Efficacy of Transcranial Direct Current Stimulation and Photobiomodulation in Improving Cognitive Abilities for Alzheimer’s Disease: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
- (1)
- English language;
- (2)
- Human participants;
- (3)
- Studies that evaluated efficacy over the cognitive functions while using tDCS or PBM;
- (4)
- Studies that evaluated safety while using tDCS or PBM;
- (5)
- Alzheimer’s disease diagnosis.
- (1)
- Simulated studies;
- (2)
- Studies that did not evaluate the efficacy over the cognitive functions;
- (3)
- Studies using only other NIBS (noninvasive brain stimulation techniques) as treatment;
- (4)
- Studies on pediatric population.
2.3. Data Collection and Outcome Measures
- (1)
- Metadata (authors names, journal and publication date);
- (2)
- Demographic data (age, sex, and number of participants);
- (3)
- Data-collecting methods (randomized controlled trial (RCT) and side-effects evaluation);
- (4)
- tDCS protocol (amperage used, active/sham stimulation, duration of stimulation, number of sessions performed, electrode size and location, and content of saline solution);
- (5)
- PBM protocol (wavelength, pulse frequency, intensity, duration of stimulation, numbers of sessions performed, way to administer (transcranial, intranasal), and active/sham);
- (6)
- Efficacy;
- (7)
- Cognitive abilities;
- (8)
- Reported medications used;
- (9)
- The author’s conclusions (whether tDCS and PBM are effective and improve cognitive abilities based on their assessments).
2.4. Risk of Bias
Quality of Studies
3. Results
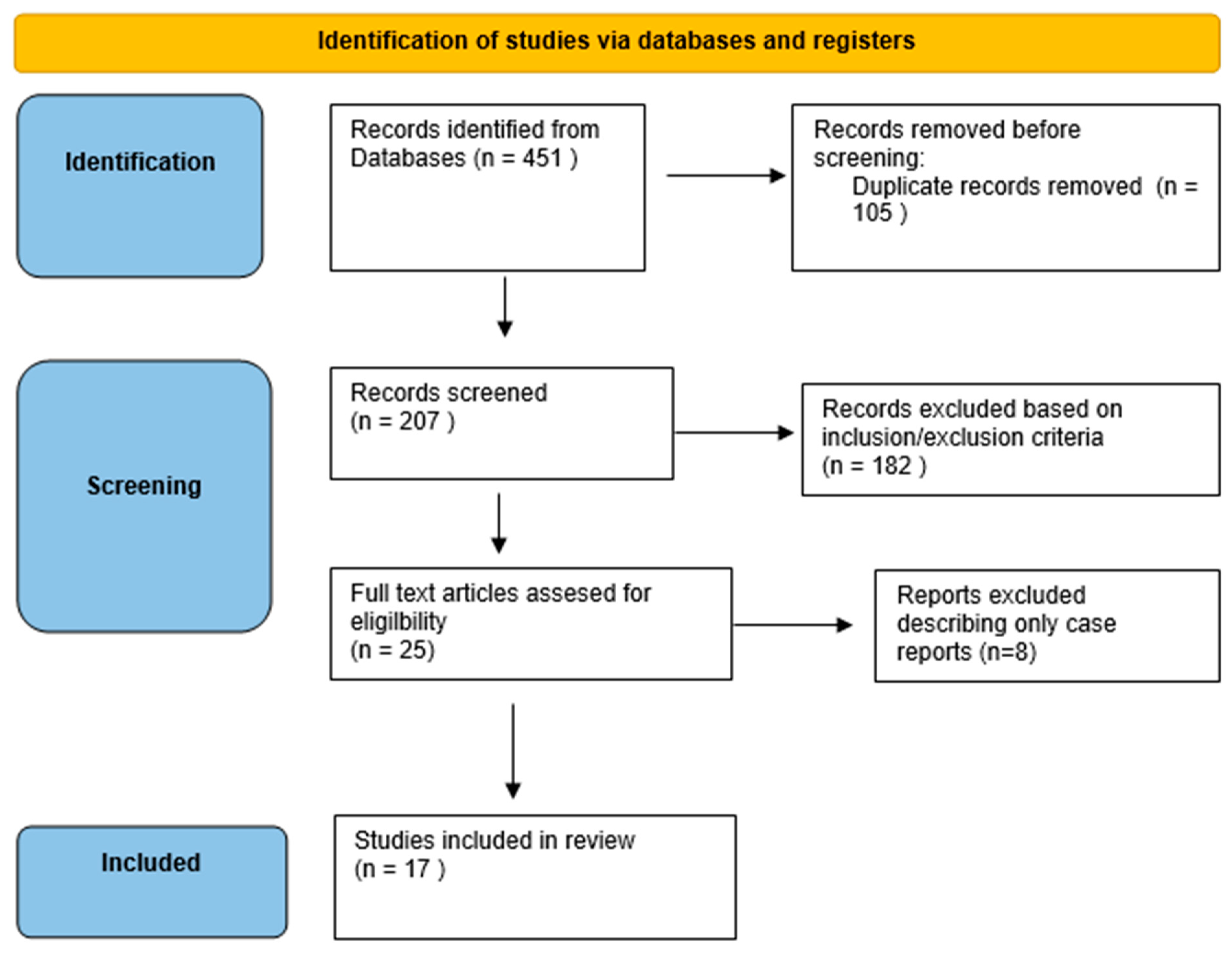
3.1. Literature Search
3.2. Efficacy and tDCS
3.2.1. Efficacy and Electrode Location
3.2.2. Electrode Size, Conducting Material, and Amperage
3.2.3. Efficacy and Medication
3.2.4. Efficacy for Specific tDCS Devices
3.2.5. Efficacy and Neuroimaging
EEG
MRI/PET
3.3. Safety and tDCS
Safety, Tolerability, and Side Effects
3.4. Efficacy and PBM
3.4.1. Efficacy and Method of Administration
3.4.2. Parameters Evaluated
3.4.3. Efficacy and Medication
3.4.4. Efficacy and Neuroimaging
3.5. Safety, Cognitive Effects, and PBM
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Disease—NHS. Available online: https://www.nhs.uk/conditions/alzheimers-disease/ (accessed on 12 January 2025).
