Association of Ankle–Brachial Index with Quality of Life and Survival Outcomes in Hemodialysis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Quality of Life Questionnaire
2.3. Data Collection
2.4. ABI Measurement Method
2.5. Statistical Analysis
3. Results
3.1. Clinical Parameters
3.2. Association of ABI ≤ 0.9 with Body Fluid Composition
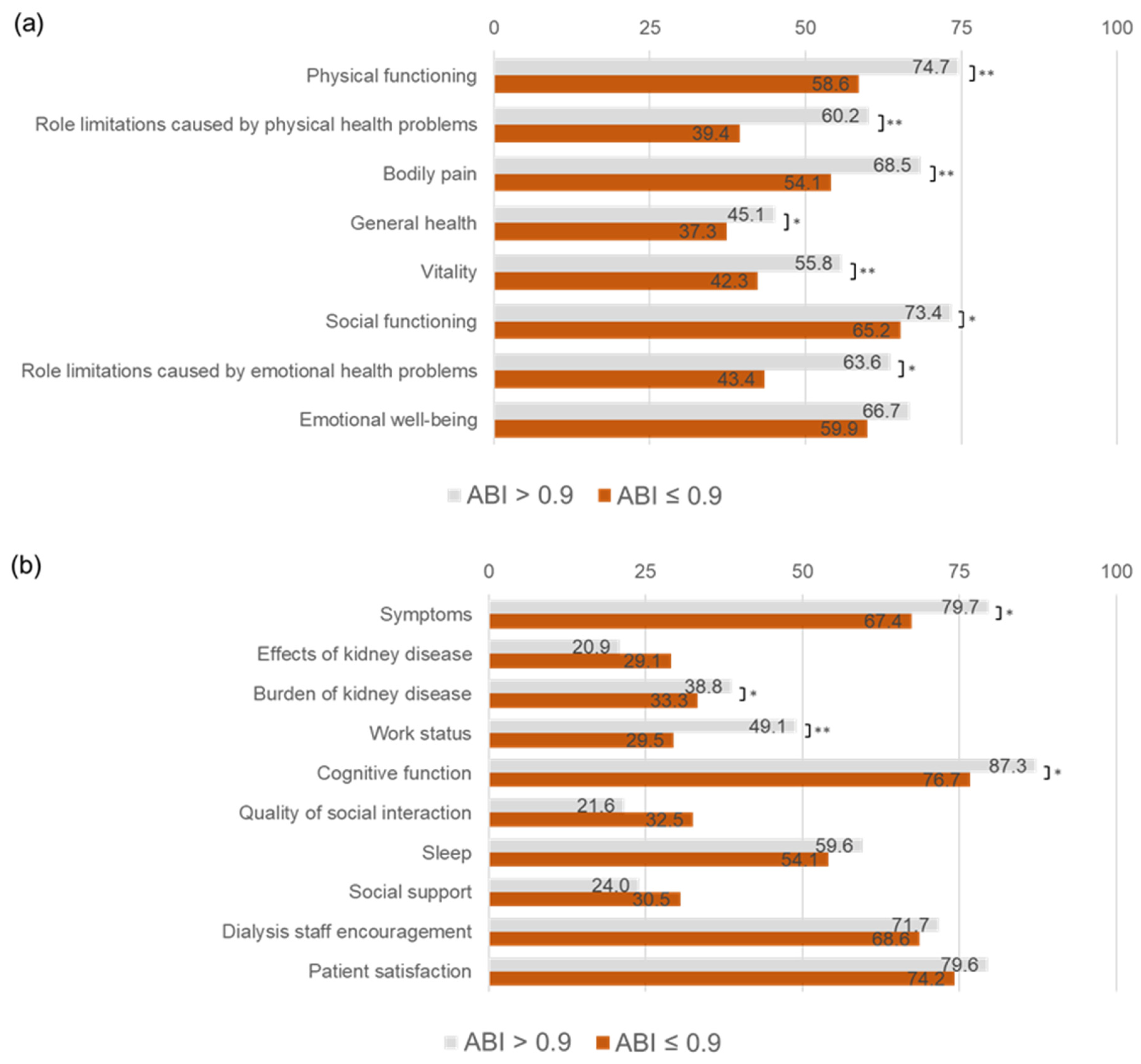
3.3. Association of ABI ≤ 0.9 with QOL Domains
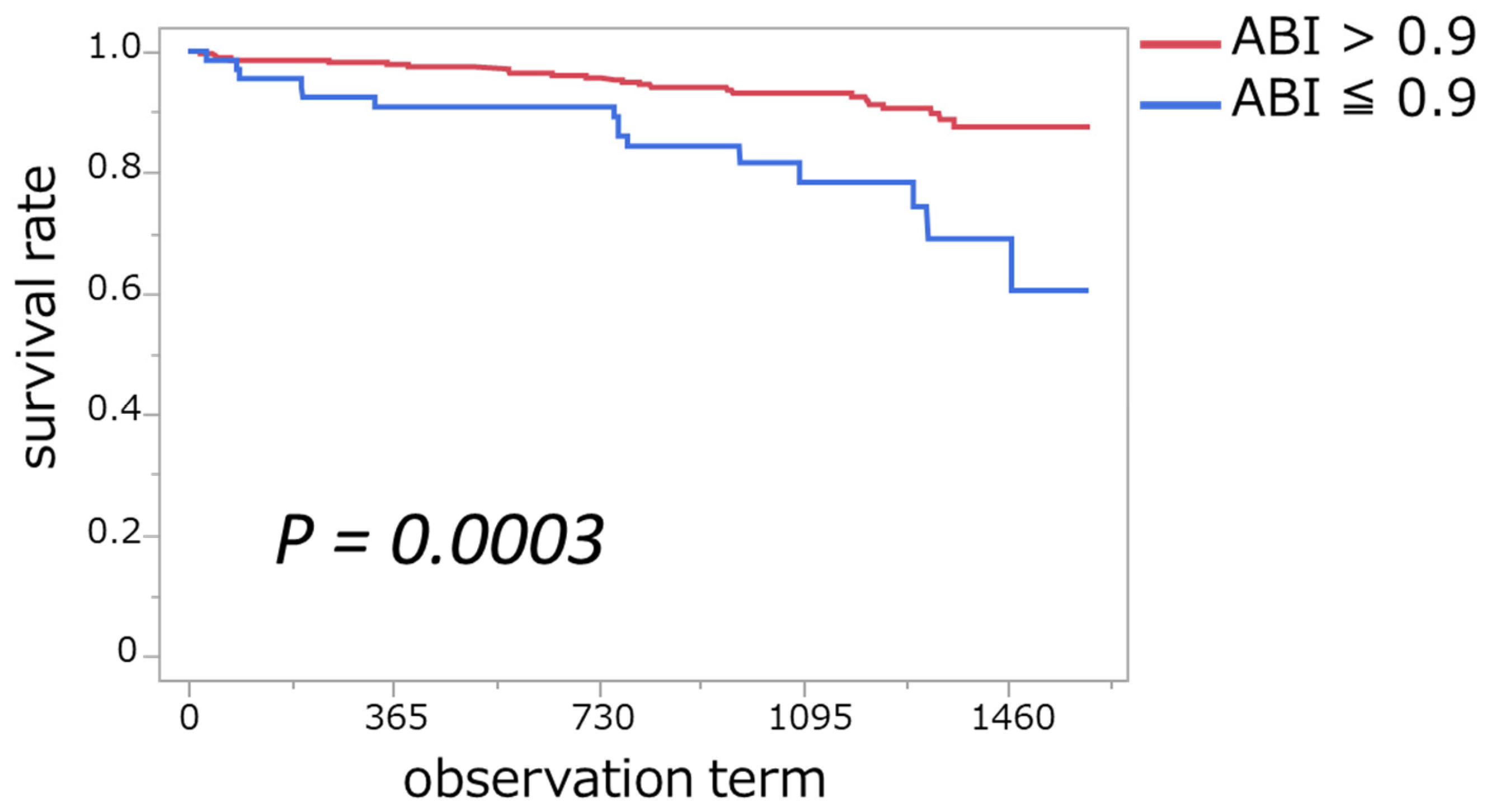
3.4. Association of ABI ≤ 0.9 with Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABI | Ankle–brachial index |
| QOL | Quality of life |
| PAD | Peripheral arterial disease |
| DM | Diabetes mellitus |
| HD | Hemodialysis |
| SBP | Systolic Blood Pressure |
| DBP | Diastolic Blood Pressure |
| BUN | Blood urea nitrogen |
| HbA1c | Glycated Hemoglobin |
| iPTH | Intact parathyroid hormone |
| CRP | C-reactive protein |
| Hb | Hemoglobin |
| NT-proBNP | N-Terminal Pro B-Type Natriuretic Peptide |
| GNRI | Geriatric Nutritional Risk Index |
| ICW | Intracellular water |
| ECW | Extracellular water |
| SMI | Skeletal muscle mass index |
| BSA | Body surface area |
References
- Hanafusa, N.; Abe, M.; Joki, N.; Hoshino, J.; Wada, A.; Kikuchi, K.; Goto, S.; Ogawa, T.; Kanda, E.; Taniguchi, M.; et al. Current status of chronic dialysis therapy in Japan (as of December 31, 2022). JSDT Ren. Data Regist. 2023, 10, 78. [Google Scholar] [CrossRef]
- Cleary, J.; Drennan, J. Quality of life of patients on haemodialysis for end-stage renal disease. J. Adv. Nurs. 2005, 51, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Coresh, J.; Selvin, E.; Tanaka, H.; Heiss, G.; Hirsch, A.T.; Jaar, B.G.; Matsushita, K. Lower Extremity Peripheral Artery Disease and Quality of Life Among Older Individuals in the Community. J. Am. Heart Assoc. 2017, 6, e004519. [Google Scholar] [CrossRef] [PubMed]
- Naseef, H.H.; Haj Ali, N.; Arafat, A.; Khraishi, S.; Abukhalil, A.D.; Al-Shami, N.M.; Ladadweh, H.; Alsheikh, M.; Rabba, A.K.; Asmar, I.T.; et al. Quality of Life of Palestinian Patients on Hemodialysis: Cross-Sectional Observational Study. Sci. World J. 2023, 2023, 4898202. [Google Scholar] [CrossRef]
