A Novel Fat-Augmented Omentum-Based Construct Is a Cost-Effective Alternative for Autologous Breast Reconstruction
Abstract
1. Introduction
2. Materials and Methods
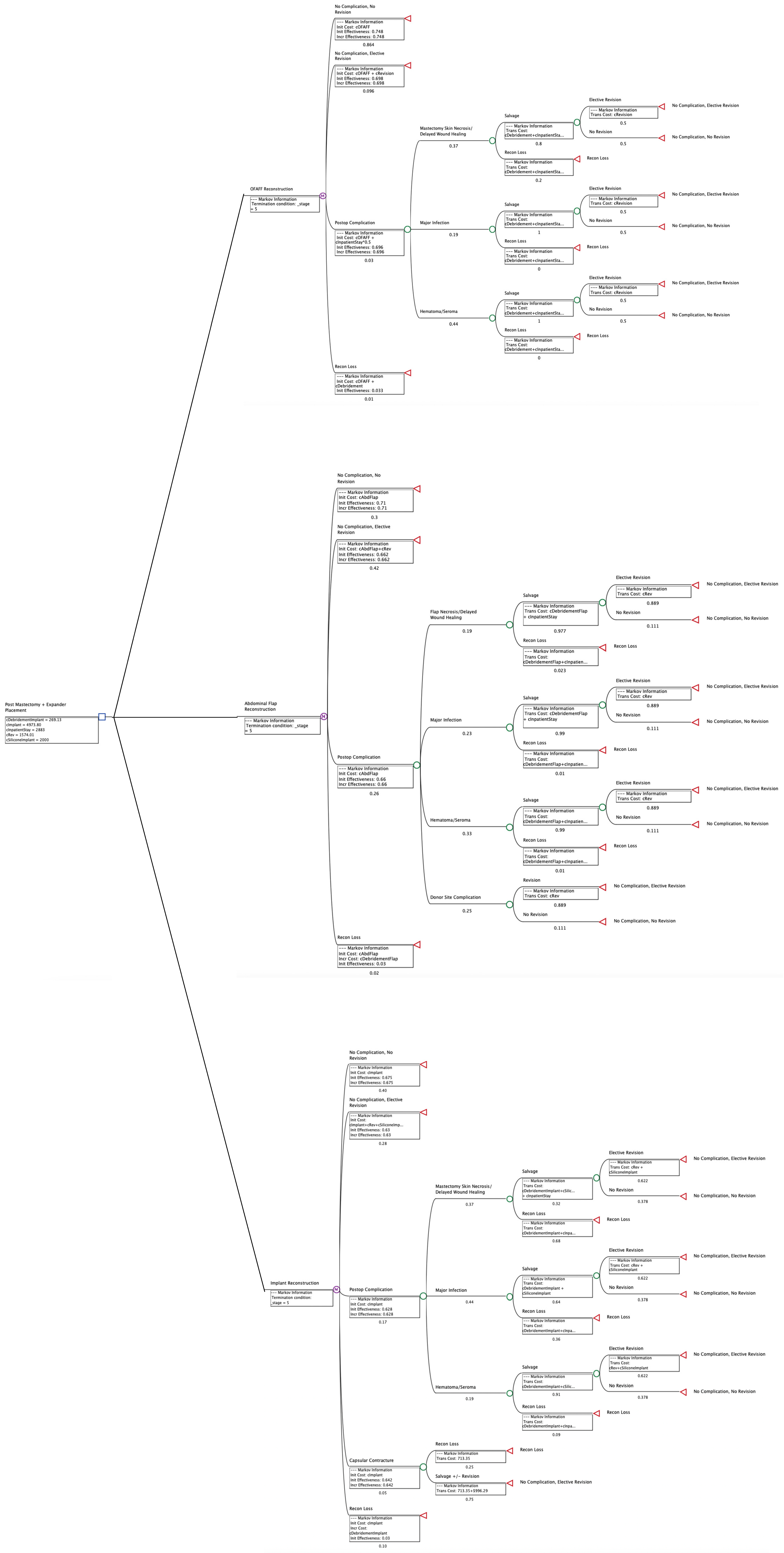
2.1. Model Development
2.2. Data Sources
2.3. Model Parameters
2.4. Model Assumptions
2.5. Patient Simulation
2.6. Study Outcomes
2.7. Sensitivity Analyses
3. Results
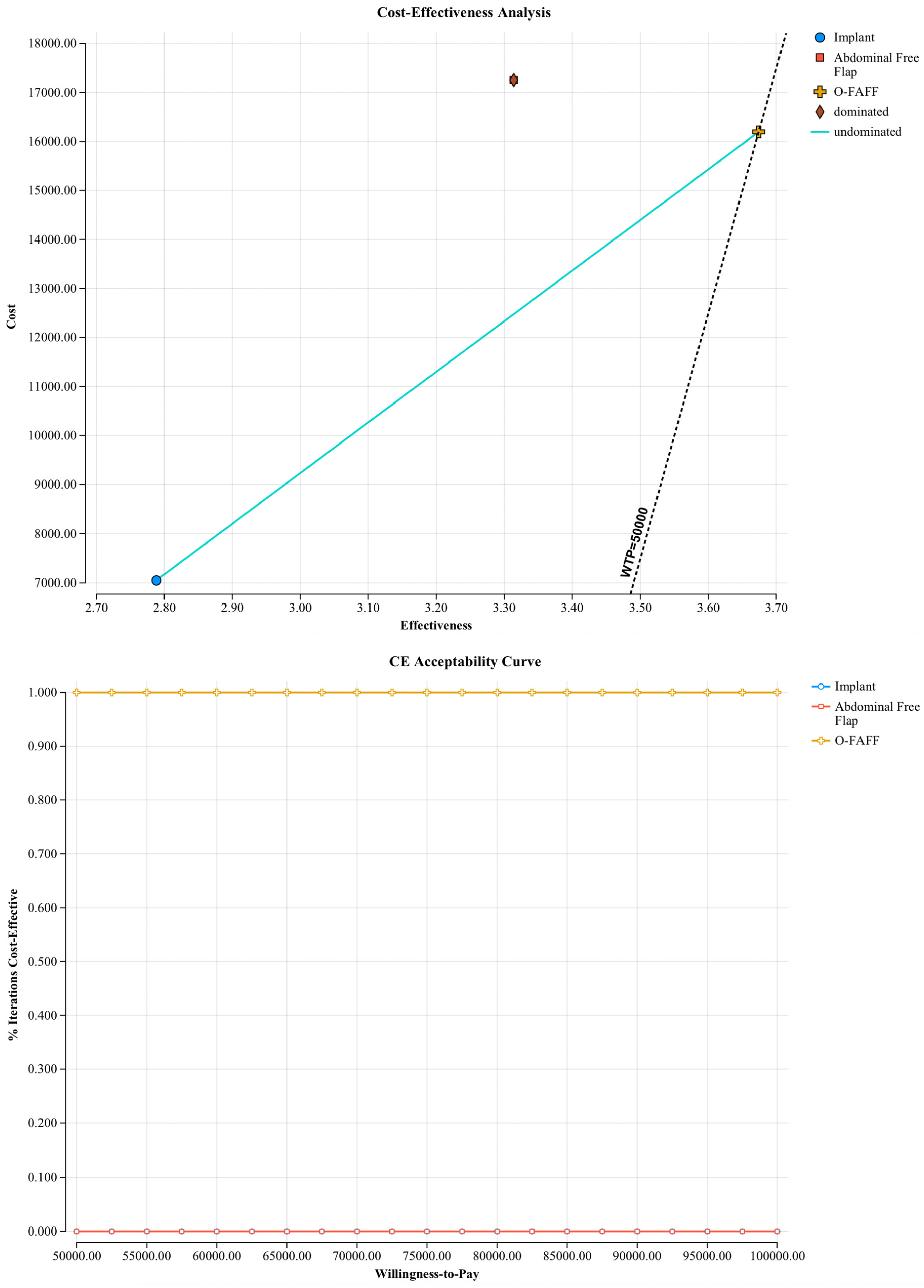
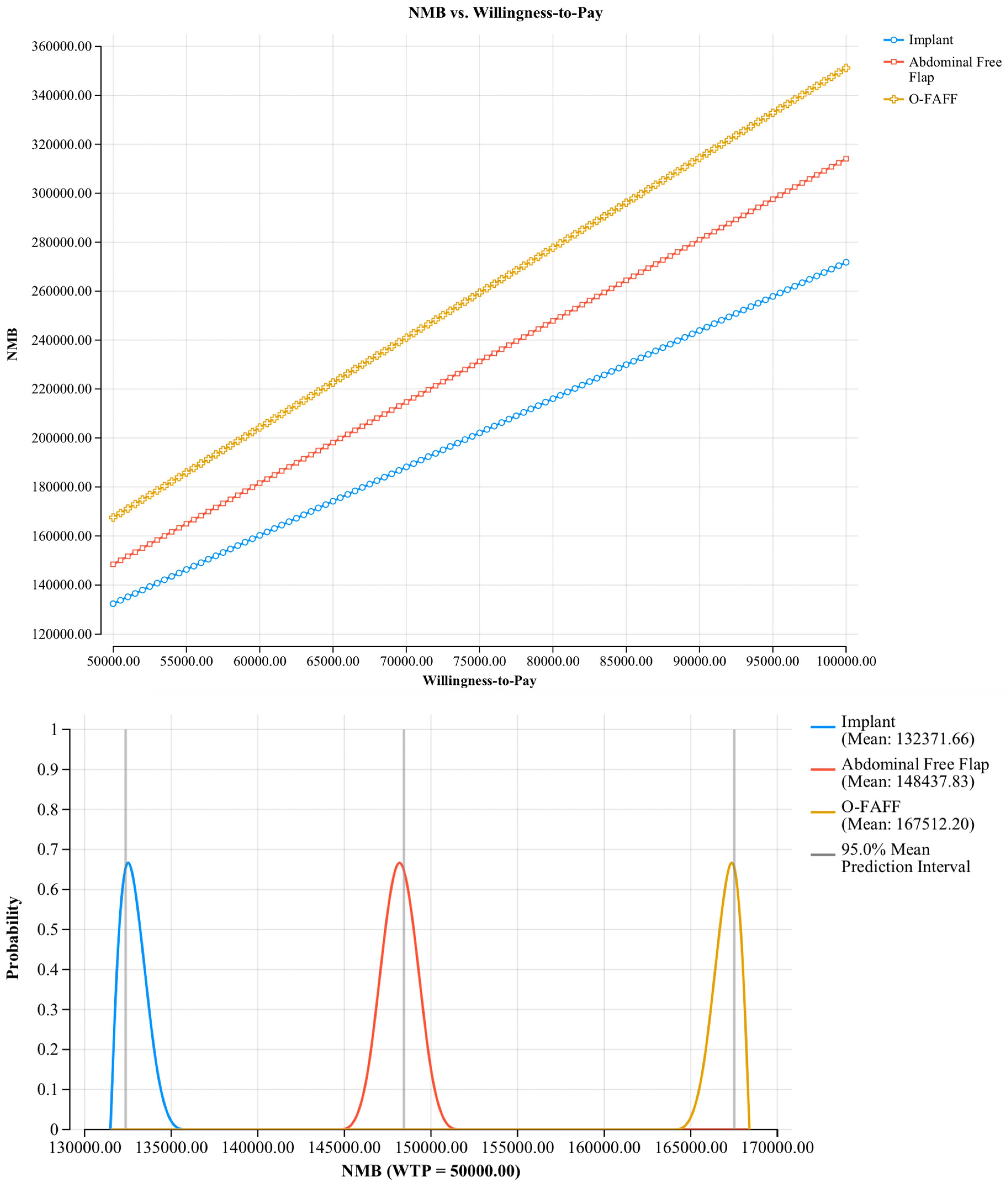
3.1. Model Outcomes
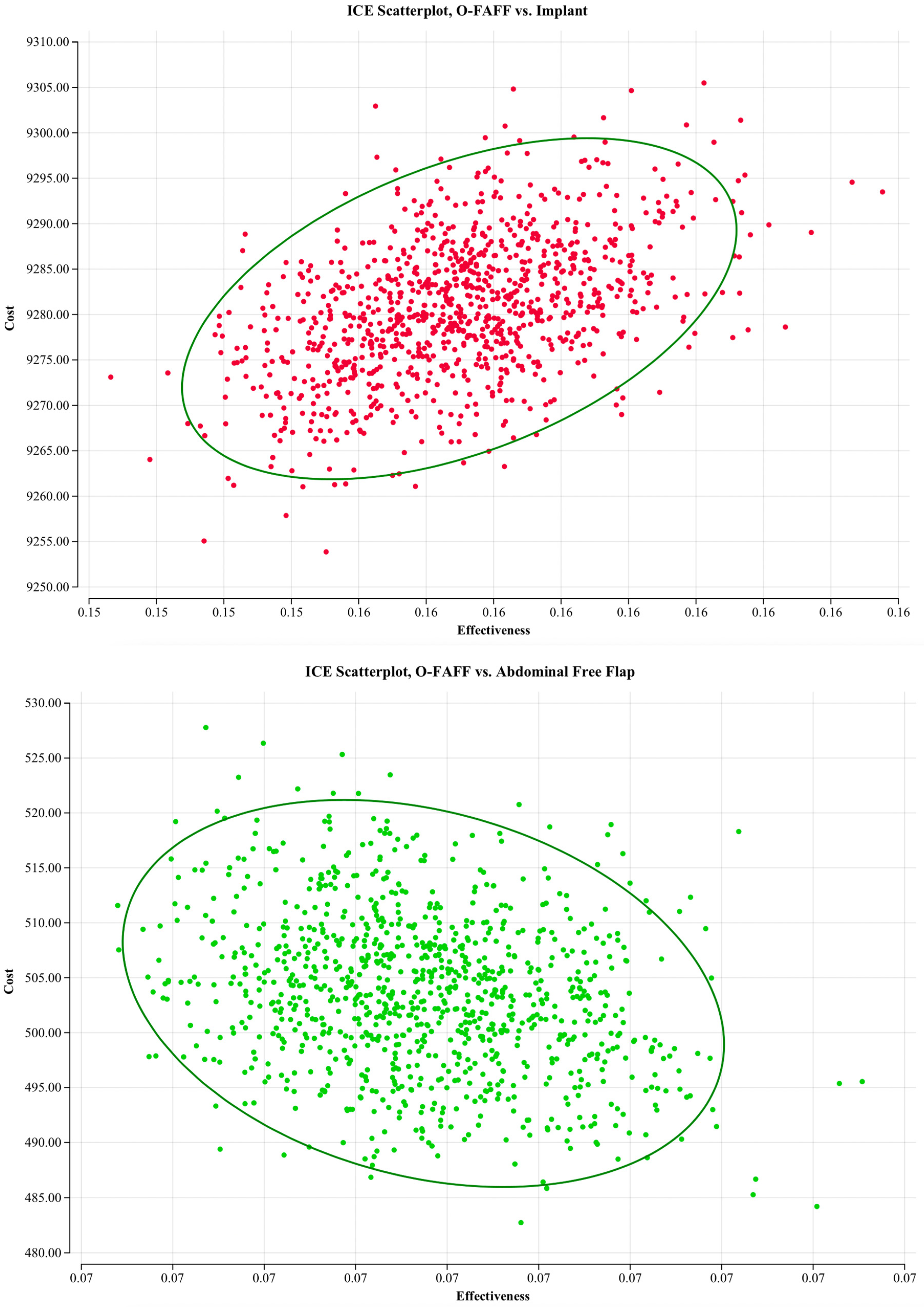
3.2. Sensitivity Analyses
3.2.1. Deterministic Sensitivity
3.2.2. Probabilistic Sensitivity Analyses
4. Discussion
4.1. Model Outcomes
4.2. Study Limitations
4.3. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| USD | United States Dollar |
| OR | Operating Room |
References
- Nguyen, D.H.; Ma, I.T.; Choi, Y.K.; Zak, Y.; Dua, M.M.; Wapnir, I.L. Creating a Biological Breast Implant with an Omental Fat-Augmented Free Flap. Plast. Reconstr. Surg. 2022, 149, 832–835. [Google Scholar] [CrossRef] [PubMed]
- Devisetti, N.; Sarpong, C.; Hu, A.C.; Yesantharao, P.S.; Liu, F.C.; Carrion, K.; Nguyen, D.H. Fat-augmented omentum-based construct for breast reconstruction. Plast. Aesthetic Res. 2024, 11, 21. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Rochlin, D.H.; Deptula, P.L.; Zak, Y.; Dua, M.; Wapnir, I.L. A Novel Fat-Augmented Omentum-Based Construct for Unilateral and Bilateral Free-Flap Breast Reconstruction in Underweight and Normal Weight Women Receiving Nipple or Skin-Sparing Mastectomies. Ann. Surg. Oncol. 2022, 30, 3048–3057. [Google Scholar] [CrossRef]