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Marchi, L.Z.; Ferreira, R.G.D.; de Lima, G.N.S.; da Silva, J.A.S.; da Cruz, D.M.C.; Fernandez-Calvo, B.; Dos Santos Andrade, S.M.M. Multisite Transcranial Direct Current Stimulation Associated with Cognitive Training in Episodic Memory and Executive Functions in Individuals with Alzheimer’s Disease: A Case Report. J. Med. Case Rep. 2021, 15, 185. [Google Scholar] [CrossRef] [PubMed]
- Crisan, R.M.; Bacila, C.I.; Neamtu, B.; Cristian, A.N.; Topîrcean, E.; Popescu, A.; Morar, S. Psychological Autopsy and Forensic Considerations in Completed Suicide of the SARS-CoV-2 Infected Patients. A Case Series and Literature Review. Appl. Sci. 2021, 11, 11547. [Google Scholar] [CrossRef]
- Murugaraja, V.; Shivakumar, V.; Sivakumar, P.T.; Sinha, P.; Venkatasubramanian, G. Clinical Utility and Tolerability of Transcranial Direct Current Stimulation in Mild Cognitive Impairment. Asian J. Psychiatry 2017, 30, 135–140. [Google Scholar] [CrossRef]
- Crișan, R.M.; Băcilă, C.I.; Morar, S. The Role of Psychological Autopsy in Investigating a Case of Atypical Suicide in Schizophrenia: A Case Report with a Brief Review of Literature. Egypt. J. Forensic Sci. 2022, 12, 30. [Google Scholar] [CrossRef]
- Negrea, M.O.; Neamtu, B.; Dobrotă, I.; Sofariu, C.R.; Crisan, R.M.; Ciprian, B.I.; Domnariu, C.D.; Teodoru, M. Causative Mechanisms of Childhood and Adolescent Obesity Leading to Adult Cardiometabolic Disease: A Literature Review. Appl. Sci. 2021, 11, 11565. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The Diagnosis of Dementia Due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef]
- Vintilă, B.I.; Anghel, C.E.; Sava, M.; Bereanu, A.S.; Codru, I.R.; Stoica, R.; Vulcu Mihai, A.M.; Grama, A.M.; Cătană, A.C.; Boicean, A.G.; et al. Evaluating Anesthesia Practices, Patient Characteristics, and Outcomes in Electroconvulsive Therapy: A Two-Year Retrospective Study. J. Clin. Med. 2024, 13, 6253. [Google Scholar] [CrossRef]
- Paroni, G.; Bisceglia, P.; Seripa, D. Understanding the Amyloid Hypothesis in Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 68, 493–510. [Google Scholar] [CrossRef]
- Mormino, E.C.; Papp, K.V. Amyloid Accumulation and Cognitive Decline in Clinically Normal Older Individuals: Implications for Aging and Early Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 64, S633–S646. [Google Scholar] [CrossRef]
- Villemagne, V.L.; Burnham, S.; Bourgeat, P.; Brown, B.; Ellis, K.A.; Salvado, O.; Szoeke, C.; Macaulay, S.L.; Martins, R.; Maruff, P.; et al. Amyloid β Deposition, Neurodegeneration, and Cognitive Decline in Sporadic Alzheimer’s Disease: A Prospective Cohort Study. Lancet Neurol. 2013, 12, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, R.H.; Khan, S.M. A “Mitochondrial Cascade Hypothesis” for Sporadic Alzheimer’s Disease. Med. Hypotheses 2004, 63, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, R.H.; Khan, S.M. The Alzheimer’s Disease Mitochondrial Cascade Hypothesis: An Update. Exp. Neurol. 2009, 218, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Vintila, B.I.; Arseniu, A.M.; Morgovan, C.; Butuca, A.; Bîrluțiu, V.; Dobrea, C.M.; Rus, L.L.; Ghibu, S.; Bereanu, A.S.; Arseniu, R.; et al. A Real-World Study on the Clinical Characteristics, Outcomes, and Relationship between Antibiotic Exposure and Clostridioides Difficile Infection. Antibiotics 2024, 13, 144. [Google Scholar] [CrossRef]
- Blonz, E.R. Alzheimer’s Disease as the Product of a Progressive Energy Deficiency Syndrome in the Central Nervous System: The Neuroenergetic Hypothesis. J. Alzheimer’s Dis. 2017, 60, 1223–1229. [Google Scholar] [CrossRef]