- Rezvani, F.; Pelt, M.; Härter, M.; Dirmaier, J. Effects of walking impairment on mental health burden, health risk behavior and quality of life in patients with intermittent claudication: A cross-sectional path analysis. PLoS ONE 2022, 17, e0273747. [Google Scholar] [CrossRef]
- Cohen, G.; Hörl, W. Immune Dysfunction in Uremia—An Update. Toxins 2012, 4, 962–990. [Google Scholar] [CrossRef]
- Nusair, M.B.; Rajpurohit, N.; Alpert, M.A. Chronic Inflammation and Coronary Atherosclerosis in Patients with End-Stage Renal Disease. Cardiorenal Med. 2012, 2, 117–124. [Google Scholar] [CrossRef]
- Mourad, J.-J.; Cacoub, P.; Collet, J.-P.; Becker, F.; Pinel, J.-F.; Huet, D.; Sevestre-Pietri, M.-A.; Priollet, P. Screening of unrecognized peripheral arterial disease (PAD) using ankle-brachial index in high cardiovascular risk patients free from symptomatic PAD. J. Vasc. Surg. 2009, 50, 572–580. [Google Scholar] [CrossRef][Green Version]
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.R.; Hamburg, N.M.; Kinlay, S.; et al. 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2017, 69, e71–e126. [Google Scholar] [CrossRef]
- Chen, H.Y.; Wei, F.; Wang, L.H.; Wang, Z.; Meng, J.; Yu, H.B.; Zhang, R.N.; Sun, G.J.; Jiang, A.L.; Wang, L. Abnormal ankle-brachial index and risk of cardiovascular or all-cause mortality in patients with chronic kidney disease: A meta-analysis. J. Nephrol. 2017, 30, 493–501. [Google Scholar] [CrossRef]
- Xu, C.; Tian, Q.; Yu, H.; Ge, W.; Zheng, H.; Huang, D. Predictive Value of the Ankle-Brachial Index for All-Cause and Cardio-Cerebrovascular Mortality. Angiology 2023, 74, 649–656. [Google Scholar] [CrossRef] [PubMed]
- García-Ortiz, L.; Recio-Rodríguez, J.I.; Mora-Simón, S.; Guillaumet, J.; Martí, R.; Agudo-Conde, C.; Rodriguez-Sanchez, E.; Maderuelo-Fernandez, J.A.; Ramos-Blanes, R.; Gómez-Marcos, M.A. Vascular structure and function and their relationship with health-related quality of life in the MARK study. BMC Cardiovasc. Disord. 2016, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Feng, Y.; Ren, J.; Sun, Y. Ankle-Brachial Index, a New Predictor for All-Cause Mortality. Angiology 2024, 75, 597. [Google Scholar] [CrossRef] [PubMed]