- Matros, E.; Albornoz, C.R.; Razdan, S.N.; Mehrara, B.J.; Macadam, S.A.; Ro, T.; McCarthy, C.M.; Disa, J.J.; Cordeiro, P.G.; Pusic, A.L. Cost-Effectiveness Analysis of Implants versus Autologous Perforator Flaps Using the BREAST-Q. Plast. Reconstr. Surg. 2015, 135, 937–946. [Google Scholar] [CrossRef]
- Duane, S.; Kennedy, A.; Pendleton, B.J.; Roweth, D. Hybrid Monte Carlo. Phys. Lett. B 1987, 195, 216–222. [Google Scholar] [CrossRef]
- Iskandar, R.; Berns, C. Markov Cohort State-Transition Model: A Multinomial Distribution Representation. Med. Decis. Mak. 2022, 43, 139–142. [Google Scholar] [CrossRef]
- Eltahir, Y.; Werners, L.L.C.H.; Dreise, M.M.; van Emmichoven, I.A.Z.; Werker, P.M.N.; de Bock, G.H. Which Breast Is the Best? Successful Autologous or Alloplastic Breast Reconstruction. Plast. Reconstr. Surg. 2015, 135, 43–50. [Google Scholar] [CrossRef]
- Blok, Y.L.M.; van Lierop, E.; Plat, V.D.; Corion, L.U.; Verduijn, P.S.; Krekel, N.M. Implant Loss and Associated Risk Factors following Implant-based Breast Reconstructions. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3708. [Google Scholar] [CrossRef] [PubMed]