- Briggs, R.; Kennelly, S.P.; O’Neill, D. Drug Treatments in Alzheimer’s Disease. Clin. Med. 2016, 16, 247–253. [Google Scholar] [CrossRef]
- Lahoz, P.E.; Guilherme, F.; Silva, D.; Lahoz Fernandez, P.E. Cognitive Outcomes of Anti-Amyloid-β Monoclonal Antibodies in Patients with Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Alzheimer’s Dement. 2021, 17, e057778. [Google Scholar] [CrossRef]
- Pritam, P.; Deka, R.; Bhardwaj, A.; Srivastava, R.; Kumar, D.; Jha, A.K.; Jha, N.K.; Villa, C.; Jha, S.K. Antioxidants in Alzheimer’s Disease: Current Therapeutic Significance and Future Prospects. Biology 2022, 11, 212. [Google Scholar] [CrossRef]
- Ciobanu, A.M.; Petrescu, C.; Anghele, C.; Manea, M.C.; Ciobanu, C.A.; Petrescu, D.M.; Antonia, M.O.; Riga, S. Severe Vitamin D Deficiency—A Possible Cause of Resistance to Treatment in Psychiatric Pathology. Medicina 2023, 59, 2056. [Google Scholar] [CrossRef]
- Gonzalez-Moreno, J.; Satorres, E.; Soria-Urios, G.; Meléndez, J.C. Cognitive Stimulation in Moderate Alzheimer’s Disease. J. Appl. Gerontol. 2022, 41, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.; Song, I.U.; Chung, Y.A. Changes in Cerebral Glucose Metabolism after 3 Weeks of Noninvasive Electrical Stimulation of Mild Cognitive Impairment Patients. Alzheimer’s Res. Ther. 2016, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, I.D.; Mittner, M.; Boayue, N.M.; Csifcsák, G.; Aslaksen, P.M. Tracking the Current in the Alzheimer’s Brain—Systematic Differences between Patients and Healthy Controls in the Electric Field Induced by TDCS. Neuroimage Rep. 2023, 3, 100172. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-Based Guidelines on the Therapeutic Use of Transcranial Direct Current Stimulation (TDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef]
- Teselink, J.; Bawa, K.K.; Koo, G.K.; Sankhe, K.; Liu, C.S.; Rapoport, M.; Oh, P.; Marzolini, S.; Gallagher, D.; Swardfager, W.; et al. Efficacy of Non-Invasive Brain Stimulation on Global Cognition and Neuropsychiatric Symptoms in Alzheimer’s Disease and Mild Cognitive Impairment: A Meta-Analysis and Systematic Review. Ageing Res. Rev. 2021, 72, 101499. [Google Scholar] [CrossRef]
- Yu, M.; Sporns, O.; Saykin, A.J. The Human Connectome in Alzheimer Disease—Relationship to Biomarkers and Genetics. Nat. Rev. Neurol. 2021, 17, 545–563. [Google Scholar] [CrossRef]
- Pievani, M.; de Haan, W.; Wu, T.; Seeley, W.W.; Frisoni, G.B. Functional Network Disruption in the Degenerative Dementias. Lancet Neurol. 2011, 10, 829–843. [Google Scholar] [CrossRef]
- Chan, C.Y.; Hounsgaard, J.; Nicholson, C. Effects of Electric Fields on Transmembrane Potential and Excitability of Turtle Cerebellar Purkinje Cells in Vitro. J. Physiol. 1988, 402, 751–771. [Google Scholar] [CrossRef]
- Woods, A.J.; Antal, A.; Bikson, M.; Boggio, P.S.; Brunoni, A.R.; Celnik, P.; Cohen, L.G.; Fregni, F.; Herrmann, C.S.; Kappenman, E.S.; et al. A Technical Guide to TDCS, and Related Non-Invasive Brain Stimulation Tools. Clin. Neurophysiol. 2016, 127, 1031–1048. [Google Scholar] [CrossRef]
