A Bioartificial Device for the Encapsulation of Pancreatic β-Cells Using a Semipermeable Biocompatible Porous Membrane
Abstract
1. Introduction
2. Materials and Methods
2.1. Adherent Cell Culture
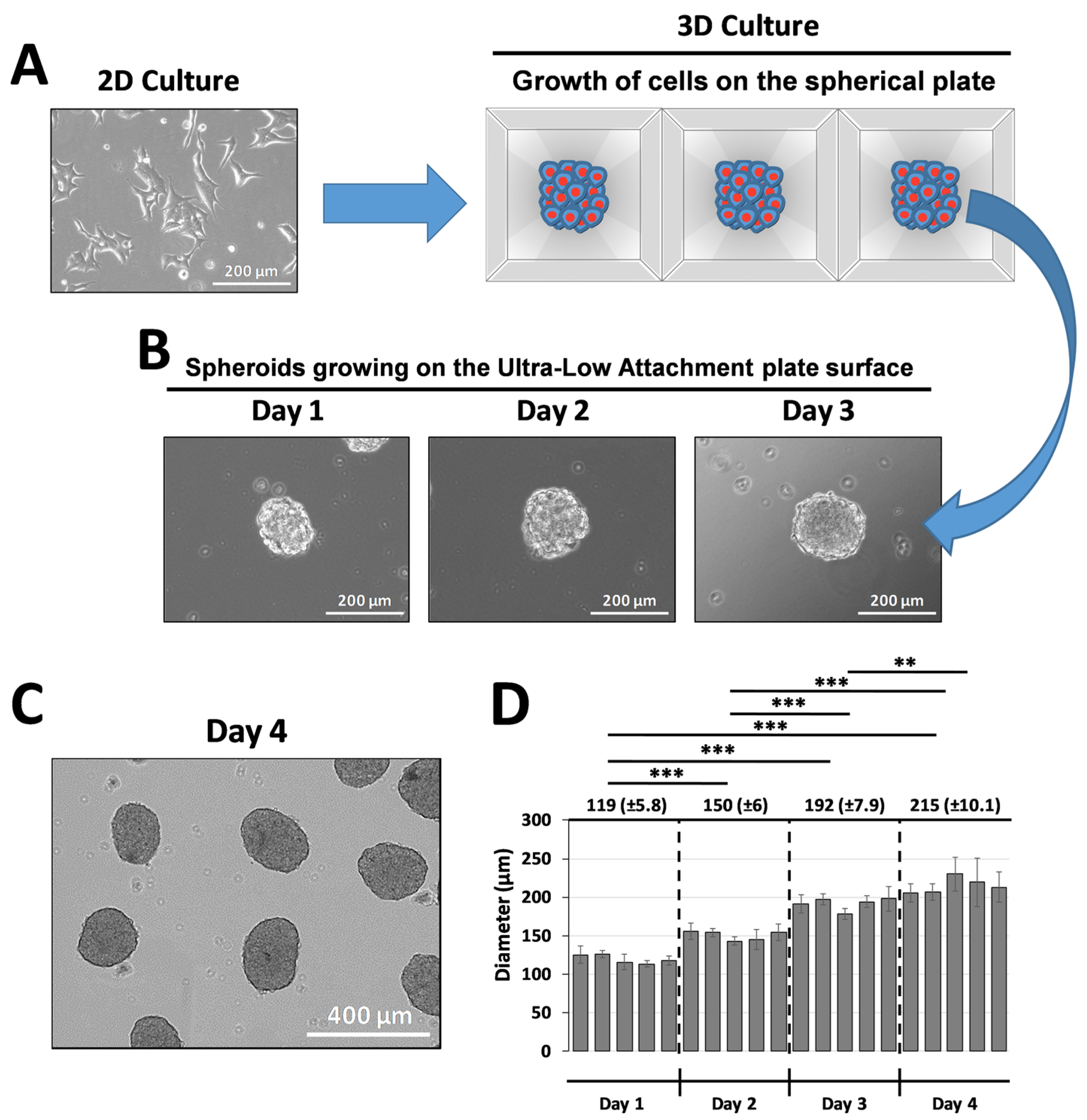
2.2. Generation of INS-1E Islet-like Spheroids (ILSs)
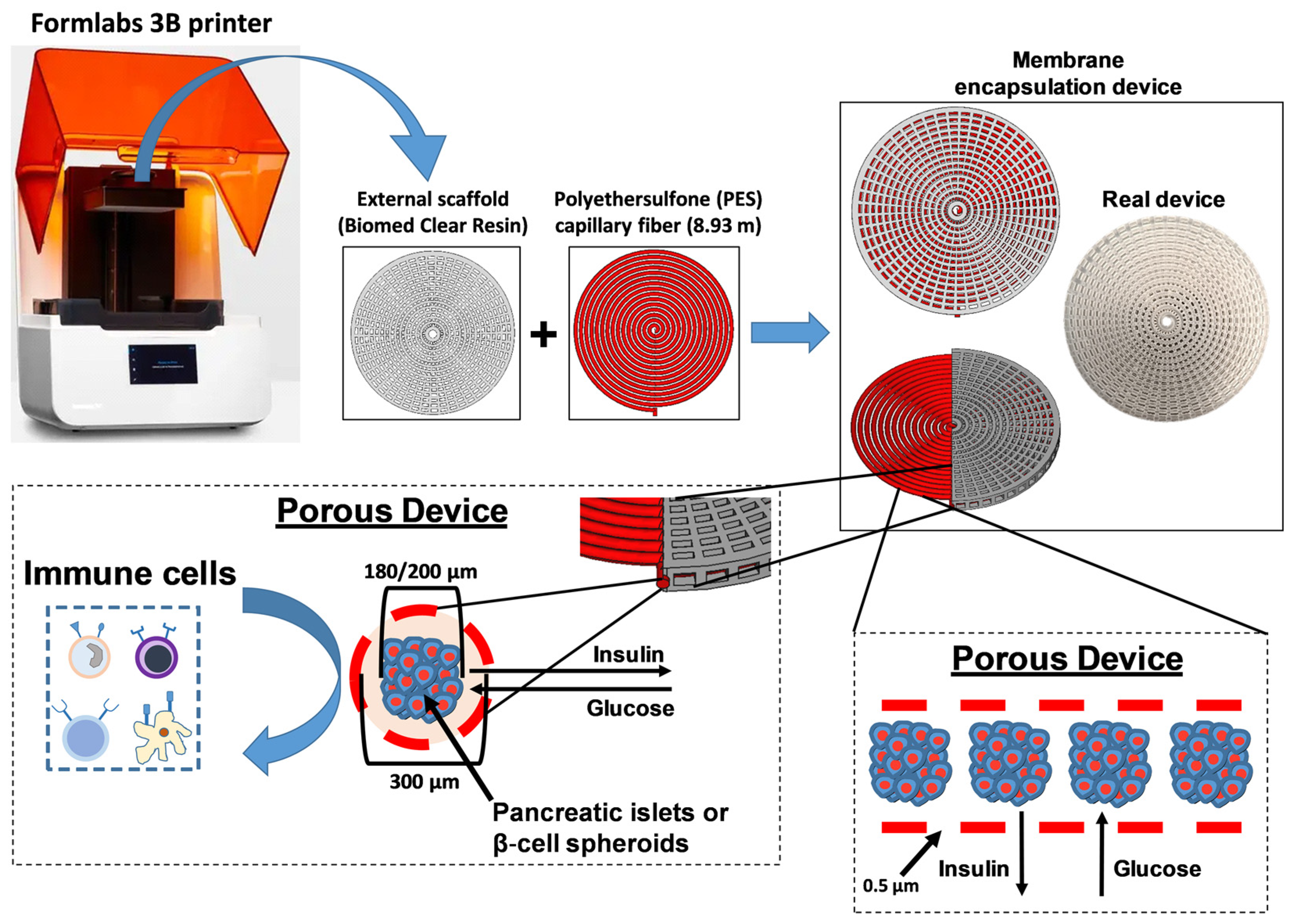
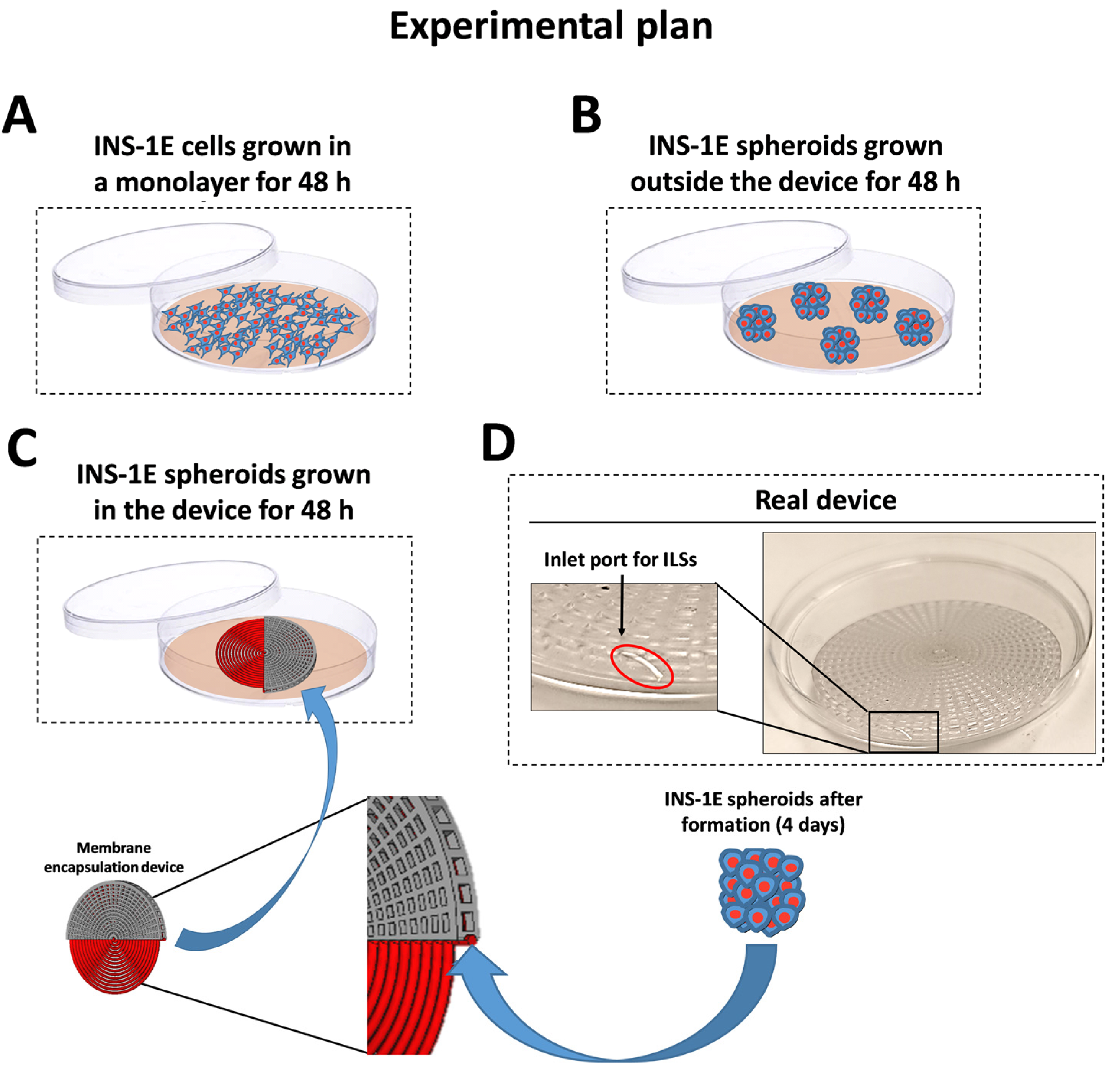
2.3. Device Fabrication and Encapsulation of ILSs
2.4. Viability Assays
2.5. Evaluation of Membrane Transport Properties by Glucose-Stimulated Insulin Secretion (GSIS)
2.6. Statistics
3. Results
3.1. Fabrication and Characterization of the Encapsulation Device
3.2. Production and Size Distribution of INS-1E Islet-like Spheroids (ILSs)
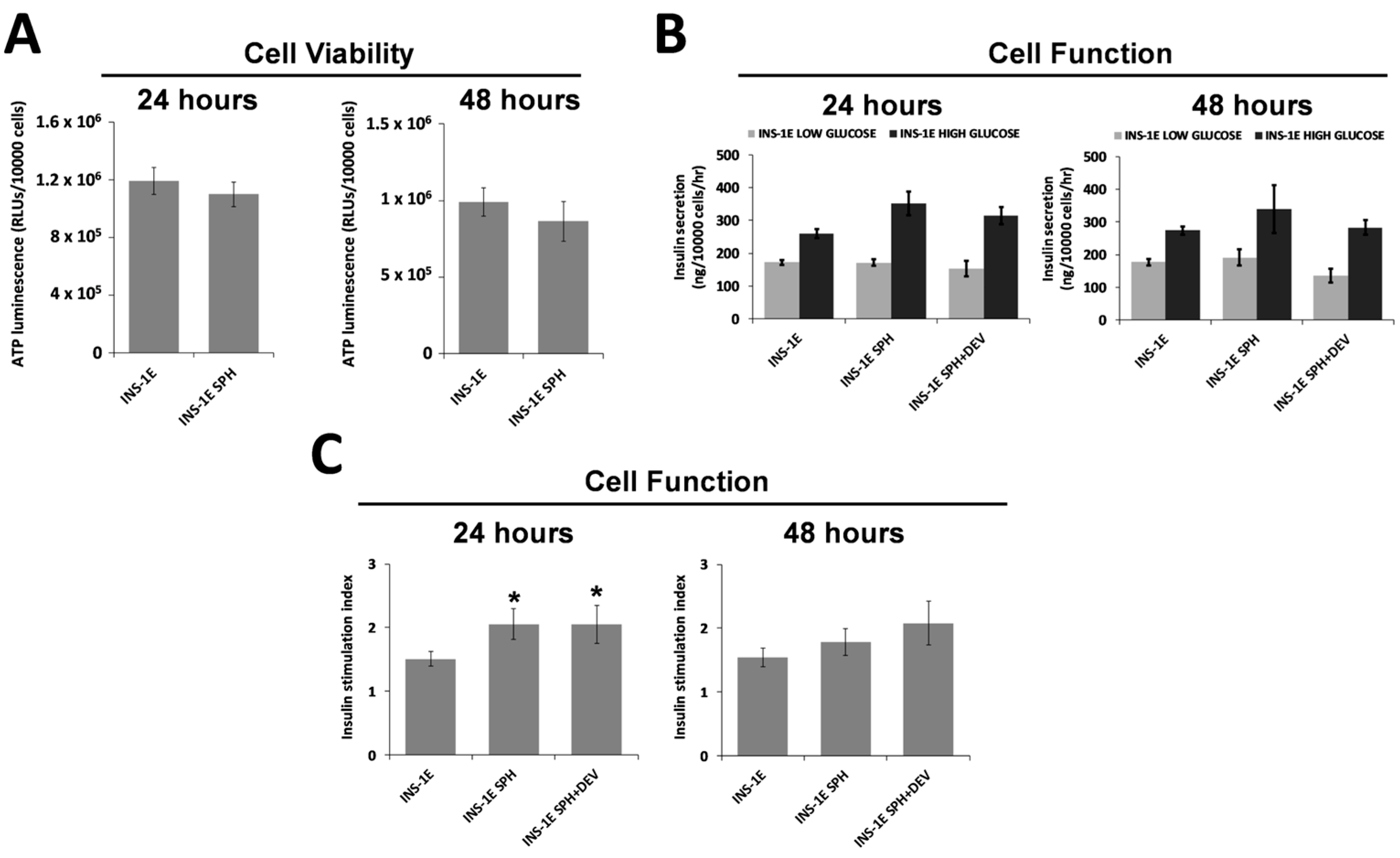
3.3. Survival of ILSs
3.4. ILS Functionality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| GSIS | Glucose-stimulated insulin secretion |
| HEPES | 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid |
| IEQ | Islet equivalent |
| ILS | Islet-like spheroid |
| INS-1E | Insulinoma-derived β-cell line (specific β-cell type used in research) |
| ITx | Islet transplantation |
| PES | Polyethersulfone |
| PVP | Polyvinylpyrrolidone |
| SI | Stimulation index |
| T1D | Type 1 diabetes |
References
- DiMeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41 (Suppl. S1), S13–S27. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Yang, W.; Xing, Y.; Lai, Y.; Shan, Z. Global, regional, and national burden of type 1 diabetes in adolescents and young adults. Pediatr. Res. 