- Hays, R.D.; Kallich, J.D.; Mapes, D.L.; Coons, S.J.; Carter, W.B. Development of the Kidney Disease Quality of Life (KDQOLTM) Instrument. Qual. Life Res. 1994, 3, 329–338. [Google Scholar] [CrossRef]
- Green, J.; Fukuhara, S.; Shinzato, T.; Miura, Y.; Wada, S.; Hays, R.D.; Tabata, R.; Otsuka, H.; Takai, I.; Maeda, K.; et al. Translation, cultural adaptation, and initial reliability and multitrait testing of the Kidney Disease Quality of Life instrument for use in Japan. Qual. Life Res. 2001, 10, 93–100. [Google Scholar] [CrossRef]
- Shinzato, T.; Nakai, S.; Fujita, Y.; Takai, I.; Morita, H.; Nakane, K.; Maeda, K. Determination of Kt/V and protein catabolic rate using pre- and postdialysis blood urea nitrogen concentrations. Nephron 1994, 67, 280–290. [Google Scholar] [CrossRef]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef]
- Doobay, A.V.; Anand, S.S. Sensitivity and Specificity of the Ankle–Brachial Index to Predict Future Cardiovascular Outcomes. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1463–1469. [Google Scholar] [CrossRef]
- Kajikawa, M.; Maruhashi, T.; Iwamoto, Y.; Iwamoto, A.; Matsumoto, T.; Hidaka, T.; Kihara, Y.; Chayama, K.; Nakashima, A.; Goto, C.; et al. Borderline Ankle-Brachial Index Value of 0.91–0.99 Is Associated with Endothelial Dysfunction. Circ. J. 2014, 78, 1740–1745. [Google Scholar] [CrossRef]
- Adragao, T.; Pires, A.; Branco, P.; Castro, R.; Oliveira, A.; Nogueira, C.; Bordalo, J.; Curto, J.D.; Prata, M.M. Ankle--brachial index, vascular calcifications and mortality in dialysis patients. Nephrol. Dial. Transplant. 2012, 27, 318–325. [Google Scholar] [CrossRef]
- Xu, D.; Zou, L.; Xing, Y.; Hou, L.; Wei, Y.; Zhang, J.; Qiao, Y.; Hu, D.; Xu, Y.; Li, J.; et al. Diagnostic value of ankle-brachial index in peripheral arterial disease: A meta-analysis. Can. J. Cardiol. 2013, 29, 492–498. [Google Scholar] [CrossRef]
- Hakamäki, M.; Lankinen, R.; Hellman, T.; Koivuviita, N.; Pärkkä, J.P.; Saarenhovi, M.; Metsärinne, K.; Järvisalo, M.J. Quality of Life Is Associated with Cardiac Biomarkers, Echocardiographic Indices, and Mortality in CKD Stage 4-5 Patients Not on Dialysis. Blood Purif. 