Management and Outcomes of Sternoclavicular Joint Infections: A Retrospective Study
Abstract
1. Introduction
2. Methods
2.1. Diagnostic Procedures
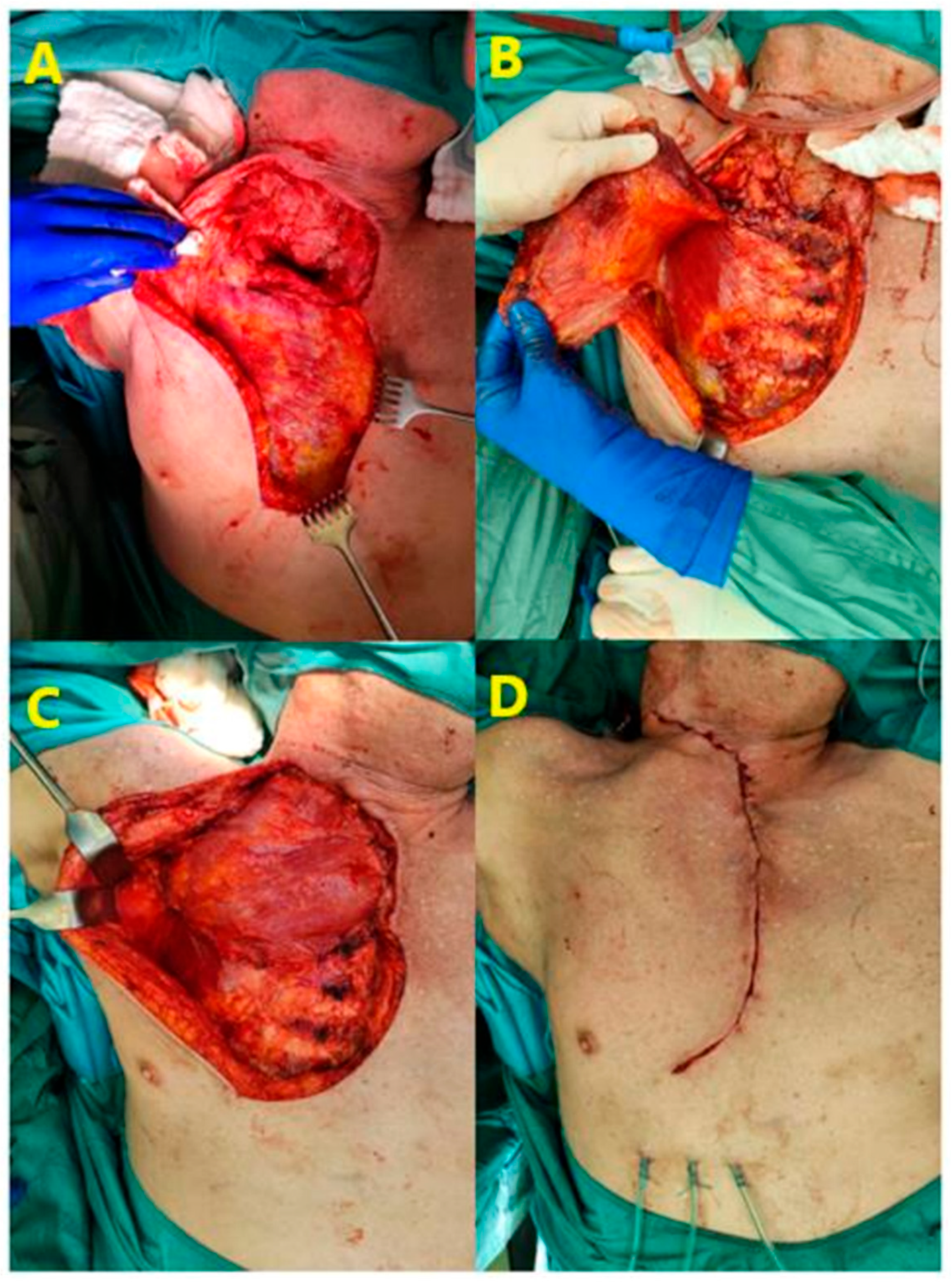
2.2. Surgical Technique
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edwin, J.; Ahmed, S.; Verma, S.; Tytherleigh-Strong, G.; Karuppaiah, K.; Sinha, J. Swellings of the sternoclavicular joint: Review of traumatic and non-traumatic pathologies. EFORT Open Rev. 2018, 3, 471–484. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McAninch, S.A.; Smithson, C., 3rd; Juergens, A.L.; Collins, J.N.; Nanda, A. Sternoclavicular Joint Infection Presenting as Nonspecific Chest Pain. J. Emerg. Med. 2018, 54, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.A.; Barlotta, K.S. Septic Arthritis of the Sternoclavicular Joint. J. Emerg. Med. 2018, 55, 128–129. [Google Scholar] [CrossRef] [PubMed]
- Abu Arab, W.; Khadragui, I.; Echavé, V.; Deshaies, A.; Sirois, C.; Sirois, M. Surgical management of sternoclavicular joint infection. Eur. J. Cardio-Thorac. Surg. 2011, 40, 630–635. [Google Scholar] [CrossRef]
- Pothini, T.; Wilmot, C.D.; Waters, J.K.; Wait, M.A.; Reznik, S.I.; Jordan, K.G.; Caire, J.T.; Ashworth, J.M.; Cady, L.C.; Lysikowski, J.R.; et al. Clinical and radiological septic joint analysis of spontaneous sternoclavicular joint infections: Achieving the best outcomes—A systems engineering approach. Eur. J. Cardio-Thorac. Surg. 2024, 65, ezae128. [Google Scholar] [CrossRef] [PubMed]
- Tasnim, S.; Shirafkan, A.; Okereke, I. Diagnosis and management of sternoclavicular joint infections: A literature review. J. Thorac. Dis. 2020, 12, 4418–4426. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nusselt, T.; Klinger, H.M.; Freche, S.; Schultz, W.; Baums, M.H. Surgical management of sternoclavicular septic arthritis. Arch. Orthop. Trauma. Surg. 2011, 131, 319–323. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Joethy, J.; Lim, C.H.; Koong, H.N.; Tan, B.K. Sternoclavicular joint infection: Classification of resection defects and reconstructive algorithm. Arch. Plast. Surg. 2012, 39, 643–648. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Mufarrej, F.; Martinez-Jorge, J.; Carlsen, B.T.; Saint-Cyr, M.; Moran, S.L.; Mardini, S. Use of the deltoid branch-based clavicular head of pectoralis major muscle flap in isolated sternoclavicular infections. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 1702–1711. [Google Scholar] [CrossRef] [PubMed]
