Google Scholar] [CrossRef] [PubMed]
- Cuff, S.M.; Reeves, N.; Lewis, E.; Jones, E.; Baker, S.; Karategos, A.; Morris, R.; Torkington, J.; Eberl, M. Inflammatory biomarker signatures in post-surgical drain fluid may detect anastomotic leaks within 48 hours of colorectal resection. Tech. Coloproctology 2023, 27, 1297–1305. [Google Scholar] [CrossRef]
- Qi, X.Y.; Liu, M.X.; Xu, K.; Gao, P.; Tan, F.; Yao, Z.D.; Zhang, N.; Yang, H.; Zhang, C.H.; Xing, J.D.; et al. Peritoneal Cytokines as Early Biomarkers of Colorectal Anastomotic Leakage Following Surgery for Colorectal Cancer: A Meta-Analysis. Front. Oncol. 2021, 11, 791462. [Google Scholar] [CrossRef]
- Alonso, S.; Pascual, M.; Salvans, S.; Mayol, X.; Mojal, S.; Gil, M.J.; Grande, L.; Pera, M. Postoperative intra-abdominal infection and colorectal cancer recurrence: A prospective matched cohort study of inflammatory and angiogenic responses as mechanisms involved in this association. Eur. J. Surg. Oncol. 2015, 41, 208–214. [Google Scholar] [CrossRef]
- Sammour, T.; Singh, P.P.; Zargar-Shoshtari, K.; Su’a, B.; Hill, A.G. Peritoneal Cytokine Levels Can Predict Anastomotic Leak on the First Postoperative Day. Dis. Colon. Rectum 2016, 59, 551–556. [Google Scholar] [CrossRef]
- Ugras, B.; Giris, M.; Erbil, Y.; Gokpinar, M.; Citlak, G.; Issever, H.; Bozbora, A.; Oztezcan, S. Early prediction of anastomotic leakage after colorectal surgery by measuring peritoneal cytokines: Prospective study. Int. J. Surg. 2008, 6, 28–35. [Google Scholar] [CrossRef]
- Cini, C.; Wolthuis, A.; D’Hoore, A. Peritoneal fluid cytokines and matrix metalloproteinases as early markers of anastomotic leakage in colorectal anastomosis: A literature review and meta-analysis. Color. Dis. 2013, 15, 1070–1077. [Google Scholar] [CrossRef]
- Bilgin, I.A.; Hatipoglu, E.; Aghayeva, A.; Arikan, A.E.; Incir, S.; Mamal Torun, M.; Dirican, A.; Erguney, S. Predicting Value of Serum Procalcitonin, C-Reactive Protein, Drain Fluid Culture, Drain Fluid Interleukin-6, and Tumor Necrosis Factor-alpha Levels in Anastomotic Leakage after Rectal Resection. Surg. Infect. 2017, 18, 350–356. [Google Scholar] [CrossRef]
- Reeves, N.; Vogel, I.; Ghoroghi, A.; Ansell, J.; Cornish, J.; Torkington, J. Peritoneal cytokines as a predictor of colorectal anastomotic leaks on postoperative day 1: A systematic review and meta-analysis. Tech. Coloproctol. 2022, 26, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Oikonomakis, I.; Jansson, D.; Horer, T.M.; Skoog, P.; Nilsson, K.F.; Jansson, K. Results of postoperative microdialysis intraperitoneal and at the anastomosis in patients developing anastomotic leakage after rectal cancer surgery. Scand. J. Gastroenterol. 2019, 54, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, X.E.; Xu, L.; Zhou, X.; Zhou, J.N.; Yu, D.S.; Li, D.Z.; Guan, X. Acidic pelvic drainage as a predictive factor for anastomotic leakage after surgery for patients with rectal cancer. Asian Pac. J. Cancer Prev. 2013, 14, 5441–5447. [Google Scholar] [CrossRef]
- Molinari, E.; Giuliani, T.; Andrianello, S.; Talamini, A.; Tollini, F.; Tedesco, P.; Pirani, P.; Panzeri, F.; Sandrini, R.; Remo, A.; et al. Drain fluid’s pH predicts anastomotic leak in colorectal surgery: Results of a prospective analysis of 173 patients. Minerva Chir. 2020, 75, 30–36. [Google Scholar] [CrossRef]