2021, 50, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F.G.; Murray, G.D.; Butcher, I.; Heald, C.L.; Lee, R.J.; Chambless, L.E.; Folsom, A.R.; Hirsch, A.T.; Dramaix, M.; deBacker, G.; et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: A meta-analysis. JAMA 2008, 300, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Kalra, P.A.; Gilbertson, D.T.; Liu, J.; Chen, S.C.; Collins, A.J.; Foley, R.N. Atherosclerotic renovascular disease in older US patients starting dialysis, 1996 to 2001. Circulation 2007, 115, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.I.; Saglam, M.; Caglar, K.; Cakir, E.; Sonmez, A.; Ozgurtas, T.; Aydin, A.; Eyileten, T.; Ozcan, O.; Acikel, C.; et al. The determinants of endothelial dysfunction in CKD: Oxidative stress and asymmetric dimethylarginine. Am. J. Kidney Dis. 2006, 47, 42–50. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Zoccali, C.; Goldsmith, D.; Agarwal, R.; Blankestijn, P.J.; Fliser, D.; Wiecek, A.; Suleymanlar, G.; Ortiz, A.; Massy, Z.; Covic, A.; et al. The complexity of the cardio-renal link: Taxonomy, syndromes, and diseases. Kidney Int. Suppl. 2011, 1, 2–5. [Google Scholar] [CrossRef]
- Silverstein, D.M. Inflammation in chronic kidney disease: Role in the progression of renal and cardiovascular disease. Pediatr. Nephrol. 2009, 24, 1445–1452. [Google Scholar] [CrossRef]
- Yu, M.D.; Zhang, H.Z.; Zhang, Y.; Yang, S.P.; Lin, M.; Zhang, Y.M.; Wu, J.B.; Hong, F.Y.; Chen, W.X. Relationship between chronic kidney disease and sarcopenia. Sci. Rep. 2021, 11, 20523. [Google Scholar] [CrossRef]
- Moorthi, R.N.; Avin, K.G. Clinical relevance of sarcopenia in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2017, 26, 219–228. [Google Scholar] [CrossRef]
- Pasko, N.; Strakosha, A.; Dedej, A.; Kapidani, L.; Nasto, F.; Rista, E.; Cadri, V.; Mumajesi, S.; Thereska, N. P1593 the association between the ancle-brachial index and markers of inflamation in chronic hemodialysis patients. Nephrol. Dial. Transplant. 2020, 35, gfaa142-P1593. [Google Scholar] [CrossRef]