- Yamada, Y.; Sumiyoshi, T. Neurobiological Mechanisms of Transcranial Direct Current Stimulation for Psychiatric Disorders; Neurophysiological, Chemical, and Anatomical Considerations. Front. Hum. Neurosci. 2021, 15, 631838. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Wendling, F. Mechanisms of Action of TDCS: A Brief and Practical Overview. Neurophysiol. Clin. 2019, 49, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Chase, H.W.; Boudewyn, M.A.; Carter, C.S.; Phillips, M.L. Transcranial Direct Current Stimulation: A Roadmap for Research, from Mechanism of Action to Clinical Implementation. Mol. Psychiatry 2020, 25, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Meinzer, M.; Lindenberg, R.; Phan, M.T.; Ulm, L.; Volk, C.; Flöel, A. Transcranial Direct Current Stimulation in Mild Cognitive Impairment: Behavioral Effects and Neural Mechanisms. Alzheimer’s Dement. 2015, 11, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Šimko, P.; Kent, J.A.; Rektorova, I. Is Non-Invasive Brain Stimulation Effective for Cognitive Enhancement in Alzheimer’s Disease? An Updated Meta-Analysis. Clin. Neurophysiol. 2022, 144, 23–40. [Google Scholar] [CrossRef]
- Cheng, C.P.W.; Wong, C.S.M.; Lee, K.K.; Chan, A.P.K.; Yeung, J.W.F.; Chan, W.C. Effects of Repetitive Transcranial Magnetic Stimulation on Improvement of Cognition in Elderly Patients with Cognitive Impairment: A Systematic Review and Meta-Analysis. Int. J. Geriatr. Psychiatry 2018, 33, e1–e13. [Google Scholar] [CrossRef]
- Chu, C.S.; Li, C.T.; Brunoni, A.R.; Yang, F.C.; Tseng, P.T.; Tu, Y.K.; Stubbs, B.; Carvalho, A.F.; Thompson, T.; Rajji, T.K.; et al. Cognitive Effects and Acceptability of Non-Invasive Brain Stimulation on Alzheimer’s Disease and Mild Cognitive Impairment: A Component Network Meta-Analysis. J. Neurol. Neurosurg. Psychiatry 2021, 92, 195–203. [Google Scholar] [CrossRef]
- Holczer, A.; Németh, V.L.; Vékony, T.; Vécsei, L.; Klivényi, P.; Must, A. Non-Invasive Brain Stimulation in Alzheimer’s Disease and Mild Cognitive Impairment-A State-of-the-Art Review on Methodological Characteristics and Stimulation Parameters. Front. Hum. Neurosci. 2020, 14, 179. [Google Scholar] [CrossRef]
- Huo, L.; Zhu, X.; Zheng, Z.; Ma, J.; Ma, Z.; Gui, W.; Li, J. Effects of Transcranial Direct Current Stimulation on Episodic Memory in Older Adults: A Meta-Analysis. J. Gerontol. B Psychol. Sci. Soc. Sci. 2021, 76, 692–702. [Google Scholar] [CrossRef]
- Gonzalez-Lima, F. Neuroprotection and Neurocognitive Augmentation by Photobiomodulation. In Modern Approaches to Augmentation of Brain Function. Contemporary Clinical Neuroscience; Springer: Cham, Switzerland, 2021; pp. 165–207. [Google Scholar] [CrossRef]
- Blanco, N.J.; Saucedo, C.L.; Gonzalez-Lima, F. Transcranial Infrared Laser Stimulation Improves Rule-Based, but Not Information-Integration, Category Learning in Humans. Neurobiol. Learn. Mem. 2017, 139, 69–75. [Google Scholar] [CrossRef]
- Dompe, C.; Moncrieff, L.; Matys, J.; Grzech-Leśniak, K.; Kocherova, I.; Bryja, A.; Bruska, M.; Dominiak, M.; Mozdziak, P.; Skiba, T.H.I.; et al. Photobiomodulation-Underlying Mechanism and Clinical Applications. J. Clin. Med. 2020, 9, 1724. [Google Scholar] [CrossRef]
- Chao, L.L. Effects of Home Photobiomodulation Treatments on Cognitive and Behavioral Function, Cerebral Perfusion, and Resting-State Functional Connectivity in Patients with Dementia: A Pilot Trial. Photobiomodul. Photomed. Laser Surg. 2019, 37, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, F.; Hamblin, M.R.; Diduro, J.O. Rapid Reversal of Cognitive Decline, Olfactory Dysfunction, and Quality of Life Using Multi-Modality Photobiomodulation Therapy: Case Report. Photobiomodul Photomed. Laser Surg. 2019, 37, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Saltmarche, A.E.; Naeser, M.A.; Ho, K.F.; Hamblin, M.R.; Lim, L. Significant Improvement in Cognition in Mild to Moderately Severe Dementia Cases Treated with Transcranial Plus Intranasal Photobiomodulation: Case Series Report. Photomed. Laser Surg. 2017, 35, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, F.; Khademi, M.; Hamblin, M.R. Photobiomodulation Therapy for Dementia: A Systematic Review of Pre-Clinical and Clinical Studies. J. Alzheimer’s Dis. 2021, 83, 1431–1452. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, e112. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Nagy, E.N.; Ali, A.Y.; Behiry, M.E.; Naguib, M.M.; Elsayed, M.M. Impact of Combined Photo-Biomodulation and Aerobic Exercise on Cognitive Function and Quality-of-Life in Elderly Alzheimer Patients with Anemia: A Randomized Clinical Trial. Int. J. Gen. Med. 2021, 14, 141–152. [Google Scholar] [CrossRef]
- Razzaghi, M.; Sheibani, F.; Kimia, N.; Razzaghi, Z.; Chenari, Z.; Ashrafi, F.; Barati, M.; Advani, S. Photobiomodulation’s Potential as a Non-Invasive Therapy for Alzheimer’s Disease and Minimal Cognitive Impairment: A 12-Week Investigation. Photodiagnosis Photodyn. Ther. 2024, 46, 103991. [Google Scholar] [CrossRef]
- Andrade, S.M.; da Silva Machado, D.G.; da Silva-Sauerc, L.; Regis, C.T.; Mendes, C.K.T.T.; de Araújo, J.S.S.; de Araújo, K.D.T.; Costa, L.P.; Queiroz, M.E.B.S.; Leitão, M.M.; et al. Effects of Multisite Anodal Transcranial Direct Current Stimulation Combined with Cognitive Stimulation in Patients with Alzheimer’s Disease and Its Neurophysiological Correlates: A Double-Blind Randomized Clinical Trial. Neurophysiol. Clin. 2022, 52, 117–127. [Google Scholar] [CrossRef]
- Gangemi, A.; Fabio, R.A. Transcranial Direct Current Stimulation for Alzheimer Disease. Asian J. Gerontol. Geriatr. 2020, 15, 5–9. [Google Scholar] [CrossRef]
- Gangemi, A.; Colombo, B.; Fabio, R.A. Effects of Short- and Long-Term Neurostimulation (TDCS) on Alzheimer’s Disease Patients: Two Randomized Studies. Aging Clin. Exp. Res. 2021, 33, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jia, Y.; Sun, Y.; Ding, Y.; Huang, Z.; Liu, C.; Wang, Y. Efficacy and Safety of Simultaneous RTMS-TDCS over Bilateral Angular Gyrus on Neuropsychiatric Symptoms in Patients with Moderate Alzheimer’s Disease: A Prospective, Randomized, Sham-Controlled Pilot Study. Brain Stimul. 2022, 15, 1530–1537. [Google Scholar] [CrossRef]
- Im, J.J.; Jeong, H.; Bikson, M.; Woods, A.J.; Unal, G.; Oh, J.K.; Na, S.; Park, J.S.; Knotkova, H.; Song, I.U.; et al. Effects of 6-Month at-Home Transcranial Direct Current Stimulation on Cognition and Cerebral Glucose Metabolism in Alzheimer’s Disease. Brain Stimul. 2019, 12, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yang, Y.S. Alterations in Cognitive Function and Blood Biomarkers Following Transcranial Direct Current Stimulation in Patients with Amyloid Positron Emission Tomography-Positive Alzheimer’s Disease: A Preliminary Study. Front. Neurosci. 2023, 17, 1327886. [Google Scholar] [CrossRef] [PubMed]
- Lane, H.Y.; Wang, S.H.; Lin, C.H. Adjunctive Transcranial Direct Current Stimulation (TDCS) plus Sodium Benzoate for the Treatment of Early-Phase Alzheimer’s Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Psychiatry Res. 2023, 328, 115461. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Yu, K.; Zhuang, W.; Zhu, H.; Xu, W.; Yan, H.; Qi, G.; Zhou, D.; Wu, S. Impact of Twice-a-Day Transcranial Direct Current Stimulation Intervention on Cognitive Function and Motor Cortex Plasticity in Patients with Alzheimer’s Disease. Gen. Psychiatry 2023, 36, e101166. [Google Scholar] [CrossRef]
