Retrospective Analysis of Balance Parameters in Pregnant Women: A Sub-Analysis of a Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Collection
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ML | Mediolateral |
| AP | Anterior-posterior |
| TBS | Total body sway |
| OSD | One-leg stance duration |
References
- Oliveira, C.S.d.; Imakawa, T.d.S.; Moisés, E.C.D. Physical Activity during Pregnancy: Recommendations and Assessment Tools. Rev. Bras. Ginecol. Obs. 2017, 39, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Ferlin, A.; De Toni, L.; Sandri, M.; Foresta, C. Relaxin and insulin-like peptide 3 in the musculoskeletal system: From bench to bedside. Br. J. Pharmacol. 2017, 174, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Shum, G.L.K.; Crosbie, J.; Lee, R.Y.W. Three-dimensional kinetics of the lumbar spine and hips in low back pain patients during sit-to-stand and stand-to-sit. Spine 2007, 32, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Alcahuz-Griñan, M.; Nieto-Gil, P.; Perez-Soriano, P.; Gijon-Nogueron, G. Morphological and Postural Changes in the Foot during Pregnancy and Puerperium: A Longitudinal Study. Int. J. Environ. Res. Public Health 2021, 18, 2423. [Google Scholar] [CrossRef]
- Nelson, R.K.; Hafner, S.M.; Cook, A.C.; Sterner, N.J.; Butler, E.L.; Jakiemiec, B.E.; Saltarelli, W.A. Exercise During Pregnancy: What Do OB/GYNs Believe and Practice? A Descriptive Analysis. Women’s Health Rep. 2022, 3, 274–280. [Google Scholar] [CrossRef]
- Kharb, A.; Saini, V.; Jain, Y.; Dhiman, S. A review of gait cycle and its parameters. Int. J. Comput. Eng. Manag. 2011, 13, 78–83. [Google Scholar]
- Catena, R.D.; Wolcott, W.C. Self-selection of gestational lumbopelvic posture and bipedal evolution. Gait Posture 2021, 89, 7–13. [Google Scholar] [CrossRef]