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ogle, G.D.; Gregory, G.A.; Wang, F.; Robinson, T.I.; Maniam, J.; Magliano, D.J.; Orchard, T.J. IDF Diabetes Atlas: Type 1 Diabetes Numbers in Children and Adults; International Diabetes Federation: Brussels, Belgium, 2023; Volume 1, pp. 1–15. [Google Scholar]
- Khunti, K.; Davies, M.; Majeed, A.; Thorsted, B.L.; Wolden, M.L.; Paul, S.K. Hypoglycemia and risk of cardiovascular disease and all-cause mortality in insulin-treated people with type 1 and type 2 diabetes: A cohort study. Diabetes Care 2015, 38, 316–322. [Google Scholar] [CrossRef] [PubMed]
- McCall, A.L. Insulin therapy and hypoglycemia. Endocrinol. Metab. Clin. N. Am. 2012, 41, 57–87. [Google Scholar] [CrossRef] [PubMed]
- Grimus, S.; Sarangova, V.; Welzel, P.B.; Ludwig, B.; Seissler, J.; Kemter, E.; Wolf, E.; Ali, A. Immunoprotection Strategies in beta-Cell Replacement Therapy: A Closer Look at Porcine Islet Xenotransplantation. Adv. Sci. 2024, 11, e2401385. [Google Scholar] [CrossRef] [PubMed]
- Kolic, J.; Johnson, J.D. Promises and pitfalls of beta cell-replacement therapies. Nat. Metab. 2021, 3, 1036–1037. [Google Scholar] [CrossRef]
- Chen, Q.D.; Liu, L.; Zhao, X.H.; Liang, J.B.; Li, S.W. Challenges and opportunities in the islet transplantation microenvironment: A comprehensive summary of inflammatory cytokine, immune cells, and vascular endothelial cells. Front. Immunol. 2023, 14, 1293762. [Google Scholar] [CrossRef]