- Philippen, S.; Hanert, A.; Schönfeld, R.; Granert, O.; Yilmaz, R.; Jensen-Kondering, U.; Splittgerber, M.; Moliadze, V.; Siniatchkin, M.; Berg, D.; et al. Transcranial Direct Current Stimulation of the Right Temporoparietal Junction Facilitates Hippocampal Spatial Learning in Alzheimer’s Disease and Mild Cognitive Impairment. Clin. Neurophysiol. 2024, 157, 48–60. [Google Scholar] [CrossRef]
- Pini, L.; Pizzini, F.B.; Boscolo-Galazzo, I.; Ferrari, C.; Galluzzi, S.; Cotelli, M.; Gobbi, E.; Cattaneo, A.; Cotelli, M.S.; Geroldi, C.; et al. Brain Network Modulation in Alzheimer’s and Frontotemporal Dementia with Transcranial Electrical Stimulation. Neurobiol. Aging 2022, 111, 24–34. [Google Scholar] [CrossRef]
- Rasmussen, I.D.; Boayue, N.M.; Mittner, M.; Bystad, M.; Grønli, O.K.; Vangberg, T.R.; Csifcsák, G.; Aslaksen, P.M.; Peter, J. High-Definition Transcranial Direct Current Stimulation Improves Delayed Memory in Alzheimer’s Disease Patients: A Pilot Study Using Computational Modeling to Optimize Electrode Position. J. Alzheimer’s Dis. 2021, 83, 753–769. [Google Scholar] [CrossRef]
- Satorres, E.; Escudero Torrella, J.; Real, E.; Pitarque, A.; Delhom, I.; Melendez, J.C. Home-Based Transcranial Direct Current Stimulation in Mild Neurocognitive Disorder Due to Possible Alzheimer’s Disease. A Randomised, Single-Blind, Controlled-Placebo Study. Front. Psychol. 2023, 13, 1071737. [Google Scholar] [CrossRef]
- Smirni, D.; Oliveri, M.; Misuraca, E.; Catania, A.; Vernuccio, L.; Picciolo, V.; Inzerillo, F.; Barbagallo, M.; Cipolotti, L.; Turriziani, P. Verbal Fluency in Mild Alzheimer’s Disease: Transcranial Direct Current Stimulation over the Dorsolateral Prefrontal Cortex. J. Alzheimer’s Dis. 2021, 81, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Maksimovich, I.V. Stimulation of Cerebral Angiogenesis and Neurogenesis with Transcatheter Intracerebral Laser Photobiomodulation Therapy during Dementia in Patients with Alzheimer’s and Binswanger’s Disease. Alzheimer’s Dement. 2021, 17, e054945. [Google Scholar] [CrossRef]
- Wang, C.S.; Chen, P.S.; Tsai, T.Y.; Hou, N.T.; Tang, C.H.; Chen, P.L.; Huang, Y.C.; Cheng, K.S. Cognitive Effect of Transcranial Direct Current Stimulation on Left Dorsolateral Prefrontal Cortex in Mild Alzheimer’s Disease: A Randomized, Double-Blind, Cross-Over Small-Scale Exploratory Study. J. Alzheimers Dis. 2024, 98, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wu, J.; Ye, R.; Liao, X. 417—Comparison of Prevalence and Disturbance of Neuropsychic Symptoms between Institution-Dwelling and Community-Dwelling Patients with Dementia. Int. Psychogeriatr. 2021, 33, 40. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Invited Faculty Abstracts from the International Neuromodulation Society’s 14th World Congress. Neuromodulation 2019, 22, e296–e584. [CrossRef]
- McLaren, M.E.; Nissim, N.R.; Woods, A.J. The Effects of Medication Use in Transcranial Direct Current Stimulation: A Brief Review. Brain Stimul. 2018, 11, 52–58. [Google Scholar] [CrossRef]
- Singh, B.; Day, C.M.; Abdella, S.; Garg, S. Alzheimer’s Disease Current Therapies, Novel Drug Delivery Systems and Future Directions for Better Disease Management. J. Control. Release 2024, 367, 402–424. [Google Scholar] [CrossRef]
- Andrade, C.; Bolwig, T.G. Electroconvulsive Therapy, Hypertensive Surge, Blood-Brain Barrier Breach, and Amnesia: Exploring the Evidence for a Connection. J. ECT 2014, 30, 160–164. [Google Scholar] [CrossRef]
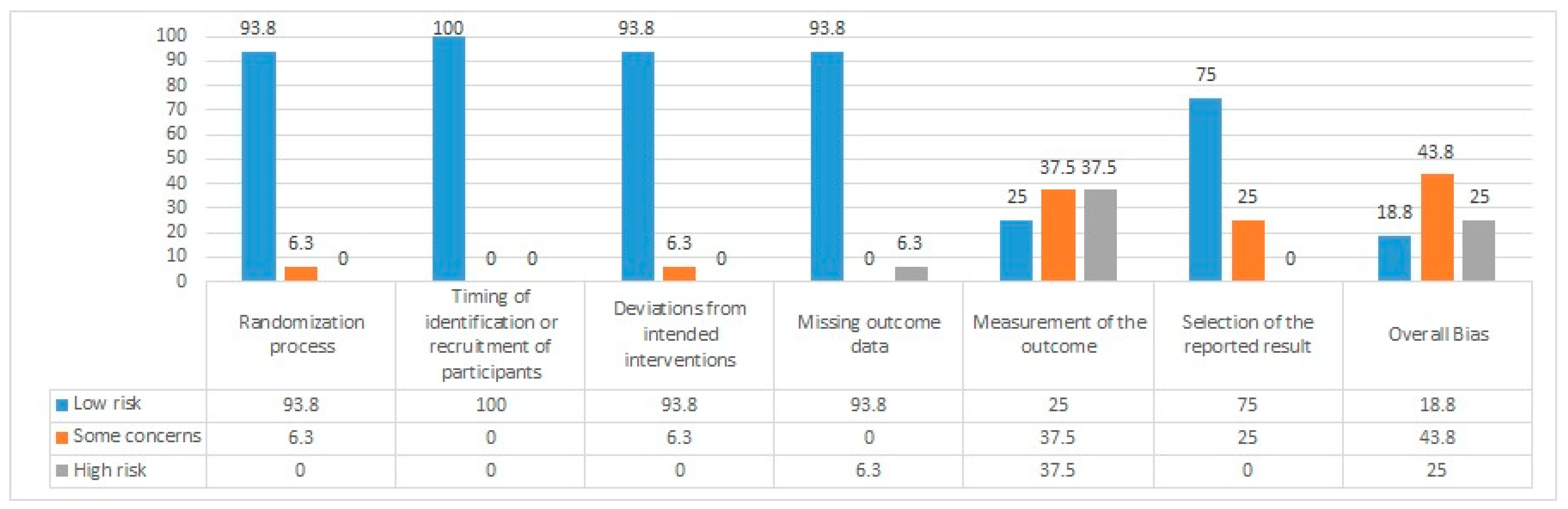