- Razdan, S.N.M.; Cordeiro, P.G.; Albornoz, C.R.M.; Ro, T.B.; Cohen, W.A.; Mehrara, B.J.; McCarthy, C.M.M.; Disa, J.J.; Pusic, A.L.; Matros, E.M. Cost-Effectiveness Analysis of Breast Reconstruction Options in the Setting of Postmastectomy Radiotherapy Using the BREAST-Q. Plast. Reconstr. Surg. 2016, 137, 510e–517e. [Google Scholar] [CrossRef]
- Zong, A.M.; Leibl, K.E.; Weichman, K.E. Effects of Elective Revision after Breast Reconstruction on Patient-Reported Outcomes. J. Reconstr. Microsurg. 2024, 41, 100–112. [Google Scholar] [CrossRef]
- Sussman, M.; Benner, J.; Neumann, P.; Menzin, J. Cost-effectiveness analysis of erenumab for the preventive treatment of episodic and chronic migraine: Results from the US societal and payer perspectives. Cephalalgia 2018, 38, 1644–1657. [Google Scholar] [CrossRef] [PubMed]
- Payne, K.A.; Varon, S.F.; Kawata, A.K.; Yeomans, K.; Wilcox, T.K.; Manack, A.; Buse, D.C.; Lipton, R.B.; Goadsby, P.J.; Blumenfeld, A.M. The International Burden of Migraine Study (IBMS): Study design, methodology, and baseline cohort characteristics. Cephalalgia 2011, 31, 1116–1130. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhuang, Y.; Momeni, A.; Luan, J.; Chung, M.T.; Wright, E.; Lee, G.K. Quality of life and patient satisfaction after microsurgical abdominal flap versus staged expander/implant breast reconstruction: A critical study of unilateral immediate breast reconstruction using patient-reported outcomes instrument BREAST-Q. Breast Cancer Res. Treat. 