- Komen, N.; Slieker, J.; Willemsen, P.; Mannaerts, G.; Pattyn, P.; Karsten, T.; de Wilt, H.; van der Harst, E.; van Leeuwen, W.; Decaestecker, C.; et al. Polymerase chain reaction for Enterococcus faecalis in drain fluid: The first screening test for symptomatic colorectal anastomotic leakage. The Appeal-study: Analysis of parameters predictive for evident anastomotic leakage. Int. J. Color. Dis. 2014, 29, 15–21. [Google Scholar] [CrossRef]
- Tominaga, T.; Nonaka, T.; Oyama, S.; Takamura, Y.; Hashimoto, S.; Shiraishi, T.; Sawai, T.; Nagayasu, T. Utility of Drain Fluid Culture and Gram Stain in Early Intervention for Occult Anastomotic Leakage in Colorectal Cancer. Anticancer Res. 2022, 42, 3091–3098. [Google Scholar] [CrossRef]
- Ge, W.; Gong, H.Y.; Xia, Y.Q.; Shao, L.H.; Shen, H.; Chen, G. Bacteriological concentration of peritoneal drainage fluid could make an early diagnosis of anastomotic leakage following rectal resection. Sci. Rep. 2021, 11, 23156. [Google Scholar] [CrossRef]



| Author | Country | Study Design | Resection | SP | P | AL | POD | CV | AUC | SEN | SPE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| YaoQi, 2019 [23] | China | Prospective | Rectal | 5 | 80 | 5 | 5 | 57.68 | 0.855 | 88 | 77 |
| Li Zhu, 2021 [24] | China | Retrospective | Colorectal | 5 | 140 | 10 | 5 | 147 | 0.984 | 100 | 95 |
| F.Klupp, 2020 [25] | Germany | Prospective | Colorectal | 3 | 38 | 18 | 3 | 62.92 | 0.703 | 72.2 | 75 |
| Dubravka Muzina Misic, 2023 [26] | Croatia | Prospective | Colorectal | 4 | 59 | 8 | 4 | 55.2 | 0.833 | 71 | 71 |
| Mark Bremholm Ellebæk, 2019 [27] | Denmark | Prospective | Rectal | 7 | 129 | 35 | 7 | 146 | 0.8 | 67 | 80 |
| Luisa-Agnello, 2023 [28] | Italy | Prospective | Colorectal | 5 | 187 | 20 | 3 | 76 | 0.752 | 72 | 87 |
| Zoran Kostic, 2016 [29] | Serbia | Prospective | Colorectal | 7 | 150 | 15 | 3 | 77 | 0.745 | 67 | 84 |
| 5 | 53 | 0.879 | 80 | 77 | |||||||
| 7 | 42 | 0.824 | 80 | 83 |
| Categories | N | SEN | SPE | PLR | NLR | DOR | AUC | H | |
|---|---|---|---|---|---|---|---|---|---|
| (95%CI) | (95%CI) | (95%CI) | (95%CI) | (95%CI) | (95%CI) | I2 | p-Value | ||
| Overall | 9 | 0.74 (0.65–0.82) | 0.83 (0.77–0.87) | 4.3 (3–6.1) | 0.31 (0.22–0.44) | 14 (7–27) | 0.84 (0.81–0.87) | 0 | 0.433 |
| Subgroup | |||||||||
| POD 3–5 | 7 | 0.76 (0.65–0.85) | 0.83 (0.75–0.89) | 4.5 (2.8–7.1) | 0.29 (0.18–0.45) | 16 (7–36) | 0.85 (0.82–0.88) | 0 | 0.395 |
| POD 5–7 | 5 | 0.82 (0.59–0.94) | 0.84 (0.75–0.90) | 5.1 (2.7–9.5) | 0.21 (0.08–0.59) | 24 (5–117) | 0.90 (0.87–0.92) | 0 | 0.421 |
| CV 40–70 | 5 | 0.77 (0.65–0.86) | 0.78 (0.74–0.82) | 3.5 (2.8–4.4) | 0.29 (0.18–0.47) | 12 (6–23) | 0.84 (0.81–0.87) | 100 | 0.500 |
| CV 70–150 | 4 | 0.77 (0.55–0.91) | 0.88 (0.80–0.93) | 6.3 (3.1 -12.8) | 0.26 (0.11–0.61) | 25 (5–112) | 0.91 (0.88–0.93) | 0 | 0.449 |
| Author | Methodology * | Inclusion | Exclusion | Limitation | Clinical Implication | Comment |
|---|---|---|---|---|---|---|