| Reference | Randomization Process | Timing of Identification or Recruitment of Participants | Deviations from Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Result | Overall Bias |
|---|---|---|---|---|---|---|---|
| Nagy et al. [49] | Low | Low | Low | High | High | Some concerns | High |
| Razzaghi et al. [50] | Low | Low | Low | Low | Low | Low | Low |
| Andrade et al. [51] | Low | Low | Low | Low | Low | Low | Low |
| Gangemi and Fabio [52] | Low | Low | Some concerns | Low | High | Some concerns | Some concerns |
| Gangemi et al. [53] | Low | Low | Low | Low | Some concerns | Low | Some concerns |
| Hu et al. [54] | Low | Low | Low | Low | Low | Low | Low |
| J.J. Im et al. [55] | Low | Low | Low | Low | Some concerns | Low | Some concerns |
| Kim J and Yang Y [56] | Low | Low | Low | Low | High | Low | High |
| H.-Y. Lane et al. [57] | Low | Low | Low | Low | High | Low | Some concerns |
| Li et al. [58] | Low | Low | Low | Low | Some concerns | Low | Some concerns |
| Philippen et al. [59] | Low | Low | Low | Low | Some concerns | Low | Some concerns |
| Pini et al. [60] | Low | Low | Low | Low | Low | Low | Low |
| Rasmussen et al. [61] | Low | Low | Low | Low | Some concerns | Low | Some concerns |
| Satorres et al. [62] | Low | Low | Low | Low | Some concerns | Low | Some concerns |
| Smirni et al. [63] | Low | Low | Low | Low | High | Some concerns | High |
| Maksimovichi [64] | Some concerns | Low | Low | Low | High | Some concerns | High |
| Wang et al. [65] | Low | Low | Low | Low | High | Low | High |
| Study | Device Used | Amperage Used (mA) | Electrode Location | Electrode Size (cm) | Soaking Solution | Medication Reported |
|---|---|---|---|---|---|---|
| Wang et al. (2024) [65] | Neuroelectrics STARSTIM | 2 | DLPFC | 5 | Saline | None to be reported |
| Smirni et al. (2021) [63] | BrainStim | 1 | DLFPC | 5 | Saline | None to be reported |
| Satorres et al. (2023) [62] | Newronika | 2 | DLFPC | 5 | Sterile water | None to be reported |
| Andrade et al. (2022) [51] | TCT-Research neurostimulator | 2 | L, R DLPFC, Broca, Wernicke, L, R PL | 5 | Saline | None to be reported |
| Gangemi (2020) [52] | Braindee | 2 | DLPFC | 2.5 | Saline | Yes |
| Gangemi (2021) [53] | Braindee | 2 | DLPFC | 2.5 | Saline | Yes |
| Hu et al. (2022) [54] | Tianjin Timus Medical | 2 | Angular gyrus | None to be reported | Saline | Yes |
| J.J. Im et al. (2019) [55] | YDS 301 | 2 | DLPFC | 6 | Saline | None to be reported |
| Kim and Yang (2023) [56] | YMS 201 | 2 | DLPFC | None to be reported | Saline | None to be reported |
| H.-Y. Lane et al. (2023) [57] | Neurocon | 2 | DLPFC | 5 | None to be reported | Yes |
| Li X et al. (2023) [58] | TDCS 20A | 2 | DLPFC | 5 | None to be reported | None to be reported |
| Philippen et al. (2024) [59] | Tarstim 32 | 2 | rTPJ | None to be reported | None to be reported | Yes |
| Pini et al. (2022) [60] | Brainstim | 1.5 | RIP DMN node, RDLPF SN node | None to be reported | 5 | None to be reported |
| Rassmusen et al. (2021) [61] | Starstim | 2 | DLPFC | 1.2 | None to be reported | None to be reported |
| Study | Country of Study and Type of Population | Other Diagnoses Beside AD | Mean Age | Participant Number | Active /Sham | Other NIBS Methods | Efficacy | Side Effects Reported |
|---|---|---|---|---|---|---|---|---|
| Wang et al. (2024) [65] | Taiwan/Asian | No | 75.6 | 30 | Yes/ yes | No | Yes | No |
| Smirni et al. (2021) [63] | Italian/ European | No | 73.17 | 40 | Yes/ no | No | Yes | Yes |
| Satorres et al. (2023) [62] | Spanish/ European | No | 75 | 33 | Yes/ yes | No | Yes | Yes |
