Medium- and Long-Term Outcomes of 597 Patients Following Minimally Invasive Multi-Vessel Coronary Off-Pump Bypass Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Surgical Technique
2.3. Statistical Analysis
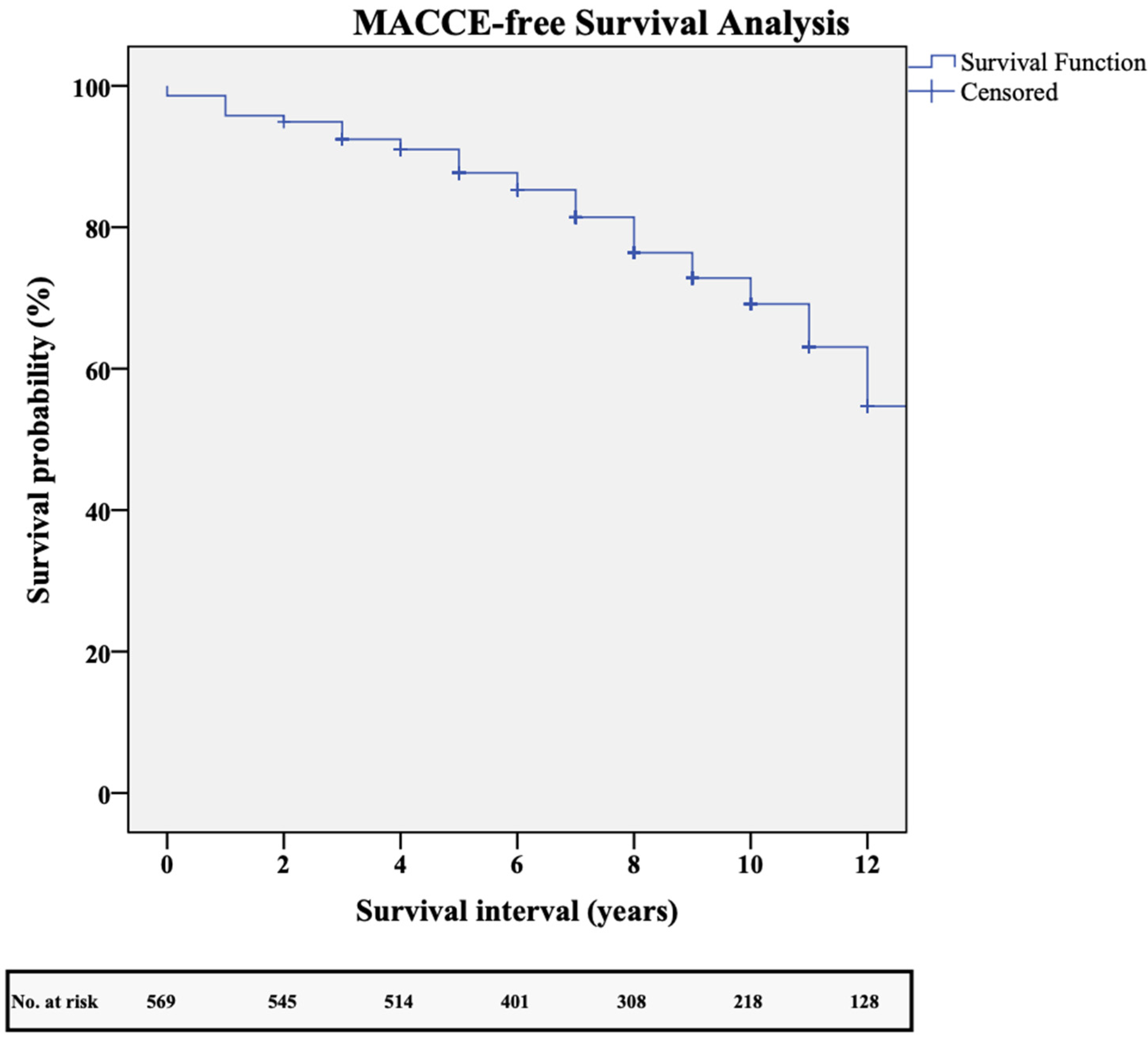
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| BITA | bilateral internal thoracic artery |
| CABG | coronary artery bypass grafting |
| CAD | coronary artery disease |
| CKD | chronic kidney disease |
| CPB | cardiopulmonary bypass |
| CPR | cardiopulmonary resuscitation |
| CVA | cerebrovascular accident |
| EuroSCORE | European System for Cardiac Operative Risk Evaluation |
| GSV | great saphenous vein |
| HCR | hybrid coronary revascularisation |
| IQR | interquartile range |
| LAD | left anterior descending artery |
| LITA | left internal thoracic artery |
| LVEF | left ventricular ejection fraction |
| MACCE | major adverse cardiac and cerebrovascular event |
| MIDCAB | minimally invasive direct coronary artery bypass |
| MICS CABG | minimally invasive multi-vessel off-pump coronary artery bypass grafting |
| PCI | percutaneous coronary intervention |
| PVD | peripheral vascular disease |
| RA | radial artery |
| RITA | right internal thoracic artery |
References
- Rodriguez, M.L.; Lapierre, H.R.; Sohmer, B.; Glineur, D.; Ruel, M. Mid-term follow- up of minimally invasive multivessel coronary artery bypass grafting: Is the early learning phase detrimental? Innovations 2017, 12, 116–120. [Google Scholar] [PubMed]
- McGinn, J.T., Jr.; Usman, S.; Lapierre, H.; Pothula, V.R.; Mesana, T.G.; Ruel, M. Minimally invasive coronary artery bypass grafting: Dual-center experience in 450 consecutive patients. Circulation 2009, 120 (Suppl. S11), S78–S84. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, P.; Kumar, S.; Mittal, C.M.; Saksena, K. Minimally invasive coronary artery bypass grafting with bilateral internal thoracic arteries: Will this be the future? J. Thorac. Cardiovasc. Surg. 2018, 155, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, H.; Chan, V.; Sohmer, B.; Mesana, T.G.; Ruel, M. Minimally invasive coronary artery bypass grafting via a small thoracotomy versus off-pump: A case-matched study. Eur. J. Cardiothorac. Surg. 2011, 40, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Sef, D.; Thet, M.S.; Hashim, S.A.; Kikuchi, K.; Ruel, M. Minimally Invasive Coronary Artery Bypass Grafting for Multivessel Coronary Artery Disease: A Systematic Review. Innovations 2024, 19, 351–359. [Google Scholar] [CrossRef]
- Guida, G.A.; Guida, G.A.; Bruno, V.D.; Zakkar, M.; De Garate, E.; Pecchinenda, M.T.; Homes, A.; Borzellino, C.; Mendoza, P.; Pecora, G.; et al. Left thoracotomy approach for off-pump coronary artery bypass grafting surgery: 15 years of experience in 2500 consecutive patients. Eur. J. Cardiothorac. Surg. 2020, 57, 271–276. [Google Scholar] [CrossRef]