- Błaszczyk, J.W.; Opala-Berdzik, A.; Plewa, M. Adaptive changes in spatiotemporal gait characteristics in women during pregnancy. Gait Posture 2016, 43, 160–164. [Google Scholar] [CrossRef]
- Dunning, K.; LeMasters, G.; Levin, L.; Bhattacharya, A.; Alterman, T.; Lordo, K. Falls in workers during pregnancy: Risk factors, job hazards, and high risk occupations. Am. J. Ind. Med. 2003, 44, 664–672. [Google Scholar] [CrossRef]
- Forczek, W.; Masłoń, A.; Frączek, B.; Curyło, M.; Salamaga, M.; Suder, A. Does the first trimester of pregnancy induce alterations in the walking pattern? McCrory JL, ed. PLoS ONE 2019, 14, e0209766. [Google Scholar] [CrossRef]
- Branco, M.; Santos-Rocha, R.; Aguiar, L.; Vieira, F.; Veloso, A. Kinematic analysis of gait in the second and third trimesters of pregnancy. J. Mech. Med. Biol. 2016, 16, 1650055. [Google Scholar] [CrossRef]
- Huang, T.H.; Lin, S.C.; Ho, C.S.; Yu, C.Y.; Chou, Y.L. The Gait Analysis of Pregnant Women. Biomed. Eng. Appl. Basis Commun. 2002, 14, 67–70. [Google Scholar] [CrossRef]
- Fitzgerald, C.M.; Segal, N.A. Musculoskeletal Health in Pregnancy and Postpartum; Fitzgerald, C.M., Segal, N.A., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Schorderet, C.; Hilfiker, R.; Allet, L. The role of the dominant leg while assessing balance performance. A systematic review and meta-analysis. Gait Posture 2021, 84, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Mottola, M.F.; Davenport, M.H.; Ruchat, S.M.; Davies, G.A.; Poitras, V.J.; Gray, C.E.; Garcia, A.J.; Barrowman, N.; Adamo, K.B.; Duggan, M.; et al. 2019 Canadian guideline for physical activity throughout pregnancy. Br. J. Sports Med. 2018, 52, 1339–1346. [Google Scholar] [CrossRef]