| Yao Qi 2019 [23] | Immunoscattering turbidimetry: Peritoneal drainage fluid collected on POD 0, 3, 5. | Laparoscopic Dixon radical resection of rectal cancer from July 2017 to July 2018; Confirmed rectal cancer by preoperative examination and postoperative pathology; No infection before or after surgery. | Severe liver and kidney dysfunction; Other primary malignant tumors; Severe postoperative infection; Crohn’s disease. | No comparison between peritoneal fluid and serum markers for predicting leakage; Small sample size. | Monitoring CRP in drainage fluid post-surgery can guide AL prevention and management. | Future studies should compare peritoneal CRP and PCT with serum markers for AL prediction. |
| Li Zhu 2021 [24] | Immunoturbidimetry (Beckman Coulter automated biochemical analyzer, Brea, CA, USA): Peritoneal drainage fluid collected on POD 1, 3, 5. | Complete clinical data; Confirmed CRC by colonoscopy and biopsy; Indication for laparoscopic radical surgery; No preoperative infection; No residual tumors confirmed by postoperative pathology. | Autoimmune diseases; Recent use of immunosuppressants or hormones; Severe multi-organs disfunction; Other malignancies; Colorectal tumors with local inflammation and elevated preoperative CRP. | Selection bias, small sample size, retrospective nature. | Dynamic CRP monitoring in serum and drainage fluid aids early AL detection on specific postoperative days. | Validate findings in larger studies; investigate optimal CRP cut-off values in serum and drainage fluid. |
| F. Klupp 2020 [25] | ELISA (Luminex®-based multiplex assay, Austin, TX, USA): Peritoneal drainage fluid collected on POD 1 to 3. | Elective surgery of CRC with a complete set of samples and without other postoperative complications. | Secondary carcinomas; Drainage removal before the POD3; Postoperative infections. | Small sample size and potential confounding factors; limited applicability. | Monitoring CRP in serum and peritoneal fluid on day 3 aids early AL detection, improving outcomes. | Use CRP in peritoneal fluid to monitor local inflammation and AL; validate findings in larger cohorts. |
| Dubravka Mužina Mišić 2020 [26] | (Latex immunoturbidimetry): Peritoneal drainage fluid collected on POD 1 to 4. | CRC resection with primary anastomosis between January 2019 and October 2019. | Under 18 years; Receiving neoadjuvant CRT; Emergency surgery; On steroid therapy. | Study’s small sample size affects generalizability; Only included patients without other complications. | Monitoring CRP in serum and peritoneal fluid with repetitive day 4 measurements helps early AL detection. | Validate findings with larger cohorts; refine CRP cut-off values; explore CRP’s utility in combination with serum levels. |
| Mark Bremholm Ellebæk 2019 [27] | Immunoturbidimetry: Peritoneal drainage fluid collected on POD 1 to 7. | Age ≥ 18 years; Biopsy-proven adenocarcinoma of the rectum (lower border within 15 cm from the anal verge by rigid proctoscopy); Potentially curative surgery, irrespective of neoadjuvant therapy; Written informed consent. | Patients not meeting the inclusion criteria were excluded. | Small sample size, single-center design, and need for larger studies noted; logistical challenges affected study duration. | CRP is valuable for early AL detection post-surgery; it complements intraperitoneal lactate in surveillance. | Explore systematic CRP use with other diagnostic tools for AL; CRP can improve early detection and management. |