- Lopes, A.A.; Tong, L.; Thumma, J.; Li, Y.; Fuller, D.S.; Morgenstern, H.; Bommer, J.; Kerr, P.G.; Tentori, F.; Akiba, T.; et al. Phosphate binder use and mortality among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS): Evaluation of possible confounding by nutritional status. Am. J. Kidney Dis. 2012, 60, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, B.; Bellasi, A.; Russo, D. Mortality in kidney disease patients treated with phosphate binders: A randomized study. Clin. J. Am. Soc. Nephrol. 2012, 7, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Nazzal, Z.A.; Hamdan, Z.; Natour, N.; Barbar, M.; Rimawi, R.; Salaymeh, E. Prevalence of Vitamin D Deficiency among Hemodialysis Patients in Palestine: A Cross-Sectional Study. Int. J. Nephrol. 2021, 2021, 6684276. [Google Scholar] [CrossRef]
- Gluba-Brzózka, A.; Franczyk, B.; Ciałkowska-Rysz, A.; Olszewski, R.; Rysz, J. Impact of Vitamin D on the Cardiovascular System in Advanced Chronic Kidney Disease (CKD) and Dialysis Patients. Nutrients 2018, 10, 709. [Google Scholar] [CrossRef]
- Hoppe, K.; Schwermer, K.; Dopierała, M.; Kałużna, M.; Hoppe, A.; Chou, J.T.; Oko, A.; Pawlaczyk, K. Can Overnutrition Lead to Wasting?-The Paradox of Diabetes Mellitus in End-Stage Renal Disease Treated with Maintenance Hemodialysis. Nutrients 2022, 14, 247. [Google Scholar] [CrossRef]
- Nakayama, Y.; Yamada, Y.; Ishii, S.; Hitaka, M.; Yamazaki, K.; Masai, M.; Joki, N.; Sakai, K.; Ohashi, Y. Association between Intra- and Extra-Cellular Water Ratio Imbalance and Natriuretic Peptides in Patients Undergoing Hemodialysis. Nutrients 2023, 15, 1274. [Google Scholar] [CrossRef]
- Ohashi, Y.; Tai, R.; Aoki, T.; Mizuiri, S.; Ogura, T.; Tanaka, Y.; Okada, T.; Aikawa, A.; Sakai, K. The Associations of Malnutrition and Aging with Fluid Volume Imbalance between Intra- and Extracellular Water in Patients with Chronic Kidney Disease. J. Nutr. Health Aging 2015, 19, 986–993. [Google Scholar] [CrossRef]
- Cherny, N.I.; Coyle, N.; Foley, K.M. Suffering in the advanced cancer patient: A definition and taxonomy. J. Palliat. Care 1994, 10, 57–70. [Google Scholar] [CrossRef]
- Karakoyun, R.; Köksoy, C.; Şener, Z.; Gündüz, U.; Karakaş, B.; Karakoyun, M. Comparison of quality of life in patients with peripheral arterial disease caused by atherosclerosis obliterans or Buerger’s disease. Cardiovasc. J. Afr. 2014, 25, 124–129. [Google Scholar] [CrossRef][Green Version]
