Association Between Diagnostic Delay and Short-Term Outcomes in Patients with Radiographic Axial Spondyloarthritis: Results from the Regisponser-AS Registry
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Collected Data
- Sociodemographic data: Sex, age, and smoking status.
- Clinical characteristics and SpA features: Age at onset of SpA, disease duration (years between SpA diagnosis and the study visit) [18], diagnostic delay (years between symptom onset and SpA diagnosis), family history of SpA, HLA-B27 antigen status, C-reactive protein level (CRP, mg/dL), synovitis, psoriasis, inflammatory bowel disease (IBD), enthesitis, dactylitis, uveitis, swollen joints, and hip replacement.
- Patient-reported outcomes (PROs): Disease activity was assessed using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) [19], the patient-reported global visual analogue scale (global VAS), and the Ankylosing Spondylitis Disease Activity Score (ASDAS) [20]. Functional status was evaluated with the Bath Ankylosing Spondylitis Functional Index (BASFI) [21]. Structural damage was assessed using the Bath Ankylosing Spondylitis Radiology Index (BASRI), as it was the available tool at the time of data collection [4]. Additionally, the participants completed the Mental Health Survey (MSF12) and the Physical Health Survey (FSF12) to evaluate health-related quality of life [22].
- Past and current treatment: Data on previous or concurrent treatments were collected, including nonsteroidal anti-inflammatory drugs (NSAIDs), conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) (sulfasalazine, methotrexate, or leflunomide) and biologic DMARDs (bDMARDs) (anti-TNF treatment). The dates of bDMARD initiation and withdrawal were also collected.
2.3. Statistical Analysis
2.4. Handling of Missing Data
3. Results
3.1. Description of the Population
3.2. Impact of Diagnostic Delay After Two Years
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rudwaleit, M.; van der Heijde, D.; Landewé, R.; Listing, J.; Akkoc, N.; Brandt, J.; Braun, J.; Chou, C.T.; Collantes-Estevez, E.; Dougados, M.; et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann. Rheum. Dis. 2009, 68, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Compán, V.; Sepriano, A.; El-Zorkany, B.; van der Heijde, D. Axial spondyloarthritis. Ann. Rheum. Dis. 2021, 80, 1511–1521. [Google Scholar] [CrossRef]