- Hinman, S.K.; Smith, K.B.; Quillen, D.M.; Smith, M.S. Exercise in Pregnancy. Sport. Health 2015, 7, 527–531. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Kayalı Vatansever, A.; Şenışık, S.; Bayraktar, D.; Demir, M.; Akercan, F. The Effect of Clinical Exercise Training on Plantar Pressure, the Subtalar Joint, and the Gait Cycle in Pregnant Women: Randomized Clinical Trial. J. Clin. Med. 2024, 13, 7795. [Google Scholar] [CrossRef]
- Activity, P.; Pregnancy, E.D. Physical Activity and Exercise During Pregnancy and the Postpartum Period. Obstet. Gynecol. 2020, 135, e178–e188. [Google Scholar] [CrossRef]
- Bayraktar, D.; Özgürbüz, C.; Öztürk, A.M.; Aktuğlu, S.K.; Özkayın, N. An investigation into the frequency and risk factors of low back pain following surgical treatment of isolated calcaneal fractures. Acta Orthop. Traumatol. Turc. 2024, 58, 45–56. [Google Scholar] [CrossRef]
- Paillard, T.; Noé, F. Does monopedal postural balance differ between the dominant leg and the non-dominant leg? A review. Hum. Mov. Sci. 2020, 74, 102686. [Google Scholar] [CrossRef]
- Muehlbauer, T.; Mettler, C.; Roth, R.; Granacher, U. One-Leg Standing Performance and Muscle Activity: Are There Limb Differences? J. Appl. Biomech. 2014, 30, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Forczek, W.; Ivanenko, Y.; Curyło, M.; Frączek, B.; Masłoń, A.; Salamaga, M.; Suder, A. Progressive changes in walking kinematics throughout pregnancy—A follow up study. Gait Posture 2019, 68, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Danna-Dos-Santos, A.; Magalhães, A.T.; Silva, B.A.; Duarte, B.S.; Barros, G.L.; Silva, M.D.F.C.; Silva, C.S.; Mohapatra, S.; Degani, A.M.; Cardoso, V.S. Upright balance control strategies during pregnancy. Gait Posture 2018, 66, 7–12. [Google Scholar] [CrossRef] [PubMed]
- El-shamy, F.F.; Ribeiro, A.P.; Abo Gazia, A.A. Effectiveness of proprioceptive training on dynamic postural balance during pregnancy: A randomized controlled trial. Physiother. Pract. Res. 2019, 40, 77–85. [Google Scholar] [CrossRef]