| Luisa Agnello 2023 [28] | Immunoscattering turbidimetry (Cobas 8000, Roche Diagnostics, Indianapolis, IN, USA): Peritoneal drainage fluid collected on POD 3, 5. | Aged > 16 years undergoing elective or emergency CRC surgery; Diverticular disease; Inflammatory bowel disease; Reversal of Hartmann’s procedure. | Age < 16 years, no informed consent, no anastomosis performed during the surgical procedure. | Single-center study and small sample size limit generalizability; Larger, multicenter studies needed. | Monitoring LDH, CRP and NLR in drainage fluid allows earlier AL detection and intervention, reducing morbidity. | Validate LDH, CRP and NLR as early AL biomarkers in larger, multicenter studies; explore other potential biomarkers. |
| Zoran Kostic 2016 [29] | Immunonephelometry autoanalyzer (DADE Behring BN II, Malvern, PA, USA): Peritoneal drainage fluid collected on POD 1, 3, 5, 7. | CRC resection and primary anastomosis. | Clinical signs of infection or inflammatory conditions preoperatively; Tumor recurrence surgery; No adequate postoperative drainage fluid samples. | CRP, as an inflammation marker, should be considered in context; limited reliability as an infection indicator. | CRP measurement in drainage fluid can detect AL early, allowing timely interventions. | Confirm findings and investigate if combining CRP with other biomarkers enhances AL detection accuracy. |
| Subgroups | Sign * | AL Level | Recommend |
|---|---|---|---|
| POD 3–5 with CV 40–70 | + | Low | Observe clinical symptoms, offer conservative treatment, and continue monitoring of CRP level. |
| POD 5–7 with CV 40–70 | − | No | Discharge. |
| + | Low | Delay discharge, offer conservative treatment, continue monitoring of CRP level, and observe clinical symptoms. | |
| POD 3–5 with CV 70–150 | − | No/Low | Observe clinical symptoms, offer conservative treatment, and continue monitoring of CRP level. |
| + | Moderate | Swiftly rule out AL with contrast-enhanced CT. | |
| POD 5–7 with CV 70–150 | − | Moderate | Swiftly rule out AL with contrast-enhanced CT. If the CT does not confirm AL, consider delaying discharge. |
| + | High | If CT confirms AL, proceed with surgery or continue conservative management. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vun, T.; Wu, Z.; Chea, C.; Liu, W.; Tao, R.; Deng, Y. C-Reactive Protein in Peritoneal Fluid for Predicting Anastomotic Leakage After Colorectal Cancer Surgery: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 2099. https://doi.org/10.3390/jcm14062099
Vun T, Wu Z, Chea C, Liu W, Tao R, Deng Y. C-Reactive Protein in Peritoneal Fluid for Predicting Anastomotic Leakage After Colorectal Cancer Surgery: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(6):2099. https://doi.org/10.3390/jcm14062099
Chicago/Turabian StyleVun, Tharith, Zhanghao Wu, Chetra Chea, Weidong Liu, Ran Tao, and Youming Deng. 2025. "C-Reactive Protein in Peritoneal Fluid for Predicting Anastomotic Leakage After Colorectal Cancer Surgery: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 6: 2099. https://doi.org/10.3390/jcm14062099
APA StyleVun, T., Wu, Z., Chea, C., Liu, W., Tao, R., & Deng, Y. (2025). C-Reactive Protein in Peritoneal Fluid for Predicting Anastomotic Leakage After Colorectal Cancer Surgery: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(6), 2099. https://doi.org/10.3390/jcm14062099