- van der Heijde, D.; Braun, J.; Deodhar, A.; Baraliakos, X.; Landewé, R.; Richards, H.B.; Porter, B.; Readie, A. Modified stoke ankylosing spondylitis spinal score as an outcome measure to assess the impact of treatment on structural progression in ankylosing spondylitis. Rheumatology 2019, 58, 388–400. [Google Scholar] [CrossRef]
- MacKay, K.; Mack, C.; Brophy, S.; Calin, A. The Bath Ankylosing Spondylitis Radiology Index (BASRI): A new, validated approach to disease assessment. Arthritis Rheum. 1998, 41, 2263–2270. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, S.; Valkenburg, H.A.; Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984, 27, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Mandl, P.; Navarro-Compán, V.; Terslev, L.; Aegerter, P.; van der Heijde, D.; D’Agostino, M.A. EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann. Rheum. Dis. 2015, 74, 1327–1339. [Google Scholar] [CrossRef]
- Nikiphorou, E.; Ramiro, S.; van der Heijde, D.; Norton, S.; Moltó, A.; Dougados, M.; van den Bosch, F.; Landewé, R. Association of comorbidities in spondyloarthritis with poor function, work disability, and quality of life: Results from the assessment of SpondyloArthritis international Society Comorbidities in Spondyloarthritis study. Arthritis Care. Res. (Hoboken) 2018, 70, 1257–1262. [Google Scholar] [CrossRef]
- Moltó, A.; Etcheto, A.; van der Heijde, D.; Landewé, R.; van den Bosch, F.; Molano, W.B.; Burgos-Vargas, R.; Cheung, P.P.; Collantes-Estevez, E.; Deodhar, A.; et al. Prevalence of comorbidities and evaluation of their screening in spondyloarthritis: Results of the international cross-sectional ASAS-COMOSPA study. Ann. Rheum. Dis. 2016, 75, 1016–1023. [Google Scholar] [CrossRef]
- López-Medina, C.; Jiménez-Gómez, Y.; Moltó, A.; Schiotis, R.E.; Marzo-Ortega, H.; van Gaalen, F.A.; Ozgocmen, S.; Dougados, M.; Calvo-Gutiérrez, J.; Castro-Villegas, M.C.; et al. Cardiovascular risk factors in patients with spondyloarthritis from Northern European and Mediterranean countries: An ancillary study of the ASAS-COMOSPA project. Jt. Bone Spine. 2018, 85, 447–453. [Google Scholar] [CrossRef]