2014, 146, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Pirro, O.; Mestak, O.; Vindigni, V.; Sukop, A.; Hromadkova, V.; Nguyenova, A.; Vitova, L.; Bassetto, F. Comparison of Patient-reported Outcomes after Implant Versus Autologous Tissue Breast Reconstruction Using the BREAST-Q. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1217. [Google Scholar] [CrossRef]
- Kandi, L.A.B.; Hammond, J.B.; Nadone, H.B.; Kosiorek, H.E.; Rebecca, A.M.M.; Casey, W.J.I.; Reece, E.M.M.; Cronin, P.A.; Pockaj, B.A. Patient Perspectives and Quality of Life after Breast Reconstruction and the Impact of Subsequent Revisions. Plast. Reconstr. Surg. Glob. Open 2023, 11, e4885. [Google Scholar] [CrossRef]
- Kim, M.B.; Vingan, P.B.; Boe, L.A.; Mehrara, B.J.; Stern, C.S.; Allen, R.J.; Nelson, J.A. Satisfaction with Breasts following Autologous Reconstruction: Assessing Associated Factors and the Impact of Revisions. Plast. Reconstr. Surg. 2024, 155, 235–244. [Google Scholar] [CrossRef]
- Nelson, J.A.; Voineskos, S.H.; Qi, J.; Kim, H.M.; Hamill, J.B.; Wilkins, E.G.; Pusic, A.L. Elective Revisions after Breast Reconstruction: Results from the Mastectomy Reconstruction Outcomes Consortium. Plast. Reconstr. Surg. 2019, 144, 1280–1290. [Google Scholar] [CrossRef]