- Miceli, V.; Pampalone, M.; Frazziano, G.; Grasso, G.; Rizzarelli, E.; Ricordi, C.; Casu, A.; Iannolo, G.; Conaldi, P.G. Carnosine protects pancreatic beta cells and islets against oxidative stress damage. Mol. Cell. Endocrinol. 2018, 474, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhao, Y.Z.; Jiang, Z.; Wang, Z.; Wang, Q.; Kou, L.; Yao, Q. Immune-Protective Formulations and Process Strategies for Improved Survival and Function of Transplanted Islets. Front. Immunol. 2022, 13, 923241. [Google Scholar] [CrossRef] [PubMed]
- Kriz, J.; Vilk, G.; Mazzuca, D.M.; Toleikis, P.M.; Foster, P.J.; White, D.J. A novel technique for the transplantation of pancreatic islets within a vascularized device into the greater omentum to achieve insulin independence. Am. J. Surg. 2012, 203, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Hu, S.; de Vos, P. A composite capsule strategy to support longevity of microencapsulated pancreatic beta cells. Biomater. Adv. 2023, 155, 213678. [Google Scholar] [CrossRef]
- Espona-Noguera, A.; Ciriza, J.; Canibano-Hernandez, A.; Orive, G.; Hernandez, R.M.M.; Saenz Del Burgo, L.; Pedraz, J.L. Review of Advanced Hydrogel-Based Cell Encapsulation Systems for Insulin Delivery in Type 1 Diabetes Mellitus. Pharmaceutics 2019, 11, 597. [Google Scholar] [CrossRef]
- Lopez-Mendez, T.B.; Santos-Vizcaino, E.; Pedraz, J.L.; Orive, G.; Hernandez, R.M. Cell microencapsulation technologies for sustained drug delivery: Latest advances in efficacy and biosafety. J. Control Release 2021, 335, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Kupikowska-Stobba, B.; Lewinska, D.; Grzeczkowicz, M. Chemical method for retrieval of cells encapsulated in alginate-polyethersulfone microcapsules. Artif. Cells Nanomed. Biotechnol. 