- Phyo, A.Z.Z.; Freak-Poli, R.; Craig, H.; Gasevic, D.; Stocks, N.P.; Gonzalez-Chica, D.A.; Ryan, J. Quality of life and mortality in the general population: A systematic review and meta-analysis. BMC Public Health 2020, 20, 1596. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.-Y.; Duan, G.-C.; Wang, C.-J.; Liu, D.-W.; Qiao, Y.-J.; Pan, S.-K.; Jiang, D.-K.; Liu, Y.; Zhao, Z.-H.; Liang, L.-L.; et al. Prevalence and risk factors of chronic kidney disease and diabetic kidney disease in a central Chinese urban population: A cross-sectional survey. BMC Nephrol. 2020, 21, 115. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.M.; Bartz-Overman, C.; Parikh Md, T.; Thielke, S.M. Associations Between Activities of Daily Living Independence and Mental Health Status Among Medicare Managed Care Patients. J. Am. Geriatr. Soc. 2020, 68, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
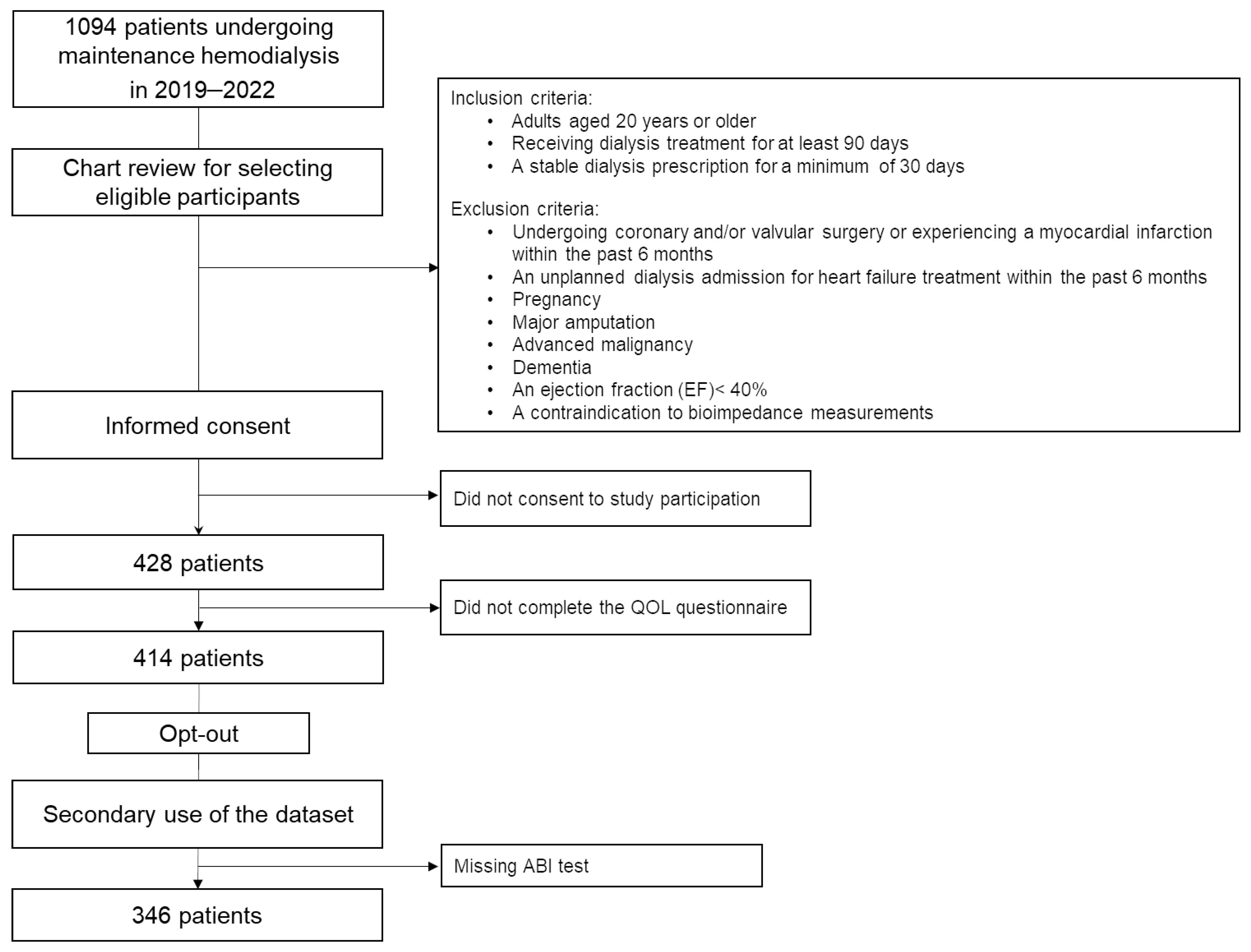
| Clinical Parameters | ABI ≤ 0.9 (n = 66, 19.1%) | ABI > 0.9 (n = 280, 80.9%) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Median/ Proportion | IQR | 95% CI | Median/ Proportion | IQR | 95% CI | ||
| Age (years) | 70 | 60–77 | 66–71 | 66 | 54–73 | 62–65 | 0.006 |
| Male gender, % | 76 | n/a | 64–84 | 70 | n/a | 65–75 | 0.38 |
| DM, % | 62.1 | n/a | 50–73 | 41.4 | n/a | 41–59 | 0.002 |
| Cardiovascular disease, % | 31.8 | n/a | 32–68 | 16.1 | n/a | 12–21 | 0.003 |
| Smoking, % | 25.8 | n/a | 17–37 | 26.1 | n/a | 21–32 | 0.96 |
| HD duration (months) | 100 | 61–226 | 116–172 | 61.5 | 30–117 | 79–98 | <0.001 |
| Body mass index, (kg/m2) | 22.2 | 19.4–24.7 | 21.1–22.8 | 22.1 | 20.0–25.4 | 22.2–23.2 | 0.29 |
| SBP (mmHg) | 150 | 132–164 | 142–154 | 145 | 129–160 | 142–147 | 0.23 |
| DBP (mmHg) | 72 | 65–81 | 71–77 | 76 | 68–86 | 76–79 | 0.043 |
| BUN (mg/dL) | 53 | 44–62 | 51–58 | 58 | 49–70 | 59–62 | 0.002 |
| Creatinine (mg/dL) | 10.01 | 8.68–11.15 | 9.47–10.38 | 10.3 | 8.83–12.03 | 10.2–10.8 | 0.11 |
| Calcium (mg/dL) | 8.7 | 8.3–9.0 | 8.5–8.8 | 8.7 | 8.3–8.9 | 8.56–8.68 | 0.69 |
| Phosphorus (mg/dL) | 5.5 | 5.0–5.9 | 5.3–5.8 | 5.6 | 5.0–6.3 | 5.5–5.8 | 0.49 |
| HbA1c (%) | 5.9 | 5.4–6.7 | 5.8–6.4 | 6.3 | 5.7–6.8 | 6.3–6.6 | 0.041 |
| iPTH (pg/mL) | 160 | 98–208 | 140–186 | 157 | 106–217 | 158–180 | 0.68 |