- Sieper, J.; Poddubnyy, D. Axial spondyloarthritis. Lancet 2017, 390, 73–84. [Google Scholar] [CrossRef]
- Sykes, M.P.; Doll, H.; Sengupta, R.; Gaffney, K. Delay to diagnosis in axial spondyloarthritis: Are we improving in the UK? Rheumatology 2015, 54, 2283–2284. [Google Scholar] [CrossRef] [PubMed]
- Hay, C.A.; Packham, J.; Ryan, S.; Mallen, C.D.; Chatzixenitidis, A.; Prior, J.A. Diagnostic delay in axial spondyloarthritis: A systematic review. Clin. Rheumatol. 2022, 41, 1939–1950. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.S.; Pittam, B.; Harrison, N.L.; Ahmed, A.E.; Goodson, N.J.; Hughes, D.M. Diagnostic delay in axial spondyloarthritis: A systematic review and meta-analysis. Rheumatology 2021, 60, 620–1628. [Google Scholar] [CrossRef]
- Jovaní, V.; Blasco-Blasco, M.; Ruiz-Cantero, M.T.; Pascual, E. Understanding how the diagnostic delay of spondyloarthritis differs between women and men: A systematic review and Metaanalysis. J. Rheumatol. 2017, 44, 174–183. [Google Scholar] [CrossRef]
- Redeker, I.; Callhoff, J.; Hoffmann, F.; Haibel, H.; Sieper, J.; Zink, A.; Poddubnyy, D. Determinants of diagnostic delay in axial spondyloarthritis: An analysis based on linked claims and patient-reported survey data. Rheumatology 2019, 58, 1634–1638. [Google Scholar] [CrossRef]
- Collantes, E.; Zarco, P.; Munoz, E.; Juanola, X.; Mulero, J.; Fernandez-Sueiro, J.L.; Torre-Alonso, J.C.; Gratacos, J.; Gonzalez, C.; Batlle, E.; et al. Disease pattern of spondyloarthropathies in Spain: Description of the first national registry (REGISPONSER) extended report. Rheumatology 2007, 46, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Schiotis, R.; Font, P.; Escudero, A.; Zarco, P.; Almodovar, R.; Gratacós, J.; Mulero, J.; Juanola, X.; Montilla, C.; Moreno, E.; et al. Usefulness of a centralized system of data collection for the development of an international multicentre registry of spondyloarthritis. Rheumatology 2011, 50, 132–136. [Google Scholar] [CrossRef]