- L’heveder, A.; Chan, M.; Mitra, A.; Kasaven, L.; Saso, S.; Prior, T.; Pollock, N.; Dooley, M.; Joash, K.; Jones, B.P. Sports Obstetrics: Implications of Pregnancy in Elite Sportswomen, a Narrative Review. J. Clin. Med. 2022, 11, 4977. [Google Scholar] [CrossRef]
- Cancela-Carral, J.M.; Blanco, B.; López-Rodríguez, A. Therapeutic Aquatic Exercise in Pregnancy: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 501. [Google Scholar] [CrossRef]
- McCrory, J.; Chambers, A.; Daftary, A.; Redfern, M. Dynamic postural stability in pregnant fallers and non-fallers. BJOG An. Int. J. Obstet. Gynaecol. 2010, 117, 954–962. [Google Scholar] [CrossRef]
- Lesinski, M.; Hortobágyi, T.; Muehlbauer, T.; Gollhofer, A.; Granacher, U. Effects of Balance Training on Balance Performance in Healthy Older Adults: A Systematic Review and Meta-analysis. Sport. Med. 2015, 45, 1721–1738. [Google Scholar] [CrossRef]
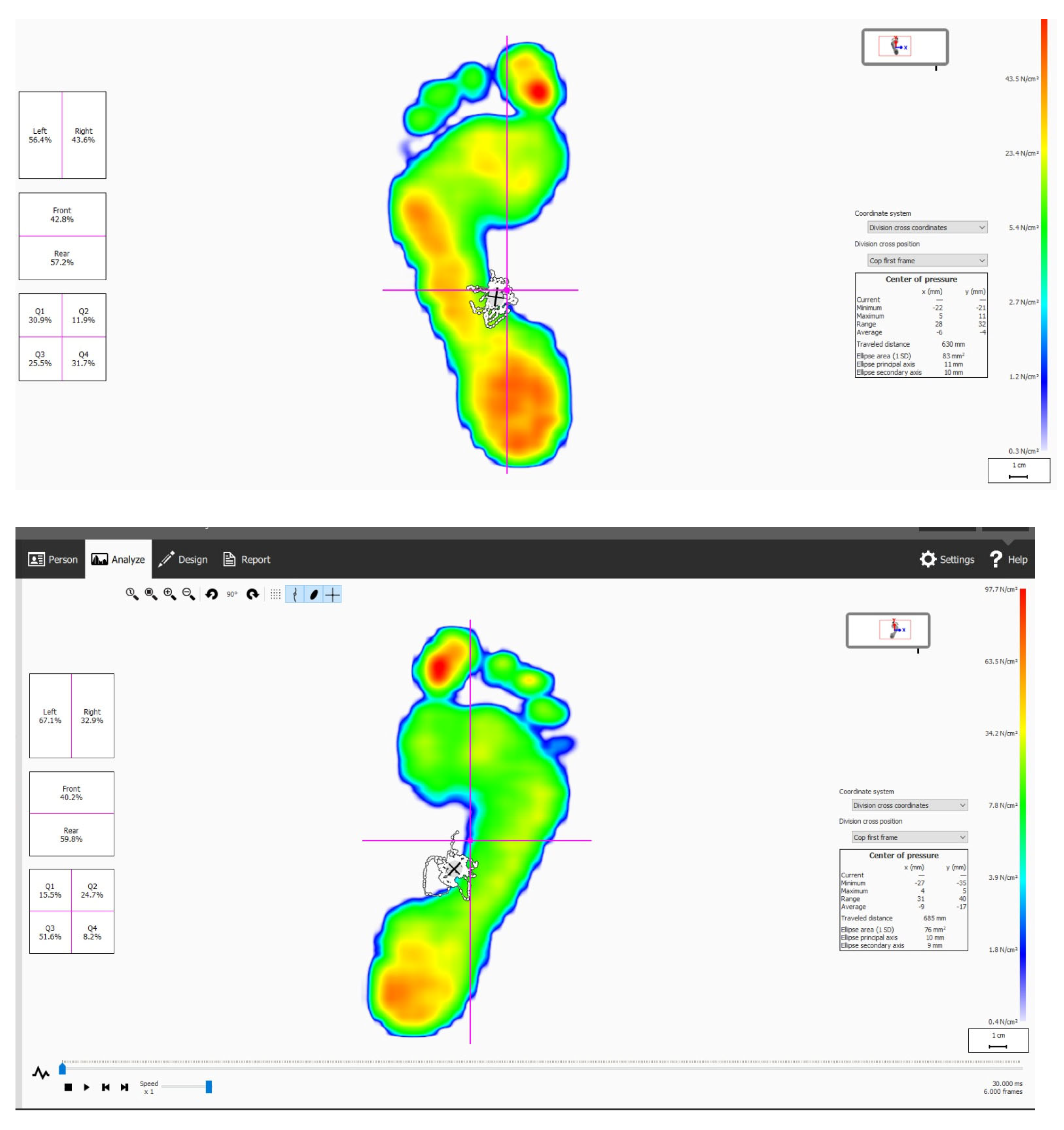

| Exercise Group (n = 50) M ± SD | Control Group (n = 51) M ± SD | p-Value | |
|---|---|---|---|
| Age (years) | 29.7 ± 3.8 | 29.1 ± 6.1 | 0.505 1 |
| Dominant side Right Left | n (%) 49 (98.0) 1 (2.0) | n (%) 49 (96.0) 2 (4.0) | 0.063 2 |
| BMI (kg/m2) | 24.5 ± 2.2 | 24.9 ± 3.4 | 0.491 1 |
| Height (cm) | 164.0 ± 6.8 | 161.7 ± 6.7 | 0.084 1 |
| Body weight (kg) | 66.0 ± 8.4 | 65.0 ± 9.8 | 0.584 1 |
| Gestational Week | 15.7 ± 3.1 | 14.8 ± 2.6 | 0.166 2 |
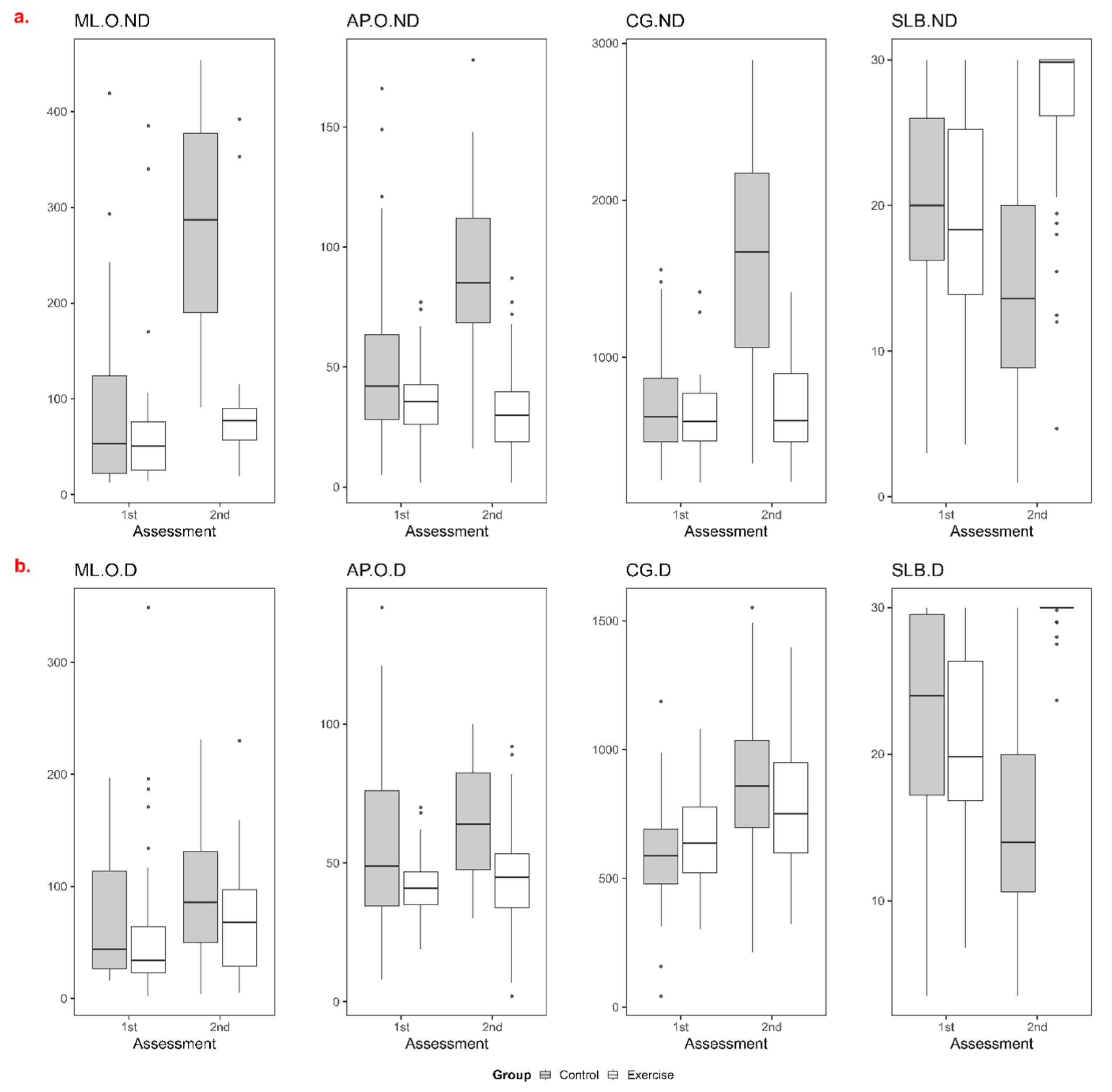
| Measurement | EG | EG | CG | CG | p-Value | p-Value | p-Value |
|---|---|---|---|---|---|---|---|
| (Baseline) | (Week 8) | (Baseline) | (Week 8) | (Group) | (Time) | (Interaction) | |
| ML sway | |||||||
| ND | 51 (14–385) | 77 (19–392) | 53 (13–419) | 287 (91–454) | 0.000 | 0.000 | 0.000 |