| CRP (mg/dL) | 0.14 | 0.06–0.36 | 0.17–0.57 | 0.1 | 0.04–0.27 | 0.23–0.38 | 0.21 |
| Hb (g/dL) | 11.5 | 10.8–12.0 | 11.2–11.7 | 11.2 | 10.7–11.9 | 11.1–11.4 | 0.06 |
| NT-proBNP (pg/mL) | 6050 | 3550–17,750 | 8822–15,941 | 2985 | 1595–6700 | 5542–7981 | <0.001 |
| KT/Vurea (D) | 1.84 | 1.68–2.09 | 1.83–1.99 | 1.80 | 1.63–2.04 | 1.82–1.9 | 0.19 |
| GNRI | 95 | 89–100 | 93–97 | 97 | 90–104 | 96–98 | 0.11 |
| Number of any phosphate binders 0/1/2 or more | 24/38/4 | n/a | n/a | 83/137/60 | n/a | n/a | 0.015 |
| Calcitriol use, % | 86.9 | n/a | 78–94 | 70.4 | n/a | 65–75 | 0.004 |
| Body Fluid Composition | ABI ≤ 0.9 (n = 66, 19.1%) | ABI > 0.9 (n = 280, 80.9%) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Median | IQR | 95% CI | Median | IQR | 95% CI | ||
| Total body water, L | 32.6 | 27.5–35.4 | 30.4–33.2 | 33.3 | 28.0–38.9 | 32.8–34.5 | 0.10 |
| Total body water, per BSA | 19.6 | 18.3–20.6 | 19.0–19.9 | 20.0 | 18.5–21.7 | 19.8–20.3 | 0.038 |
| Intracellular water, L | 19.7 | 16.6–21.8 | 18.4–20.1 | 20.3 | 17.1–24.1 | 20.0–21.2 | 0.06 |
| Intracellular water, L per BSA | 11.8 | 11.0–12.6 | 11.5–12.0 | 12.2 | 11.3–13.3 | 12.1–12.4 | 0.006 |
| Extracellular water, L | 12.9 | 10.8–13.8 | 12.1–13.1 | 12.9 | 10.8–14.8 | 12.7–13.3 | 0.31 |
| Extracellular water, L per BSA | 7.7 | 7.3–8.1 | 7.5–7.9 | 7.8 | 7.2–8.4 | 7.7–7.9 | 0.49 |
| Extracellular water-to-intracellular water ratio | 0.66 | 0.63–0.67 | 0.65–0.66 | 0.63 | 0.61–0.63 | 0.63–0.64 | <0.001 |
| Phase angle, ° | 4.8 | 4.2–5.1 | 4.4–4.9 | 5.3 | 4.6–6.2 | 5.3–5.5 | <0.001 |
| Skeletal muscle mass index, kg/m2 | 8.4 | 7.7–9.3 | 6.5–7.1 | 9.0 | 8.0–10.1 | 7.0–7.4 | 0.007 |
| QOL Domains | ABI ≤ 0.9 (n = 66, 19.1%) | ABI > 0.9 (n = 280, 80.9%) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Median | IQR | 95% CI | Median | IQR | 95% CI | ||
| Physical functioning | 65 | 45–75 | 52–65 | 80 | 65–95 | 72–78 | <0.001 |
| Role limitations caused by physical health problems | 0 | 0–100 | 28–50 | 75 | 0–100 | 55–65 | <0.001 |
| Bodily pain | 56 | 33–80 | 47–61 | 70 | 53–90 | 65–72 | <0.001 |
| General health | 40 | 25–60 | 33–42 | 50 | 35–55 | 43–47 | 0.004 |
| Vitality | 45 | 25–60 | 36–48 | 55 | 40–75 | 53–59 | <0.001 |