- Raymond, D. Surgical intervention for thoracic infections. Surg. Clin. N. Am. 2014, 94, 1283–1303. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ji, X.; Hao, M.; Zhang, Q.; Tang, P. A three-stage procedure using bone transportation for the treatment of sternoclavicular infectious arthritis. J. Orthop. Surg. Res. 2016, 11, 152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bendon, C.L.; Giele, H.P. Second toe metatarsophalangeal joint transfer for sternoclavicular joint reconstruction. J. Hand Surg. Am. 2014, 39, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.J.; Shamsuddin, H. Sternoclavicular septic arthritis: Review of 180 cases. Medicine 2004, 83, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Murga, A.; Copeland, H.; Hargrove, R.; Wallen, J.M.; Zaheer, S. Treatment for sternoclavicular joint infections: A multi-institutional study. J. Thorac. Dis. 2017, 9, 1503–1508. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kachala, S.S.; D’Souza, D.M.; Teixeira-Johnson, L.; Murthy, S.C.; Raja, S.; Blackstone, E.H.; Raymond, D.P. Surgical Management of Sternoclavicular Joint Infections. Ann. Thorac. Surg. 2016, 101, 2155–2160. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, W.; Ludolph, I.; Dudek, W.; Horch, R.E.; Sirbu, H. Negative Pressure Wound Therapy Combined with Instillation for Sternoclavicular Joint Infection. Ann. Thorac. Surg. 2020, 110, 1722–1725. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kato, H.; Shirai, K.; Nakajima, Y.; Yamada, N.; Okada, H.; Yoshida, T.; Toyoda, I.; Ogura, S. Sternoclavicular joint septic arthritis with chest wall abscess in a healthy adult: A case report. J. Med. Case Rep. 2016, 10, 69. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Ali, B.; Petersen, T.R.; Shetty, A.; Demas, C.; Schwartz, J.D. Muscle flaps for sternoclavicular joint septic arthritis. J. Plast. Surg. Hand Surg. 2021, 55, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.R.; Kim, T.; Kim, M.C.; Sup Sung, H.; Kim, M.N.; Kim, M.J.; Kim, S.H.; Lee, S.O.; Choi, S.H.; Woo, J.H.; et al. Sternoclavicular septic arthritis caused by Staphylococcus aureus: Excellent results from medical treatment and limited surgery. Infect. Dis. 2019, 51, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Puri, V.; Meyers, B.F.; Kreisel, D.; Patterson, G.A.; Crabtree, T.D.; Battafarano, R.J.; Krupnick, A.S. Sternoclavicular joint infection: A comparison of two surgical approaches. Ann. Thorac. Surg. 2011, 91, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Bakaeen, F.G.; Huh, J.; Fagan, S.P.; Bellows, C.F. Surgical treatment of sternoclavicular joint infections in cirrhotic patients. Am. J. Surg. 2008, 195, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Shetty, A.; Qeadan, F.; Demas, C.; Schwartz, J.D. Sternoclavicular Joint Infections: Improved Outcomes with Myocutaneous Flaps. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 369–376. [Google Scholar] [CrossRef] [PubMed]
- von Glinski, A.; Yilmaz, E.; Rausch, V.; Koenigshausen, M.; Schildhauer, T.A.; Seybold, D.; Geßmann, J. Surgical management of sternoclavicular joint septic arthritis. J. Clin. Orthop. Trauma. 2019, 10, 406–413. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chuang, M.J.; Jancosko, J.J.; Mendoza, V.; Nottage, W.M. The Incidence of Propionibacterium acnes in Shoulder Arthroscopy. Arthroscopy 2015, 31, 1702–1707. [Google Scholar] [CrossRef] [PubMed]
- Mook, W.R.; Klement, M.R.; Green, C.L.; Hazen, K.C.; Garrigues, G.E. The Incidence of Propionibacterium acnes in Open Shoulder Surgery: A Controlled Diagnostic Study. J. Bone Joint Surg. Am. 2015, 97, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Phadnis, J.; Gordon, D.; Krishnan, J.; Bain, G.I. Frequent isolation of Propionibacterium acnes from the shoulder dermis despite skin preparation and prophylactic antibiotics. J. Shoulder Elbow Surg. 2016, 25, 304–310. [Google Scholar] [CrossRef] [PubMed]

| Variable | Number (%) |
|---|---|
| Comorbidities total | 37 (67.27) |
| Obesity | 14 (25.45) |
| Diabetes Mellitus | 9 (16.36) |
| Renal Insufficiency | 8 (14.55) |
| Immunodeficiency | 2 (3.6) |
| I.V. Drug Abuse | 3 (5.45) |
| Variable | Number (%) |
|---|---|
| Positive blood cultures | 36 (65.45) |
| Sepsis | 9 (16.36) |
| Pleural involvement | 21 (38.18) |
| Primary joint resection and flap closure | 5 (9.09) |
| Secondary flap closure | 15 (27.27) |
| Total number of flap closure | 20 (36.36) |
| Hyperbaric oxygen treatment | 8 (14.54) |
| Postoperative complications | 20 (36.36) |
| Recurrence | 3 (5.45) |
| 30-day mortality | 0 (0) |
| NPWT changes [Mean (Std. Dev.)] | 3.09 [2.55] |
| Length of hospital stay [Mean (Std. Dev.)] | 20.75 [12.48] |
| Variable | Sepsis (OR) | Sepsis (95% CI) | p-Value | Complications (OR) | Complications (95% CI) | p-Value | Hospital Stay (Coefficient) | p-Value |
|---|---|---|---|---|---|---|---|---|
| Gender (Male) | 1.17 | 0.97–1.41 | 0.836 | 1.09 | 0.94–1.27 | 0.87 | −3.93 | 0.26 |