- Feldtkeller, E.; Erlendsson, J. Definition of disease duration in ankylosing spondylitis. Rheumatol. Int. 2008, 28, 693–696. [Google Scholar] [CrossRef]
- Garrett, S.; Jenkinson, T.; Kennedy, L.G.; Whitelock, H.; Gaisford, P.; Calin, A. A new approach to defining disease status in ankylosing spondylitis: The Bath Ankylosing Spondylitis Disease Activity Index. J. Rheumatol. 1994, 21, 2286–2291. [Google Scholar]
- Lukas, C.; Landewé, R.; Sieper, J.; Dougados, M.; Davis, J.; Braun, J.; van der Linden, S.; van der Heijde, D.; Assessment of SpondyloArthritis international Society. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann. Rheum. Dis. 2009, 68, 18–24. [Google Scholar] [CrossRef]
- Calin, A.; Jones, S.D.; Garrett, S.L.; Kennedy, L.G. Bath Ankylosing Spondylitis Functional Index. Br. J. Rheumatol. 1995, 34, 793–794. [Google Scholar] [CrossRef] [PubMed]
- Gandek, B.; Ware, J.E.; Aaronson, N.K.; Apolone, G.; Bjorner, J.B.; Brazier, J.E.; Bullinger, M.; Kaasa, S.; Leplege, A.; Prieto, L.; et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: Results from the IQOLA Project. International Quality of Life Assessment. J. Clin. Epidemiol. 1998, 51, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Ibn, Y.a.c.o.u.b.Y.; Amine, B.; Laatiris, A.; Bensabbah, R.; Hajjaj-Hassouni, N. Relationship between diagnosis delay and disease features in Moroccan patients with ankylosing spondylitis. Rheumatol. Int. 2012, 32, 357–360. [Google Scholar]
- Merino, M.; Braçe, O.; González-Domínguez, A.; Hidalgo-Vega, Á.; Garrido-Cumbrera, M.; Gratacós, J. Social economic costs of ankylosing spondylitis in Spain. Clin. Exp. Rheumatol. 2021, 39, 357–364. [Google Scholar] [CrossRef]
- Behar, V.M.; Dougados, M.; Etcheto, A.; Kreis, S.; Fabre, S.; Hudry, C.; Dadoun, S.; Rein, C.; Pertuiset, E.; Fautrel, B.; et al. Diagnostic delay in axial spondyloarthritis: A cross-sectional study of 432 patients. Jt. Bone Spine. 2017, 84, 467–471. [Google Scholar] [CrossRef]
- Seo, M.R.; Baek, H.L.; Yoon, H.H.; Ryu, H.J.; Choi, H.-J.; Baek, H.J.; Ko, K.-P. Delayed diagnosis is linked to worse outcomes and unfavourable treatment responses in patients with axial spondyloarthritis. Clin. Rheumatol. 2015, 34, 1397–1405. [Google Scholar] [CrossRef]