2014, 42, 151–160. [Google Scholar] [CrossRef]
- Neumann, M.; Arnould, T.; Su, B.L. Encapsulation of stem-cell derived beta-cells: A promising approach for the treatment for type 1 diabetes mellitus. J. Colloid Interface Sci. 2023, 636, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Jakutowicz, T.; Wasyleczko, M.; Plonczak, M.; Wojciechowski, C.; Chwojnowski, A.; Czubak, J. Comparative Study of Autogenic and Allogenic Chondrocyte Transplants on Polyethersulfone Scaffolds for Cartilage Regeneration. Int. J. Mol. Sci. 2024, 25, 9075. [Google Scholar] [CrossRef]
- Wasyleczko, M.; Wojciechowski, C.; Chwojnowski, A. Polyethersulfone Polymer for Biomedical Applications and Biotechnology. Int. J. Mol. Sci. 2024, 25, 4233. [Google Scholar] [CrossRef] [PubMed]
- Keymeulen, B.; De Groot, K.; Jacobs-Tulleneers-Thevissen, D.; Thompson, D.M.; Bellin, M.D.; Kroon, E.J.; Daniels, M.; Wang, R.; Jaiman, M.; Kieffer, T.J.; et al. Encapsulated stem cell-derived beta cells exert glucose control in patients with type 1 diabetes. Nat. Biotechnol. 2024, 42, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Bruin, J.E.; Rezania, A.; Xu, J.; Narayan, K.; Fox, J.K.; O’Neil, J.J.; Kieffer, T.J. Maturation and function of human embryonic stem cell-derived pancreatic progenitors in macroencapsulation devices following transplant into