| Age | 1.04 | 0.47–2.28 | 0.232 | 0.99 | 0.81–1.22 | 0.82 | 0.42 | 0.10 |
| Pleural involvement | 2.34 | 1.09–5.04 | 0.249 | 1.56 | 0.87–2.79 | 0.43 | 3.33 | 0.34 |
| NPWT | 1.10 | 0.63–1.91 | 0.459 | 1.22 | 1.13–1.31 | 0.94 | 3.96 | 0.00 |
| Myoplastic procedure | 4.57 | 1.54–13.5 | 0.050 | 3.53 | 1.11–11.19 | 0.03 | 8.41 | 0.015 |
| HBO therapy | - | - | - | 1.05 | 0.98–1.13 | 0.94 | −2.33 | 0.62 |
| Pathogen (positive) | 2.25 | 1.16–4.38 | 0.344 | 2.25 | 0.97–5.2 | 0.19 | 4.23 | 0.22 |
| Comorbidities | 0.54 | 0.3–0.98 | 0.410 | 1.77 | 0.91–3.42 | 0.35 | 0.28 | 0.93 |
| Obesity | 1.03 | 0.99–1.07 | 0.966 | 1.17 | 0.91–1.5 | 0.78 | 1.53 | 0.67 |
| Diabetes mellitus | 0.21 | 0.09–0.5 | 0.161 | 2.36 | 0.95–5.84 | 0.14 | −1.52 | 0.67 |
| Renal insufficiency | 1.59 | 1.05–2.41 | 0.606 | 0.44 | 0.23–0.86 | 0.34 | 0.43 | 0.92 |
| I.V. drug abuse | - | - | - | 3.77 | 1.83–7.76 | 0.29 | −5.72 | 0.44 |
| Pain | 1.53 | 1.02–2.28 | 0.622 | 2.08 | 0.98–4.42 | 0.26 | 2.02 | 0.59 |
| Swelling | 1.20 | 1.03–1.4 | 0.870 | 1.50 | 1.02–2.2 | 0.64 | −4.71 | 0.35 |
| Fever | 3.17 | 1.24–8.1 | 0.120 | 3.37 | 1.1–10.29 | 0.04 | 7.48 | 0.036 |
| CRP | 1.01 | 0.25–4.01 | 0.003 | 1.00 | 0.45–2.23 | 0.22 | 0.01 | 0.23 |
| WBC | 1.16 | 0.3–4.5 | 0.004 | 0.95 | 0.45–2.0 | 0.27 | 0.02 | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmic, E.; Swatek, P.; Mykoliuk, I.; Busau, A.; Bamberg, P.; Smolle, J.; Smolle-Juettner, F.M.; Lindenmann, J. Management and Outcomes of Sternoclavicular Joint Infections: A Retrospective Study. J. Clin. Med. 2025, 14, 1893. https://doi.org/10.3390/jcm14061893
Ahmic E, Swatek P, Mykoliuk I, Busau A, Bamberg P, Smolle J, Smolle-Juettner FM, Lindenmann J. Management and Outcomes of Sternoclavicular Joint Infections: A Retrospective Study. Journal of Clinical Medicine. 2025; 14(6):1893. https://doi.org/10.3390/jcm14061893
Chicago/Turabian StyleAhmic, Edin, Paul Swatek, Iurii Mykoliuk, Anton Busau, Paul Bamberg, Josef Smolle, Freyja Maria Smolle-Juettner, and Jörg Lindenmann. 2025. "Management and Outcomes of Sternoclavicular Joint Infections: A Retrospective Study" Journal of Clinical Medicine 14, no. 6: 1893. https://doi.org/10.3390/jcm14061893
APA StyleAhmic, E., Swatek, P., Mykoliuk, I., Busau, A., Bamberg, P., Smolle, J., Smolle-Juettner, F. M., & Lindenmann, J. (2025). Management and Outcomes of Sternoclavicular Joint Infections: A Retrospective Study. Journal of Clinical Medicine, 14(6), 1893. https://doi.org/10.3390/jcm14061893