- Haroon, M.; Gallagher, P.; FitzGerald, O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann. Rheum. Dis. 2015, 74, 1045–1050. [Google Scholar] [CrossRef]
- Mennini, F.S.; Viti, R.; Marcellusi, A.; Sciattella, P.; Viapiana, O.; Rossini, M. Economic evaluation of spondyloarthritis: Economic impact of diagnostic delay in Italy. Clin. Outcomes Res. 2018, 10, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Berbel-Arcobé, L.; Aparicio, M.; Calvet, J.; Arévalo, M.; Nack, A.; Juanola, X.; Toniolo, E.; Maratia, S.; Lizán, L.; Gratacós, J. Association between diagnostic delay and economic and clinical burden in axial spondyloarthritis: A multicentre retrospective observational study. Rheumatol. Ther. 2025, 1–12. [Google Scholar] [CrossRef]
- Yi, E.; Ahuja, A.; Rajput, T.; George, A.T.; Park, Y. Clinical, economic, and humanistic burden associated with delayed diagnosis of axial spondyloarthritis: A systematic review. Rheumatol. Ther. 2020, 7, 65–87. [Google Scholar] [CrossRef]
- Qaiyum, Z.; Lim, M.; Inman, R.D. The gut-joint axis in spondyloarthritis: Immunological, microbial, and clinical insights. Semin. Immunopathol. 2021, 43, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Guggino, G.; Mauro, D.; Rizzo, A.; Alessandro, R.; Raimondo, S.; Bergot, A.; Rahman, M.A.; Ellis, J.J.; Milling, S.; Lories, R.; et al. Inflammasome Activation in Ankylosing Spondylitis Is Associated With Gut Dysbiosis. Arthritis Rheumatol. 2021, 73, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Ciccia, F.; Rizzo, A.; Triolo, G. Subclinical gut inflammation in ankylosing spondylitis. Curr. Opin. Rheumatol. 2016, 28, 89–96. [Google Scholar] [CrossRef]
- Rizzo, A.; Guggino, G.; Ferrante, A.; Ciccia, F. Role of Subclinical Gut Inflammation in the Pathogenesis of Spondyloarthritis. Front. Med. (Lausanne) 2018, 5, 63. [Google Scholar] [CrossRef]