- Asaad, M.; Slovacek, C.B.; Mitchell, D.B.; Liu, J.; Selber, J.C.M.; Clemens, M.W.; Chu, C.K.M.; Mericli, A.F.; Butler, C.E. Surgical and Patient-Reported Outcomes of Autologous versus Implant-Based Reconstruction following Infected Breast Device Explantation. Plast. Reconstr. Surg. 2022, 149, 1080e–1089e. [Google Scholar] [CrossRef]
- Francis, S.D.M.; Thawanyarat, K.B.; Johnstone, T.M.B.; Yesantharao, P.S.; Kim, T.S.B.; Rowley, M.A.B.; Sheckter, C.C.; Nazerali, R.S.M. How Postoperative Infection Affects Reoperations after Implant-based Breast Reconstruction: A National Claims Analysis of Abandonment of Reconstruction. Plast. Reconstr. Surg. Glob. Open 2023, 11, e5040. [Google Scholar] [CrossRef]
- Grover, R.; Padula, W.V.; Van Vliet, M.; Ridgway, E.B. Comparing Five Alternative Methods of Breast Reconstruction Surgery. Plast. Reconstr. Surg. 2013, 132, 709e–723e. [Google Scholar] [CrossRef]
- Toyserkani, N.M.; Jørgensen, M.G.; Tabatabaeifar, S.; Damsgaard, T.; Sørensen, J.A. Autologous versus implant-based breast reconstruction: A systematic review and meta-analysis of Breast-Q patient-reported outcomes. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Thoma, A.; Veltri, K.; Khuthaila, D.; Rockwell, G.; Duku, E. Comparison of the Deep Inferior Epigastric Perforator Flap and Free Transverse Rectus Abdominis Myocutaneous Flap in Postmastectomy Reconstruction: A Cost-Effectiveness Analysis. Plast. Reconstr. Surg. 2004, 113, 1650–1661. [Google Scholar] [CrossRef] [PubMed]
| Direct Costs | O-FAFF | Abdominal Flap | Implant | References |