mice. Diabetologia 2013, 56, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Agulnick, A.D.; Ambruzs, D.M.; Moorman, M.A.; Bhoumik, A.; Cesario, R.M.; Payne, J.K.; Kelly, J.R.; Haakmeester, C.; Srijemac, R.; Wilson, A.Z.; et al. Insulin-Producing Endocrine Cells Differentiated In Vitro From Human Embryonic Stem Cells Function in Macroencapsulation Devices In Vivo. Stem Cells Transl. Med. 2015, 4, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Flanders, J.A.; Wang, L.H.; Liu, Q.; Bowers, D.T.; Wang, K.; Chiu, A.; Wang, X.; Ernst, A.U.; Shariati, K.; et al. A Safe, Fibrosis-Mitigating, and Scalable Encapsulation Device Supports Long-Term Function of Insulin-Producing Cells. Small 2022, 18, e2104899. [Google Scholar] [CrossRef] [PubMed]
- Clua-Ferre, L.; De Chiara, F.; Rodriguez-Comas, J.; Comelles, J.; Martinez, E.; Godeau, A.L.; Garcia-Alaman, A.; Gasa, R.; Ramon-Azcon, J. Collagen-Tannic Acid Spheroids for beta-Cell Encapsulation Fabricated Using a 3D Bioprinter. Adv. Mater. Technol. 2022, 7, 2101696. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Idris, A. Overview of PES biocompatible/hemodialysis membranes: PES-blood interactions and modification techniques. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 56, 574–592. [Google Scholar] [CrossRef] [PubMed]
- Ntamo, Y.; Samodien, E.; Burger, J.; Muller, N.; Muller, C.J.F.; Chellan, N. In vitro Characterization of Insulin-Producing beta-Cell Spheroids. Front. Cell Dev. Biol. 2020, 8, 623889. [Google Scholar]