| D | 34 (2–349) | 68 (5–230) | 44 (16–197) | 86 (4–231) | 0.047 | 0.000 | 0.512 |
| AP sway | |||||||
| ND | 35.5 (2–77) | 30 (2–87) | 42 (5–166) | 85 (16–178) | 0.000 | 0.005 | 0.000 |
| D | 41 (19–70) | 45 (2–92) | 49 (8–142) | 64 (30–100) | 0.000 | 0.000 | 0.002 |
| TBS | |||||||
| ND | 592 (205–1416) | 598.5 (209–1416) | 620 (218–1558) | 1672 (324–2894) | 0.000 | 0.011 | 0.000 |
| D | 637.5 (303–1080) | 752 (322–1397) | 587 (42–1187) | 858 (213–1552) | 0.000 | 0.000 | 0.000 |
| OSD second | |||||||
| ND | 18.35 (3.6–30) | 29.85 (4.7–30) | 20 (3–30) | 13.6 (1–30) | 0.000 | 0.000 | 0.000 |
| D | 19.81 (6.8–30) | 30 (23.7–30) | 24 (3.5–30) | 14 (3.5–30) | 0.000 | 0.000 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayraktar, D.; Şenışık, S.; Kayalı Vatansever, A.; Dadaş, Ö.F.; Akercan, F. Retrospective Analysis of Balance Parameters in Pregnant Women: A Sub-Analysis of a Randomized Controlled Trial. J. Clin. Med. 2025, 14, 1892. https://doi.org/10.3390/jcm14061892
Bayraktar D, Şenışık S, Kayalı Vatansever A, Dadaş ÖF, Akercan F. Retrospective Analysis of Balance Parameters in Pregnant Women: A Sub-Analysis of a Randomized Controlled Trial. Journal of Clinical Medicine. 2025; 14(6):1892. https://doi.org/10.3390/jcm14061892
Chicago/Turabian StyleBayraktar, Dilek, Seçkin Şenışık, Ayşe Kayalı Vatansever, Ömer Faruk Dadaş, and Fuat Akercan. 2025. "Retrospective Analysis of Balance Parameters in Pregnant Women: A Sub-Analysis of a Randomized Controlled Trial" Journal of Clinical Medicine 14, no. 6: 1892. https://doi.org/10.3390/jcm14061892
APA StyleBayraktar, D., Şenışık, S., Kayalı Vatansever, A., Dadaş, Ö. F., & Akercan, F. (2025). Retrospective Analysis of Balance Parameters in Pregnant Women: A Sub-Analysis of a Randomized Controlled Trial. Journal of Clinical Medicine, 14(6), 1892. https://doi.org/10.3390/jcm14061892