|---|---|---|---|---|
| Surgical + Materials Cost | USD 7974.48 | USD 2703.94 | USD 4973.82 | Institutional Data, Center for Medicare and Medicaid Services |
| Anesthesia Cost | USD 828.29 | USD 580.35 | USD 197.54 | |
| Hospital Stay | USD 8649.00 | USD 8649.00 | USD 0 | |
| Reconstructive Salvage | USD 350.87 | USD 3205.04 | USD 2269.13 | |
| Reconstructive Loss | USD 350.87 | USD 350.87 | USD 269.13 | |
| Revision Surgery | USD 1574.01 | USD 1574.01 | USD 1574.01 |
| Expected B-QALYs | References | |||
|---|---|---|---|---|
| O-FAFF | Abdominal Flap | Implant | ||
| No Complications | 3.74 | 3.55 | 3.38 | Institutional Data |
| Reconstructive Loss | 0.03 | 0.03 | 0.08 | |
| Reconstructive Salvage | 3.48 | 3.30 | 3.14 | |
| Revision Surgery | 3.49 | 3.31 | 3.15 | |
| O-FAFF | Abdominal Flap | Implant | References | |
|---|---|---|---|---|
| Age—years (SD) | 49 (8) | 51 (9) | 55 (9) | Institutional Data |
| BMI—mean (SD) | 22.1 (3) | 27.4 (5) | 26.1 (4) | |
| Nipple Sparing Mastectomy | 78% | 35% | 59% | |
| Follow Up—months (SD) | 22(8) | 49(10) | 72(12) |
| Payer Perspective | |
|---|---|
| O-FAFF versus Implant | |
| Incremental costs per patient | +USD 9227 |
| Incremental QALYs per patient | +0.95 |
| ICER | USD 9712.64 |
| O-FAFF versus Abdominal Flap | |
| Incremental costs per patient | −USD 1410.10 |
| Incremental QALYs per patient | +0.36 |
