Oligometastatic Mixed Neuroendocrine Adenocarcinoma of the Esophago-Gastric Junction: A Case of Successful Multidisciplinary Management, the Lessons Learnt and Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
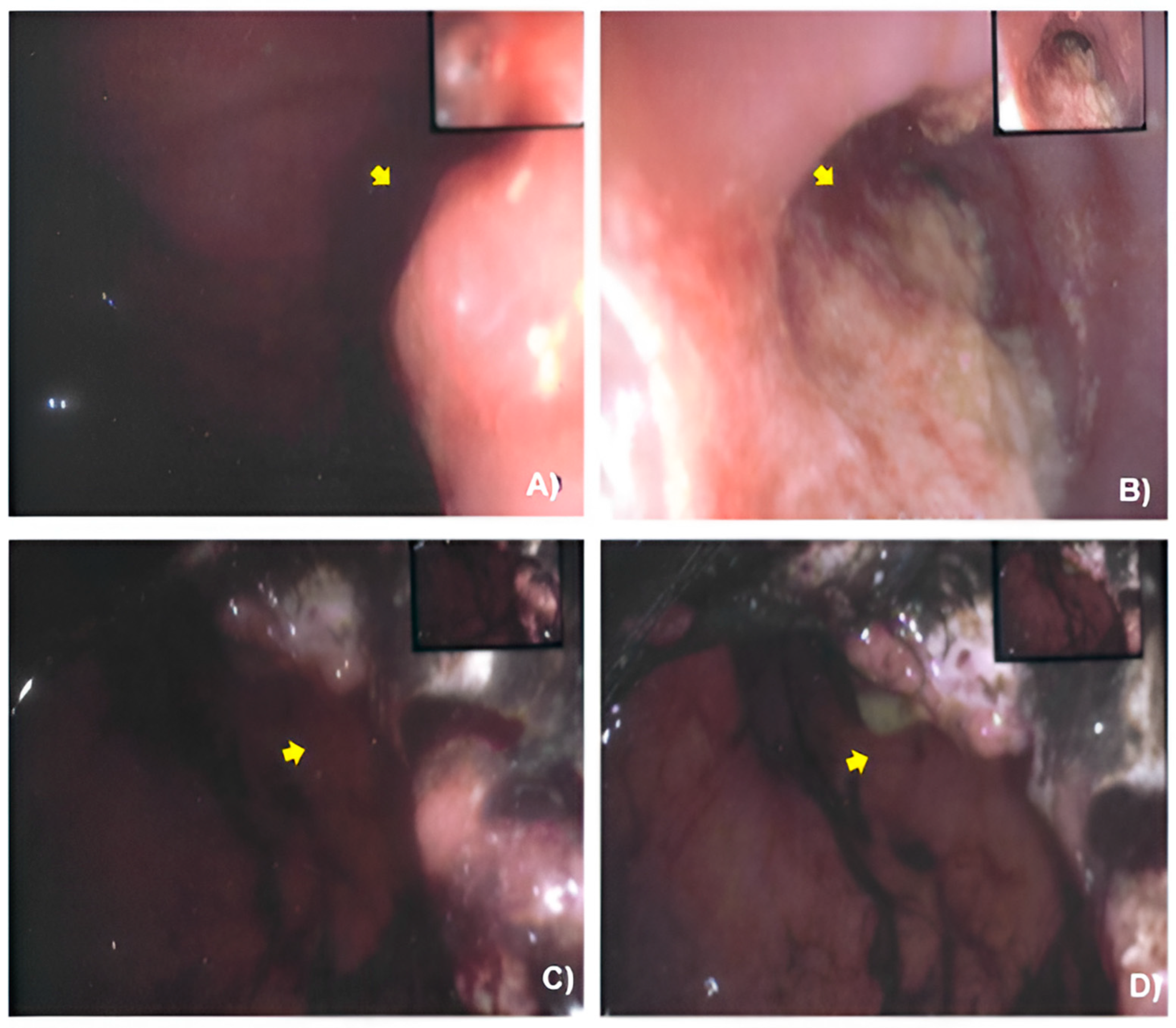
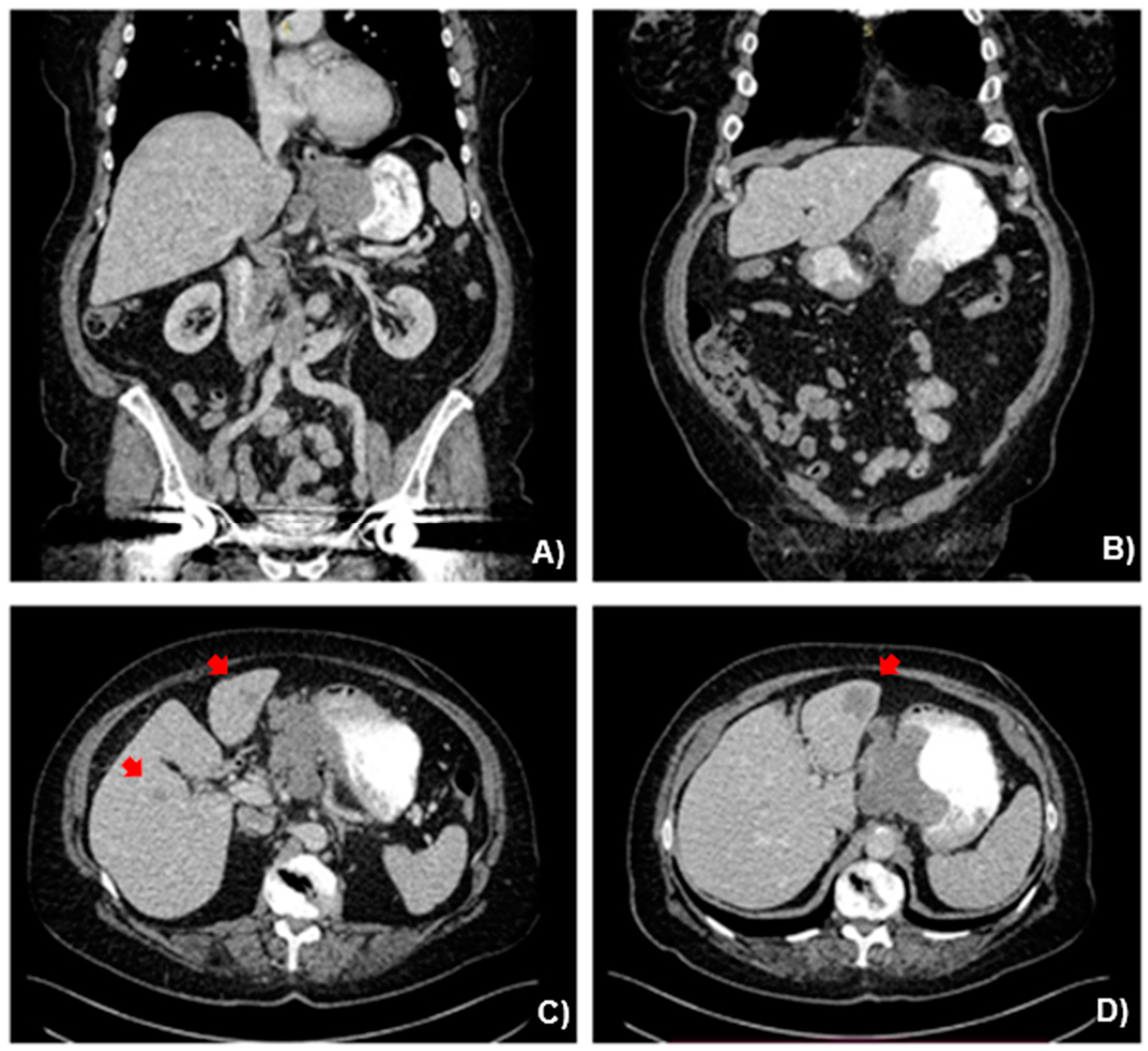