| Andrade et al. (2022) [51] | Brazil/ South American | No | 76.3 | 36 | Yes/ yes | No | Yes | No |
| Gangemi (2020) [52] | Italy/ European | No | None reported | 26 | Yes/ yes | No | Yes | No |
| Gangemi (2021) [53] | Italy/ European | No | 68.6 | 18 | Yes/ yes | No | Yes | No |
| Hu et al. (2022) [54] | China/ Asian | No | 76.2 | 21 | Yes/ yes | TMS | Yes | Yes |
| J.J. Im et al. (2019) [55] | Republic of Korea/Asian | No | 73.4 | 20 | Yes/ yes | No | Yes | No |
| Kim and Yang (2023) [56] | Republic of Korea/ Asian | No | 70.5 | 16 | Yes/ yes | No | Yes | No |
| H.-Y. Lane et al. (2023) [57] | Taiwan/ Asian | No | 74.3 | 48 | Yes/ no | No | Yes | Yes |
| Li X et al. (2023) [58] | China/ Asian | No | 76.14 | 124 | Yes/ yes | No | Yes | No |
| Philippen et al. (2024) [59] | Germany/ European | MCI | None reported (only for tDCS) | 12 | Yes/ yes | No | Yes | Yes |
| Pini et al. (2022) [60] | Italy/ European | bvFTD behavioral variant frontotemporal dementia | 72.5 | 26 | Yes/ no | No | Yes | Yes |
| Rassmusen et al. (2021) [61] | Norway/ European | No | 78.80 | 19 | Yes/ yes | No | Yes | No |
| Study Included | Device Used/ | Administration Mode | Wavelength, nm | Beam Size | Timing of Treatment/Session | Numbers of Sessions | Active /Sham | Efficacy | Side Effects Reported |
|---|---|---|---|---|---|---|---|---|---|
| Razzaghi [50] | Hoosh Yar Gamma | Transcranial | 810 | 3.14 cm2 | 20 min | 6×/week, 12 weeks | Yes/yes | Yes | None to be reported |
| Maksimovich [64] | Helium–neon laser ULF-01 | Transcatheter Intracerebral | 632.8 | 1–2 mm | 1200–2400 s | 1 time only, on both the right and left hemispheres | Yes/ no | Yes | None to be reported |
| Nagy [49] | LASPOT | Nasal and wrist laser acupuncture watch | 650 nm | None to be reported | 30 min per session | 2 times/day, 3 days/week for 3 months | Yes/ yes | Yes | None to be reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cornea, M.; Vintilă, B.I.; Bucuța, M.; Ștef, L.; Anghel, C.E.; Grama, A.M.; Lomnasan, A.; Stetiu, A.A.; Boicean, A.; Sava, M.; et al. Efficacy of Transcranial Direct Current Stimulation and Photobiomodulation in Improving Cognitive Abilities for Alzheimer’s Disease: A Systematic Review. J. Clin. Med. 2025, 14, 1766. https://doi.org/10.3390/jcm14051766
Cornea M, Vintilă BI, Bucuța M, Ștef L, Anghel CE, Grama AM, Lomnasan A, Stetiu AA, Boicean A, Sava M, et al. Efficacy of Transcranial Direct Current Stimulation and Photobiomodulation in Improving Cognitive Abilities for Alzheimer’s Disease: A Systematic Review. Journal of Clinical Medicine. 2025; 14(5):1766. https://doi.org/10.3390/jcm14051766
Chicago/Turabian StyleCornea, Monica, Bogdan Ioan Vintilă, Mihaela Bucuța, Laura Ștef, Claudia Elena Anghel, Andreea Maria Grama, Andrei Lomnasan, Andreea Angela Stetiu, Adrian Boicean, Mihai Sava, and et al. 2025. "Efficacy of Transcranial Direct Current Stimulation and Photobiomodulation in Improving Cognitive Abilities for Alzheimer’s Disease: A Systematic Review" Journal of Clinical Medicine 14, no. 5: 1766. https://doi.org/10.3390/jcm14051766
APA StyleCornea, M., Vintilă, B. I., Bucuța, M., Ștef, L., Anghel, C. E., Grama, A. M., Lomnasan, A., Stetiu, A. A., Boicean, A., Sava, M., Paziuc, L. C., Manea, M. C., Tîbîrnă, A., & Băcilă, C.-I. (2025). Efficacy of Transcranial Direct Current Stimulation and Photobiomodulation in Improving Cognitive Abilities for Alzheimer’s Disease: A Systematic Review. Journal of Clinical Medicine, 14(5), 1766. https://doi.org/10.3390/jcm14051766