- Michelena, X.; Zhao, S.S.; Marco-Pascual, C.; Almirall, M.; Collantes-Estevez, E.; Font-Ugalde, P.; López-Medina, C.; Wei, J.C.-C.; Morgan, A.W.; Rodríguez, J.; et al. Diagnostic delay is associated with uveitis and inflammatory bowel disease in AS: A study of extra-musculoskeletal manifestations in SpA. Rheumatology 2024, 63, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Rudwaleit, M.; Listing, J.; Brandt, J.; Braun, J.; Sieper, J. Prediction of a major clinical response (BASDAI 50) to tumour necrosis factor alpha blockers in ankylosing spondylitis. Ann. Rheum. Dis. 2004, 63, 665–670. [Google Scholar] [CrossRef]
- Willesen, S.T.; Hadsbjerg, A.E.; Moller, J.M.; Vladimirova, N.; Vora, B.M.K.; Seven, S.; Pedersen, S.J.; Østergaard, M. MRI-based synthetic CT: A new method for structural damage assessment in the spine in patients with axial spondyloarthritis—A comparison with low-dose CT and radiography. Ann. Rheum. Dis. 2024, 83, 807–815. [Google Scholar] [CrossRef]
| Total N = 565 | Diagnostic Delay < 5 Years N = 325 | Diagnostic Delay ≥ 5 Years N = 240 | p-Value * | |
|---|---|---|---|---|
| Age | 44.9 (10.9) | 45.2 (12.4) | 41.8 (10.6) | 0.022 |
| Sex (male) | 157 (27.8%) | 241 (74.2%) | 167 (69.6%) | 0.231 |
| Age at diagnosis (years) | 34.4 (10.9) | 33.0 (11.9) | 31.5 (10.4) | 0.188 |
| Disease duration (years) | 16 (8.2) | 12.2 (8.0) | 11.3 (7.6) | 0.210 |
| HLA-B27 positive | 436 (80.1%) | 248 (80.5%) | 188 (79.7%) | 0.804 |
| Family history of SpA | 310 (54.9%) | 177 (54.5%) | 133 (55.4%) | 0.563 |
| Psoriasis | 56 (9.9%) | 35 (10.8%) | 21 (8.8%) | 0.413 |
| Inflammatory bowel disease | 38 (6.7%) | 18 (5.5%) | 20 (8.3%) | 0.190 |
| Uveitis | 115 (20.5%) | 58 (18.0%) | 57 (23.8%) | 0.087 |
| Enthesitis | 176 (31.6%) | 104 (32.3%) | 72 (30.6%) | 0.677 |
| Dactylitis | 41 (7.3%) | 30 (9.3%) | 11 (4.6%) | 0.036 |
| Synovitis | 181 (32.1%) | 113 (34.8%) | 68 (28.5%) | 0.112 |
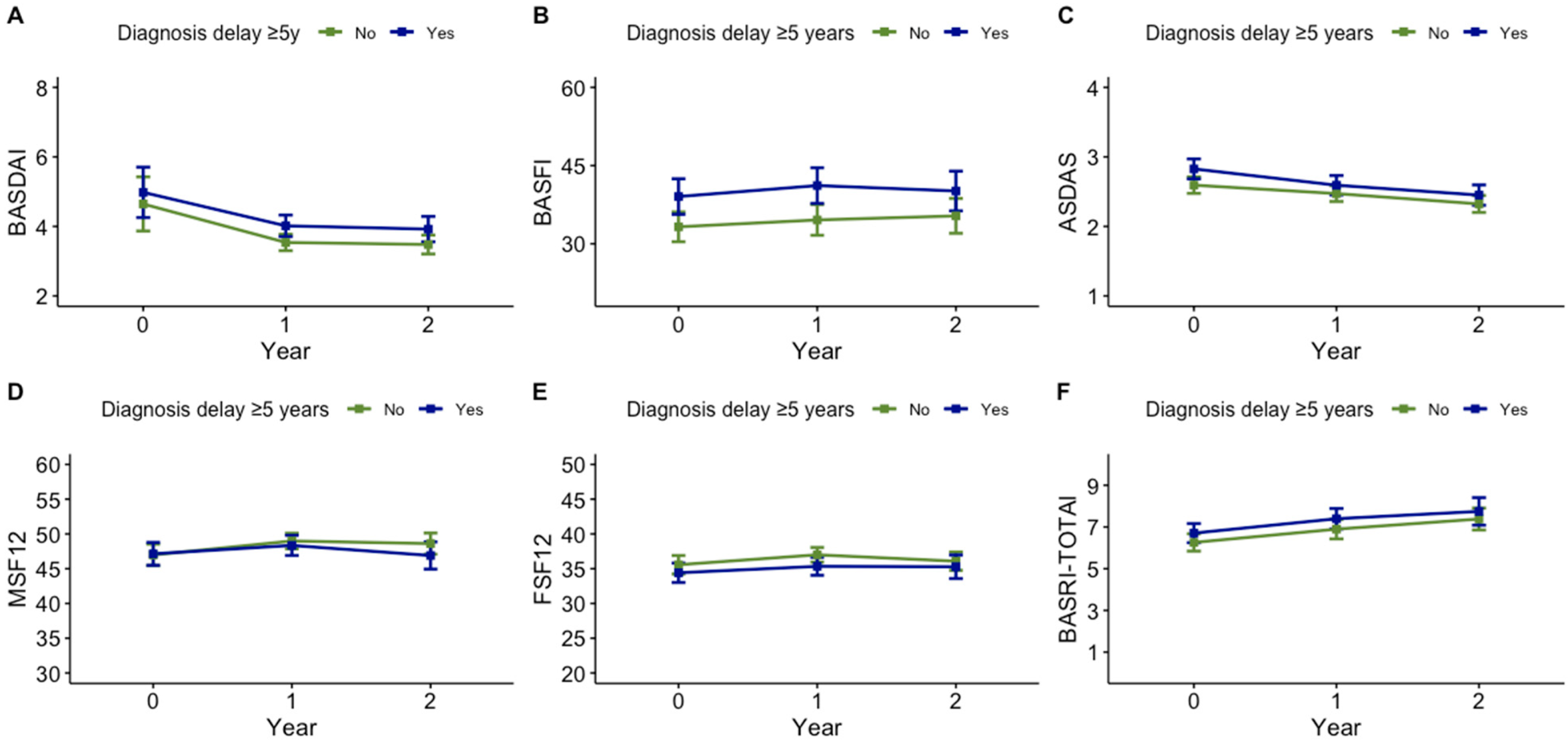
| ASDAS-CRP | 2.7 (1.1) | 2.5 (1.0) | 2.4 (1.0) | 0.321 |
| BASDAI | 4.2 (2.4) | 3.7 (2.4) | 3.5 (2.2) | 0.281 |
| BASFI | 35.8 (26.6) | 32.7 (26.7) | 27.8 (23.8) | 0.103 |
| Total BASRI | 6.5 (3.6) | 6.3 (3.7) | 5.8 (3.6) | 0.213 |
| csDMARDs ever | 154/345 (44.6%) | 90/196 (45.9%) | 64/149 (43.0%) | 0.583 |
| Anti-TNF ever | 105/342 (30.7%) | 58/195 (29.7%) | 47/147 (32.0%) | 0.658 |