- Guo, M.H.; Toubar, O.; Issa, H.; Glineur, D.; Ponnambalam, M.; Vo, T.X.; Rahmouni, K.; Chong, A.Y.; Ruel, M. Long-term survival, cardiovascular, and functional outcomes after minimally invasive coronary artery bypass grafting in 566 patients. J. Thorac. Cardiovasc. Surg. 2024, 168, 1080–1088. [Google Scholar] [CrossRef]
- Ashenhurst, C.; Toubar, O.; Guo, M.H.; Issa, H.; Ponnambalam, M.; Ruel, M. Early and long-term outcomes of less invasive approaches to coronary artery bypass surgery. Vessel. Plus. 2024, 8, 3. [Google Scholar] [CrossRef]
- Bonaros, N.; Schachner, T.; Lehr, E.; Kofler, M.; Wiedemann, D.; Hong, P.; Wehman, B.; Zimrin, D.; Vesely, M.K.; Friedrich, G.; et al. Five hundred cases of robotic totally endoscopic coronary artery bypass grafting: Predictors of success and safety. Ann. Thorac. Surg. 2013, 95, 803–812. [Google Scholar] [CrossRef]
- Rufa, M.I.; Ursulescu, A.; Dippon, J.; Aktuerk, D.; Nagib, R.; Albert, M.; Franke, U.F.W. Is minimally invasive multi-vessel off-pump coronary surgery as safe and effective as MIDCAB? Front. Cardiovasc. Med. 2024, 11, 1385108. [Google Scholar] [CrossRef]
- Rufa, M.I.; Ursulescu, A.; Dippon, J.; Aktuerk, D.; Ahad, S.; Nagib, R.; Albert, M.; Franke, U. The impact of incomplete revascularisation on survival in minimal invasive off-pump coronary artery surgery: A propensity score analysis of 1149 cases. J. Thorac. Dis. 2024, 16, 4504–4514. [Google Scholar] [CrossRef] [PubMed]
- Rufa, M.; Ursulescu, A.; Nagib, R.; Albert, M.; Franke, U.F.W. Hybrid total arterial minimally invasive off-pump coronary revascularisation and percutaneous coronary intervention strategy for multivessel coronary artery disease: A cohort study with a median 11-year follow-up. Cardiovasc. Diagn. Ther. 2024, 14, 272–282. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Totsugawa, T.; Tamura, K.; Hiraoka, A.; Ryomoto, M.; Sekiya, N.; Chikazawa, G.; Yoshitaka, H. Minimally invasive coronary artery bypass grafting: Useful routine option for coronary revascularisation in selected cases. Gen. Thorac. Cardiovasc. Surg. 2020, 68, 1128–1133. [Google Scholar] [CrossRef]
- Ziankou, A.; Ostrovsky, Y. Early and Midterm Results of No-Touch Aorta Multivessel Small Thoracotomy Coronary Artery Bypass Grafting: A Propensity Score-Matched Study. Innovations 2015, 10, 258–267, discussion 267. [Google Scholar]
- Lamy, A.; Devereaux, P.J.; Prabhakaran, D.; Taggart, D.P.; Hu, S.; Straka, Z.; Piegas, L.S.; Avezum, A.; Akar, A.R.; Lanas Zanetti, F.; et al. Five-Year Outcomes after Off-Pump or On-Pump Coronary-Artery Bypass Grafting. N. Engl. J. Med. 2016, 375, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Mohr, F.W.; Morice, M.C.; Kappetein, A.P.; Feldman, T.E.; Ståhle, E.; Colombo, A.; Mack, M.J.; Holmes, D.R., Jr.; Morel, M.A.; Van Dyck, N.; et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013, 381, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Thuijs, D.J.F.M.; Kappetein, A.P.; Serruys, P.W.; Mohr, F.W.; Morice, M.C.; Mack, M.J.; Holmes, D.R., Jr.; Curzen, N.; Davierwala, P.; Noack, T.; et al. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet 2019, 394, 1325–1334. [Google Scholar] [CrossRef]