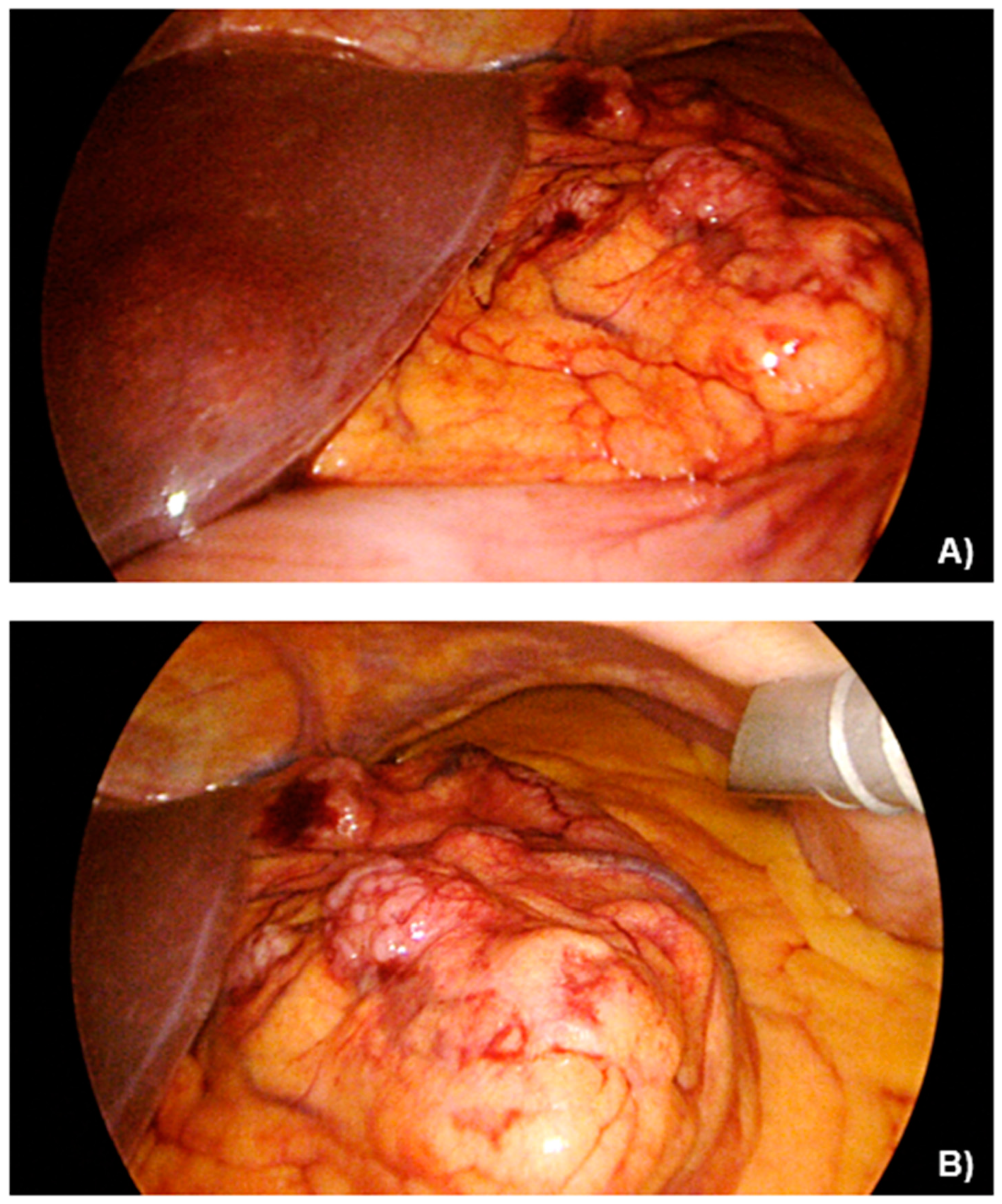
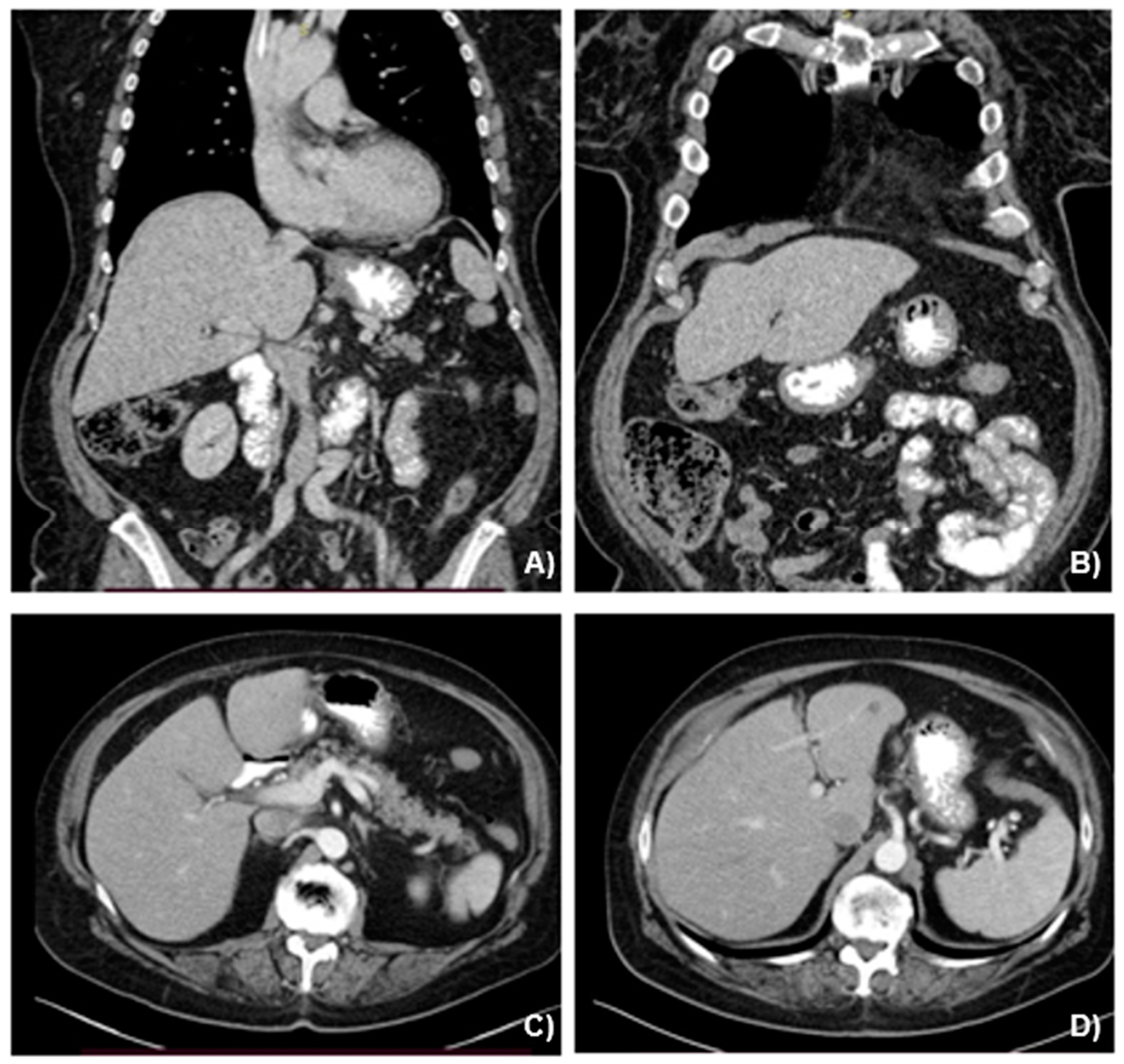