| Inability to work | 110 (19.7%) | 69 (17.2%) | 41 (13.4%) | 0.426 |
| Diagnostic Delay < 5 Years N = 325 | Diagnostic Delay ≥ 5 Years N = 240 | OR (95%CI) Adjusted for Disease Duration | |
|---|---|---|---|
| Psoriasis | 37 (11.7%) | 23 (9.9%) | 0.83 (0.48–1.45) |
| Inflammatory bowel disease | 19 (6.0%) | 23 (10.0%) | 2.01 (1.06–3.83) |
| Uveitis | 72 (22.9%) | 52 (22.4%) | 0.92 (0.61–1.39) |
| Enthesitis | 53 (18.3%) | 48 (21.4%) | 1.17 (0.76–1.82) |
| Dactylitis | 36 (11.7%) | 8 (3.4%) | 0.24 (0.11–0.55) |
| Synovitis | 114 (36.2%) | 66 (28.8%) | 0.68 (0.47–0.98) |
|
Diagnostic Delay < 5 Years N = 325 |
Diagnostic Delay ≥ 5 Years N = 240 | OR (95%CI) Adjusted for Disease Duration | |
|---|---|---|---|
| ASDAS-CRP, mean (SD) | 2.6 (1.1) | 2.3 (0.9) | 1.09 (0.92–1.30) |
| BASDAI, mean (SD) | 3.6 (2.2) | 3.3 (2.1) | 1.08 (1.00–1.16) |
| BASFI, mean (SD) | 33.8 (26.8) | 30.3 (25.0) | 1.01 (0.99–1.01) |
| Physician VAS (cm) | 3.0 (1.9) | 2.8 (2.2) | 1.07 (0.98–1.16) |
| Nocturn VAS (cm) | 3.6 (2.8) | 3.2 (2.4) | 1.03 (0.97–1.09) |
| Global VAS (cm) | 4.0 (2.7) | 3.6 (2.4) | 1.05 (0.98–1.11) |
| Pain VAS (cm) | 3.9 (2.7) | 3.4 (2.4) | 1.03 (0.96–1.09) |
| SF-12 physical component | 2.4 (0.7) | 2.3 (0.8) | 0.87 (0.69–1.09) |
| SF-12 mental component | 3.5 (0.7) | 3.5 (0.9) | 0.96 (0.77–1.20) |
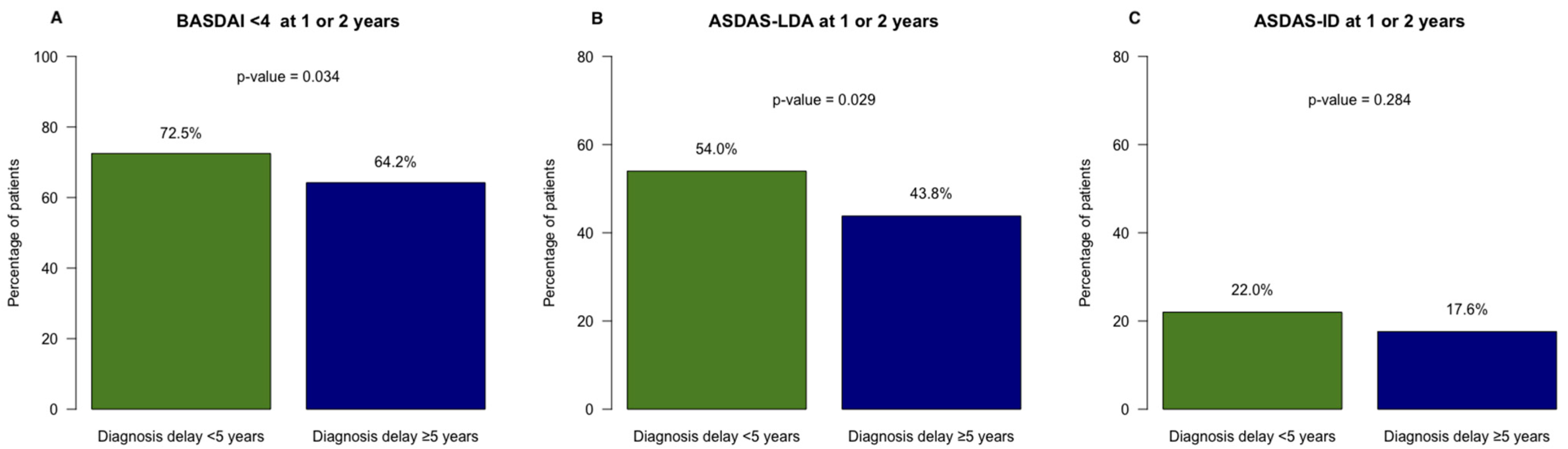
| ASDAS LDA | 113 (37.7%) | 71 (32.7%) | 0.87 (0.60–1.27) |
| ASDAS ID | 44 (14.7%) | 25 (11.5%) | 0.83 (0.49–1.42) |
| ASDAS LDA at 1 or 2 years | 161 (54.0%) | 91 (43.8%) | 0.66 (0.46–0.94) |
| ASDAS ID at 1 or 2 years | 64 (22.0%) | 36 (17.6%) | 0.76 (0.48–1.19) |
| BASDAI < 4 | 200 (61.7%) | 128 (53.3%) | 0.75 (0.53–1.06) |
| BASDAI < 4 at 1 or 2 years | 235 (72.5%) | 154 (64.2%) | 0.74 (0.51–1.07) |
| Spine BASRI | 6.4 (3.3) | 5.8 (3.3) | 1.01 (0.94–1.08) |
| Total BASRI | 7.1 (3.8) | 6.3 (3.5) | 1.00 (0.94–1.06) |
| Schober (cm) | 3.2 (1.5) | 3.8 (1.7) | 0.96 (0.87–1.07) |
| Chest expansion (cm) | 4.3 (2.4) | 4.5 (2.0) | 0.95 (0.87–1.03) |
| Distance to the ground (cm) | 15.5 (13.7) | 15.6 (13.3) | 1.01 (0.99–1.02) |
| Occiput wall distance (cm) | 3.4 (6.0) | 3.1 (6.1) | 0.99 (0.96–1.02) |