- Taggart, D.P.; Benedetto, U.; Gerry, S.; Altman, D.G.; Gray, A.M.; Lees, B.; Gaudino, M.; Zamvar, V.; Bochenek, A.; Buxton, B.; et al. Bilateral versus Single Internal-Thoracic-Artery Grafts at 10 Years. N. Engl. J. Med. 2019, 380, 437–446. [Google Scholar] [CrossRef]
- Chikwe, J.; Lee, T.; Itagaki, S.; Adams, D.H.; Egorova, N.N. Long-Term Outcomes After Off-Pump Versus On-Pump Coronary Artery Bypass Grafting by Experienced Surgeons. J. Am. Coll. Cardiol. 2018, 72, 1478–1486. [Google Scholar] [CrossRef]
- Repossini, A.; Di Bacco, L.; Nicoli, F.; Passaretti, B.; Stara, A.; Jonida, B.; Muneretto, C. Minimally invasive coronary artery bypass: Twenty-year experience. J. Thorac. Cardiovasc. Surg. 2019, 158, 127–138.e1. [Google Scholar] [CrossRef]
- Bonatti, J.; Wallner, S.; Crailsheim, I.; Grabenwöger, M.; Winkler, B. Minimally invasive and robotic coronary artery bypass grafting—A 25-year review. J. Thorac. Dis. 2021, 13, 1922–1944. [Google Scholar] [CrossRef] [PubMed]
- Manuel, L.; Fong, L.S.; Betts, K.; Bassin, L.; Wolfenden, H. LIMA to LAD grafting returns patient survival to age-matched population: 20-year outcomes of MIDCAB surgery. Interact. Cardiovasc. Thorac. Surg. 2022, 35, ivac243. [Google Scholar] [CrossRef] [PubMed]


| Variable | No (%)/Median (IQR, Q1–Q3) |
|---|---|
| Age (y) | 69 (61–76) |
| Male gender | 550 (92.1) |
| BMI | 26.3 (24.3–28.4) |
| EuroSCORE II | 1.5 (1–2.2) |
| COPD | 37 (6.2) |
| Tobacco use | 124 (20.8) |
| AF | 80 (13.5) |
| PVD | 54 (9) |
| Medically treated type II diabetes | 113 (18.9) |
| CKD (GFR < 50 mL/min/1.73 m2) | 74 (12.4) |
| Renal replacement therapy | 5 (0.8) |
| Carotid stenosis | 67 (11.2) |
| Previous CVA | 26 (4.4) |
| Previous MI | |
| 3 (0.5) |
| 65 (10.9) |
| 49 (8.2) |
| 68 (11.4) |
| Previous PCI | 167 (28) |
| Coronary angiography data | |
|
|
|
|
|
|
| LVEF (%) | |
|
|
|
|
|
|
| Priority | |
|
|
|
|
| Variable | No (%)/Median (IQR, Q1–Q3) |
|---|---|
| Number of grafts | |
|
|
|
|
| Use of LITA | 597 (100) |
| Use of RITA | 33 (5.5) |
| Use of RA | 411 (68.8) |
| Use of GSV | 47 (7.9) |
| Aortic touch | 2 (0.3) |
| Number of total arterial revascularisations | 552 (92.5) |
| Conversion to sternotomy | 8 (1.3) |
| Ventilation time (hours) | 2 (0–5) |
| RBC transfusion (per patient) | 0 (0–0) |
| Number of patients with RBC transfusion | 63 (10.6) |
| FFP transfusion (per patient) | 0 (0–0) |
| Number of patients with FFP transfusion | 12 (2) |
| Platelet transfusion (per patient) | 0 (0–0) |
| Number of patients with platelet transfusion | 15 (2.5) |
| Number of grafts to the anterior wall | |