| Social functioning | 63 | 50–75 | 57–73 | 75 | 50–100 | 70–77 | 0.048 |
| Role limitations caused by emotional health problems | 0 | 0–100 | 32–55 | 100 | 0–100 | 58–69 | 0.002 |
| Emotional well-being | 68 | 48–81 | 53–67 | 68 | 56–84 | 64–69 | 0.17 |
| Symptoms | 79 | 64–86 | 60–75 | 83 | 73–92 | 78–82 | 0.003 |
| Effects of kidney disease | 78 | 53–88 | 59–74 | 78 | 66–91 | 72–77 | 0.10 |
| Burden of kidney disease | 31 | 13–45 | 27–40 | 38 | 25–50 | 36–41 | 0.039 |
| Work status | 25 | 0–50 | 21–38 | 50 | 0–100 | 44–54 | <0.001 |
| Cognitive function | 87 | 73–100 | 67–84 | 93 | 80–100 | 85–90 | 0.017 |
| Quality of social interaction | 87 | 67–100 | 67–83 | 93 | 73–100 | 81–86 | 0.24 |
| Sleep | 58 | 47–73 | 48–60 | 63 | 48–75 | 57–62 | 0.25 |
| Social support | 67 | 67–83 | 58–73 | 67 | 67–83 | 68–74 | 0.51 |
| Dialysis staff encouragement | 75 | 50–88 | 61–76 | 75 | 50–100 | 57–62 | 0.99 |
| Patient satisfaction | 83 | 67–100 | 67–82 | 83 | 67–100 | 68–74 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, N.; Tanaka, T.; Suzuki, Y.; Takahashi, S.; Hitaka, M.; Ishii, S.; Yamazaki, K.; Ohashi, Y. Association of Ankle–Brachial Index with Quality of Life and Survival Outcomes in Hemodialysis Patients. J. Clin. Med. 2025, 14, 1625. https://doi.org/10.3390/jcm14051625
Yoshida N, Tanaka T, Suzuki Y, Takahashi S, Hitaka M, Ishii S, Yamazaki K, Ohashi Y. Association of Ankle–Brachial Index with Quality of Life and Survival Outcomes in Hemodialysis Patients. Journal of Clinical Medicine. 2025; 14(5):1625. https://doi.org/10.3390/jcm14051625
Chicago/Turabian StyleYoshida, Norihito, Tatsuki Tanaka, Yusuke Suzuki, Sadamu Takahashi, Mai Hitaka, Shingo Ishii, Keisuke Yamazaki, and Yasushi Ohashi. 2025. "Association of Ankle–Brachial Index with Quality of Life and Survival Outcomes in Hemodialysis Patients" Journal of Clinical Medicine 14, no. 5: 1625. https://doi.org/10.3390/jcm14051625
APA StyleYoshida, N., Tanaka, T., Suzuki, Y., Takahashi, S., Hitaka, M., Ishii, S., Yamazaki, K., & Ohashi, Y. (2025). Association of Ankle–Brachial Index with Quality of Life and Survival Outcomes in Hemodialysis Patients. Journal of Clinical Medicine, 14(5), 1625. https://doi.org/10.3390/jcm14051625