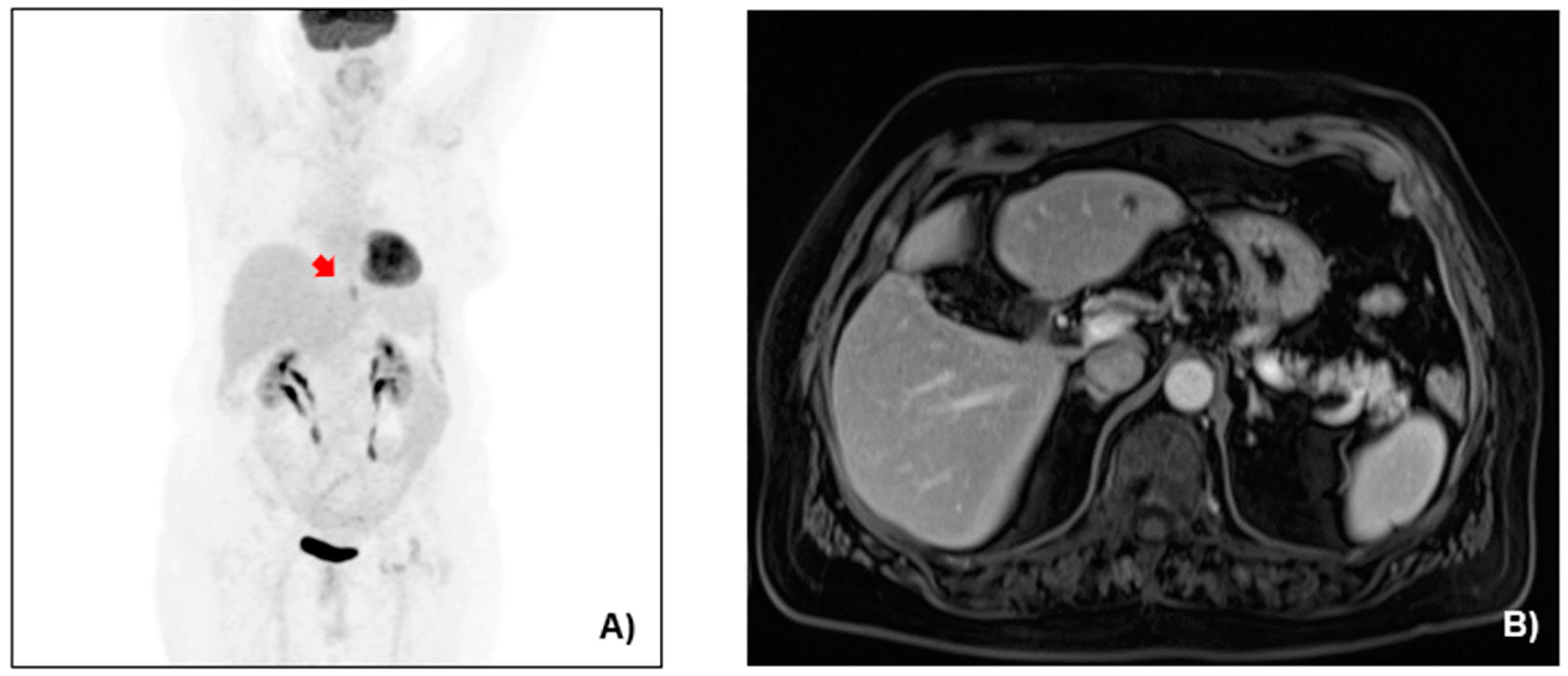
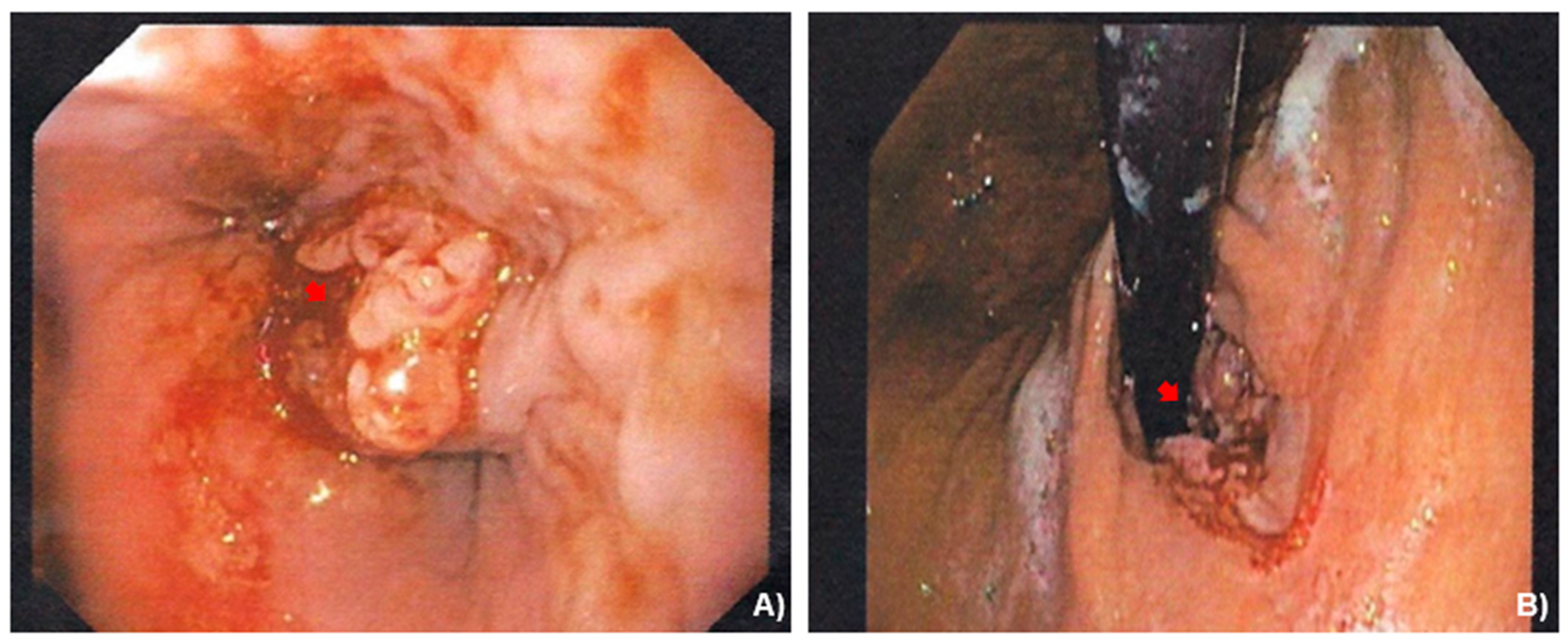