|
|
|
|
|
|
| Number of grafts to the posterior wall | |
|
|
|
|
| Number of grafts to the lateral wall | |
|
|
|
|
|
|
| Complete revascularisation | 539 (90.3) |
| Planned as hybrid procedures | 61 (10.2) |
| Variable | No (%)/Median (IQR, Q1–Q3) |
|---|---|
| New renal failure requiring dialysis | 6 (1.0) |
| New-onset AF | 13 (2.2) |
| Postoperative CVA | 2 (0.3) |
| SSI | 10 (1.7) |
| Postoperative CPR | 5 (0.8) |
| Postoperative MI | 7 (1.2) |
| Postoperative PCI as a revision | 5 (0.8) |
| Postoperative PCI planned as part of hybrid procedure (30 d) | 8 (1.3) |
| Reoperation for bleeding | 13 (2.2) |
| Reoperation with bypass revision | 5 (0.8) |
| Postoperative ventricular arrhythmia | 6 (1.0) |
| Length of ICU stay (d) | 1 (1–1) |
| Length of hospital stay (d) | 7 (6–8) |
| 30 d mortality | 3 (0.5) |
| Variable | Late Mortality | MACCE | ||||
|---|---|---|---|---|---|---|
| p Value | HR | 95% CI | p Value | HR | 95% CI | |
| LVEF < 30% | 0.042 | 2.652 | 1.037–6.780 | 0.005 | 3.441 | 1.444–8.198 |
| LVEF 30–50% | 0.025 | 1.753 | 1.072–2.866 | 0.018 | 1.556 | 1.078–2.246 |
| PVD | 0.002 | 2.306 | 1.365–3.894 | 0.010 | 1.736 | 1.142–2.461 |
| CKD | 0.001 | 2.461 | 1.448–4.182 | <0.001 | 2.271 | 1.520–3.393 |
| Previous PCI | 0.002 | 0.390 | 0.215–0.709 | |||
| Carotid stenosis | 0.029 | 1.783 | 1.060–3.001 | |||
| CVA | <0.001 | 3.581 | 1.964–6.529 | 0.003 | 2.298 | 1.333–3.961 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rufa, M.I.; Ursulescu, A.; Ahad, S.; Nagib, R.; Albert, M.; Ghinescu, M.; Shavahatli, T.; Ayala, R.; Göbel, N.; Franke, U.F.W.; et al. Medium- and Long-Term Outcomes of 597 Patients Following Minimally Invasive Multi-Vessel Coronary Off-Pump Bypass Surgery. J. Clin. Med. 2025, 14, 1707. https://doi.org/10.3390/jcm14051707
Rufa MI, Ursulescu A, Ahad S, Nagib R, Albert M, Ghinescu M, Shavahatli T, Ayala R, Göbel N, Franke UFW, et al. Medium- and Long-Term Outcomes of 597 Patients Following Minimally Invasive Multi-Vessel Coronary Off-Pump Bypass Surgery. Journal of Clinical Medicine. 2025; 14(5):1707. https://doi.org/10.3390/jcm14051707
Chicago/Turabian StyleRufa, Magdalena I., Adrian Ursulescu, Samir Ahad, Ragi Nagib, Marc Albert, Mihnea Ghinescu, Tunjay Shavahatli, Rafael Ayala, Nora Göbel, Ulrich F. W. Franke, and et al. 2025. "Medium- and Long-Term Outcomes of 597 Patients Following Minimally Invasive Multi-Vessel Coronary Off-Pump Bypass Surgery" Journal of Clinical Medicine 14, no. 5: 1707. https://doi.org/10.3390/jcm14051707
APA StyleRufa, M. I., Ursulescu, A., Ahad, S., Nagib, R., Albert, M., Ghinescu, M., Shavahatli, T., Ayala, R., Göbel, N., Franke, U. F. W., & Rylski, B. (2025). Medium- and Long-Term Outcomes of 597 Patients Following Minimally Invasive Multi-Vessel Coronary Off-Pump Bypass Surgery. Journal of Clinical Medicine, 14(5), 1707. https://doi.org/10.3390/jcm14051707