| ICER | Dominant |
| Payer Perspective | |||
|---|---|---|---|
| Low | Base | High | |
| O-FAFF versus Implant | |||
| Surgical Costs | USD 6201.39 | USD 8024.12 | USD 12,102.20 |
| Time Horizon | USD 7231.04 | USD 9584.39 | USD 10,390.10 |
| O-FAFF versus Abdominal Flap | |||
| Surgical Costs | D | D | D |
| Time Horizon | D | D | D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yesantharao, P.S.; Carrion, K.; Nguyen, D.H. A Novel Fat-Augmented Omentum-Based Construct Is a Cost-Effective Alternative for Autologous Breast Reconstruction. J. Clin. Med. 2025, 14, 1706. https://doi.org/10.3390/jcm14051706
Yesantharao PS, Carrion K, Nguyen DH. A Novel Fat-Augmented Omentum-Based Construct Is a Cost-Effective Alternative for Autologous Breast Reconstruction. Journal of Clinical Medicine. 2025; 14(5):1706. https://doi.org/10.3390/jcm14051706
Chicago/Turabian StyleYesantharao, Pooja S., Kassandra Carrion, and Dung H. Nguyen. 2025. "A Novel Fat-Augmented Omentum-Based Construct Is a Cost-Effective Alternative for Autologous Breast Reconstruction" Journal of Clinical Medicine 14, no. 5: 1706. https://doi.org/10.3390/jcm14051706
APA StyleYesantharao, P. S., Carrion, K., & Nguyen, D. H. (2025). A Novel Fat-Augmented Omentum-Based Construct Is a Cost-Effective Alternative for Autologous Breast Reconstruction. Journal of Clinical Medicine, 14(5), 1706. https://doi.org/10.3390/jcm14051706