| Lumbar lateral flexion (cm) | 23.7 (19.2) | 22.6 (18.8) | 0.99 (0.99–1.01) |
| Hip arthroplasty | 9 (2.9%) | 5 (2.2%) | 0.73 (0.24–2.24) |
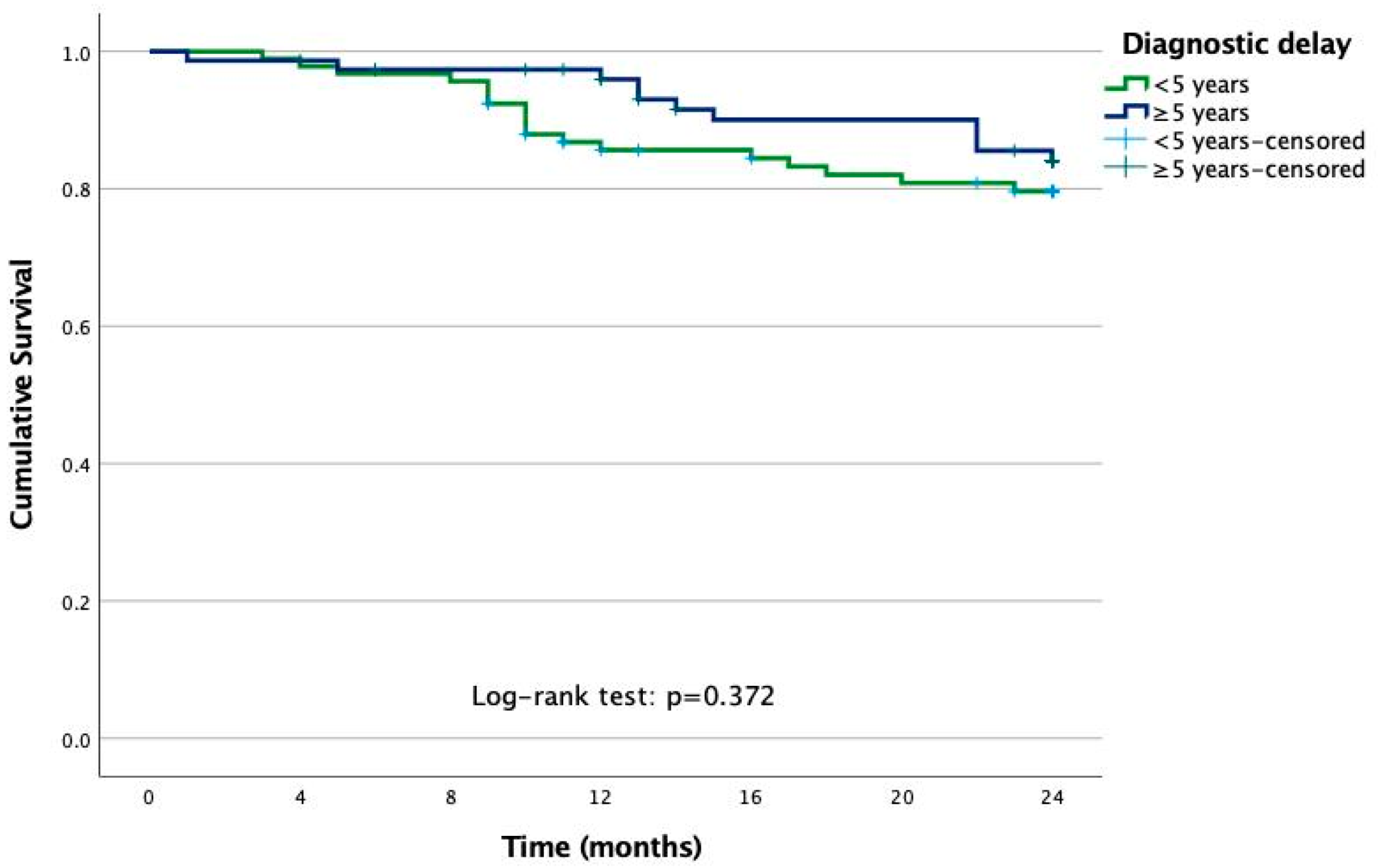
| Inability to work | 80 (26.5%) | 65 (28.9%) | 0.94 (0.63–1.41) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ladehesa-Pineda, M.L.; Ruiz-Vilchez, D.; Barranco, A.M.; Puche-Larrubia, M.Á.; Font-Ugalde, P.; Granados, R.E.M.; Gratacós-Mastmijà, J.; Juanola, X.; Escudero-Contreras, A.; Collantes-Estévez, E.; et al. Association Between Diagnostic Delay and Short-Term Outcomes in Patients with Radiographic Axial Spondyloarthritis: Results from the Regisponser-AS Registry. J. Clin. Med. 2025, 14, 1977. https://doi.org/10.3390/jcm14061977
Ladehesa-Pineda ML, Ruiz-Vilchez D, Barranco AM, Puche-Larrubia MÁ, Font-Ugalde P, Granados REM, Gratacós-Mastmijà J, Juanola X, Escudero-Contreras A, Collantes-Estévez E, et al. Association Between Diagnostic Delay and Short-Term Outcomes in Patients with Radiographic Axial Spondyloarthritis: Results from the Regisponser-AS Registry. Journal of Clinical Medicine. 2025; 14(6):1977. https://doi.org/10.3390/jcm14061977
Chicago/Turabian StyleLadehesa-Pineda, María Lourdes, Desirée Ruiz-Vilchez, Antonio Manuel Barranco, María Ángeles Puche-Larrubia, Pilar Font-Ugalde, Raquel Ena María Granados, Jordi Gratacós-Mastmijà, Xavier Juanola, Alejandro Escudero-Contreras, Eduardo Collantes-Estévez, and et al. 2025. "Association Between Diagnostic Delay and Short-Term Outcomes in Patients with Radiographic Axial Spondyloarthritis: Results from the Regisponser-AS Registry" Journal of Clinical Medicine 14, no. 6: 1977. https://doi.org/10.3390/jcm14061977
APA StyleLadehesa-Pineda, M. L., Ruiz-Vilchez, D., Barranco, A. M., Puche-Larrubia, M. Á., Font-Ugalde, P., Granados, R. E. M., Gratacós-Mastmijà, J., Juanola, X., Escudero-Contreras, A., Collantes-Estévez, E., & López-Medina, C. (2025). Association Between Diagnostic Delay and Short-Term Outcomes in Patients with Radiographic Axial Spondyloarthritis: Results from the Regisponser-AS Registry. Journal of Clinical Medicine, 14(6), 1977. https://doi.org/10.3390/jcm14061977