- Miceli, V.; Fornasier, M.; Bulati, M.; Amico, G.; Conaldi, P.G.; Casu, A.; Murgia, S. In Vitro Evaluation of Nanoerythrosome Cytotoxicity and Uptake in Pancreatic Endothelial Cells: Implications for beta-Cell Imaging Applications. Langmuir ACS J. Surf. Colloids 2022, 38, 3403–3411. [Google Scholar] [CrossRef]
- Miceli, V.; Meli, V.; Blanchard-Desce, M.; Bsaibess, T.; Pampalone, M.; Conaldi, P.G.; Caltagirone, C.; Obiols-Rabasa, M.; Schmidt, J.; Talmon, Y.; et al. In vitro imaging of β-cells using fluorescent cubic bicontinuous liquid crystalline nanoparticles. RSC Adv. 2016, 6, 62119–62127. [Google Scholar] [CrossRef]
- Huang, H.H.; Ramachandran, K.; Stehno-Bittel, L. A replacement for islet equivalents with improved reliability and validity. Acta Diabetol. 2013, 50, 687–696. [Google Scholar] [CrossRef]
- Lovett, M.; Lee, K.; Edwards, A.; Kaplan, D.L. Vascularization strategies for tissue engineering. Tissue Eng. Part B Rev. 2009, 15, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Dulong, J.L.; Legallais, C. A theoretical study of oxygen transfer including cell necrosis for the design of a bioartificial pancreas. Biotechnol. Bioeng. 2007, 96, 990–998. [Google Scholar] [CrossRef] [PubMed]
- McCall, M.D.; Toso, C.; Baetge, E.E.; Shapiro, A.M. Are stem cells a cure for diabetes? Clin. Sci. 2009, 118, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, R.; Zuellig, R.A.; Kugelmeier, P.; Baenninger, P.B.; Moritz, W.; Perren, A.; Clavien, P.A.; Weber, M.; Spinas, G.A. Superiority of small islets in human islet transplantation. Diabetes 2007, 56, 594–603. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuscino, N.; Castelbuono, S.; Centi, C.; Tinnirello, R.; Cimino, M.; Zito, G.; Orlando, A.; Pinzani, M.; Conaldi, P.G.; Mattina, A.; et al. A Bioartificial Device for the Encapsulation of Pancreatic β-Cells Using a Semipermeable Biocompatible Porous Membrane. J. Clin. Med. 2025, 14, 1631. https://doi.org/10.3390/jcm14051631
Cuscino N, Castelbuono S, Centi C, Tinnirello R, Cimino M, Zito G, Orlando A, Pinzani M, Conaldi PG, Mattina A, et al. A Bioartificial Device for the Encapsulation of Pancreatic β-Cells Using a Semipermeable Biocompatible Porous Membrane. Journal of Clinical Medicine. 2025; 14(5):1631. https://doi.org/10.3390/jcm14051631
Chicago/Turabian StyleCuscino, Nicola, Salvatore Castelbuono, Claudio Centi, Rosaria Tinnirello, Maura Cimino, Giovanni Zito, Andrea Orlando, Massimo Pinzani, Pier Giulio Conaldi, Alessandro Mattina, and et al. 2025. "A Bioartificial Device for the Encapsulation of Pancreatic β-Cells Using a Semipermeable Biocompatible Porous Membrane" Journal of Clinical Medicine 14, no. 5: 1631. https://doi.org/10.3390/jcm14051631
APA StyleCuscino, N., Castelbuono, S., Centi, C., Tinnirello, R., Cimino, M., Zito, G., Orlando, A., Pinzani, M., Conaldi, P. G., Mattina, A., & Miceli, V. (2025). A Bioartificial Device for the Encapsulation of Pancreatic β-Cells Using a Semipermeable Biocompatible Porous Membrane. Journal of Clinical Medicine, 14(5), 1631. https://doi.org/10.3390/jcm14051631