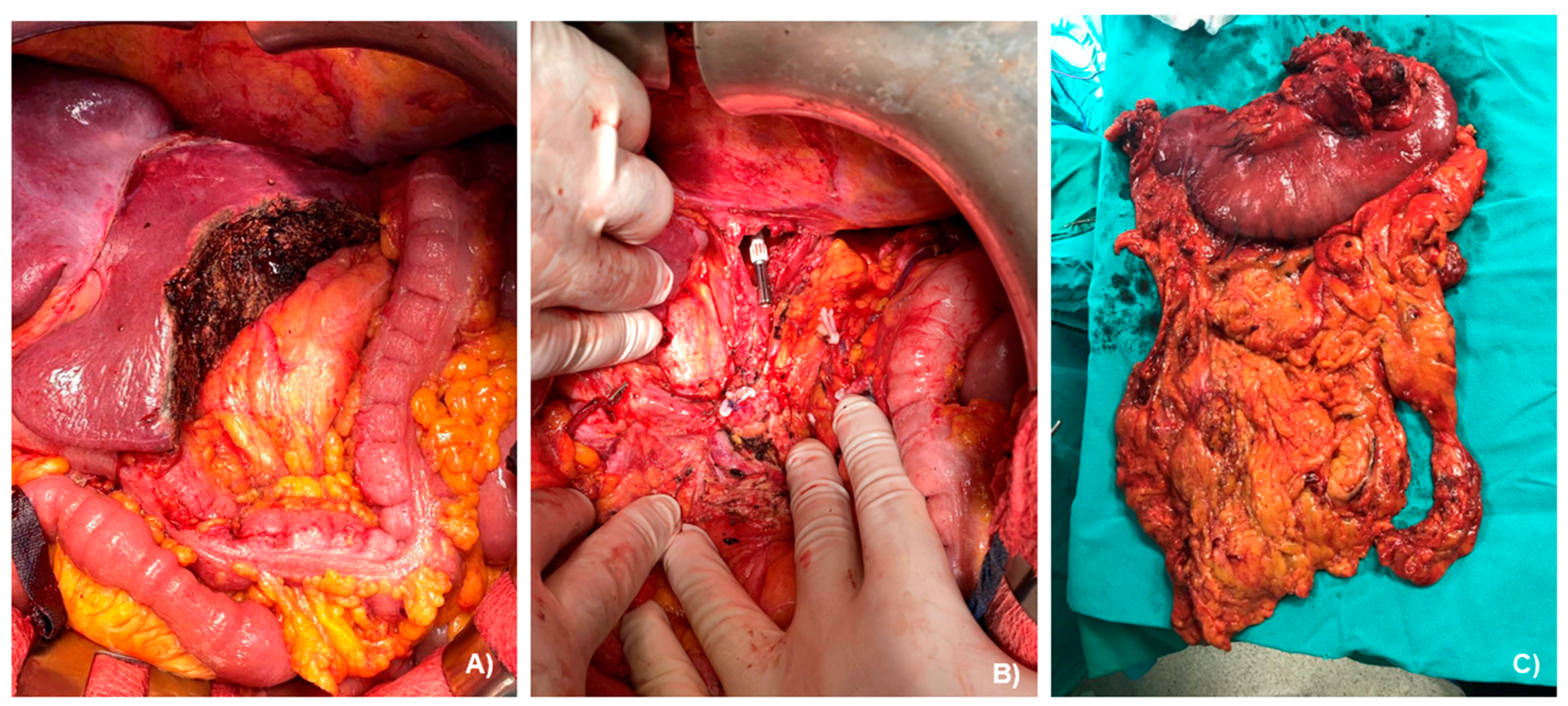
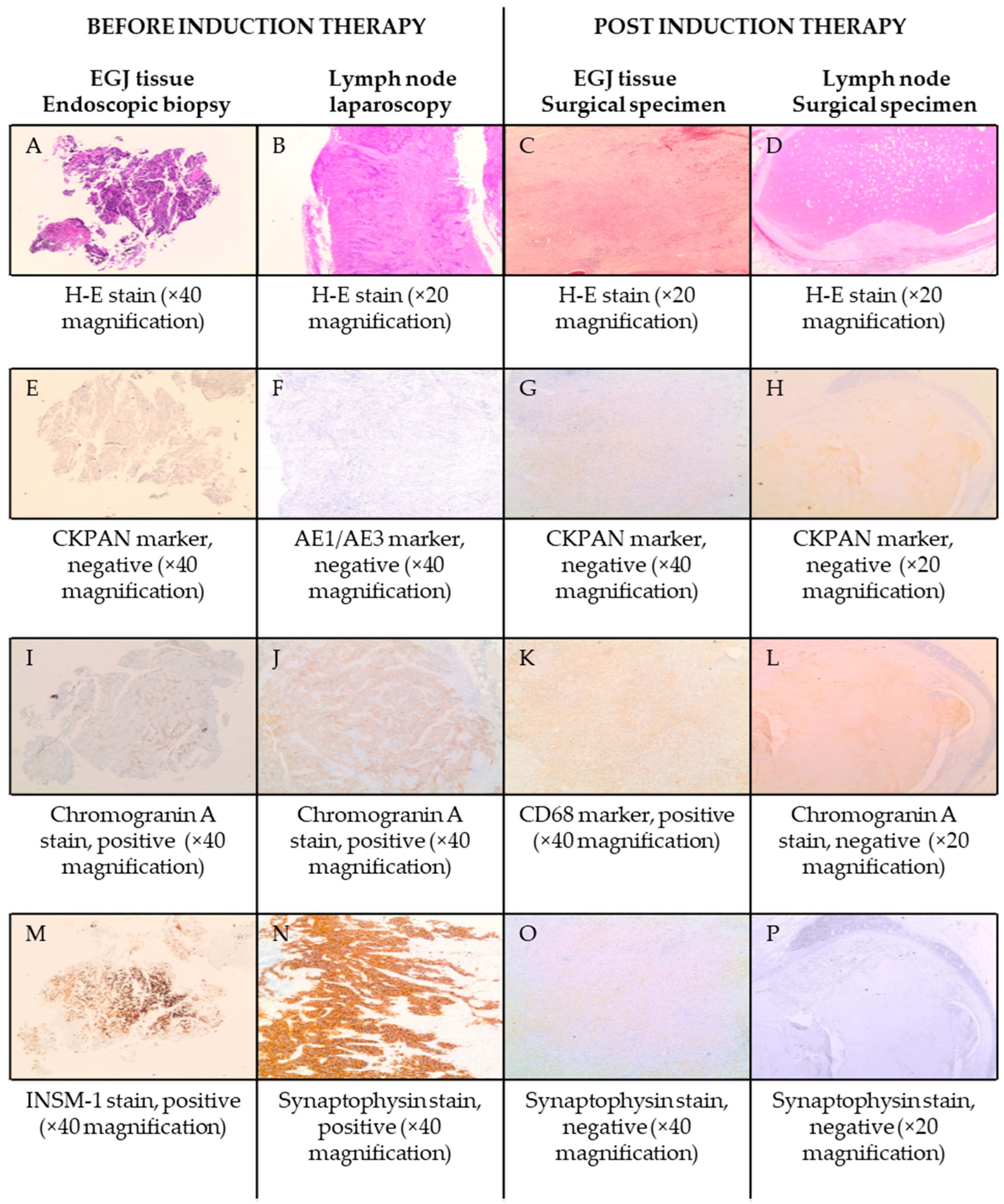
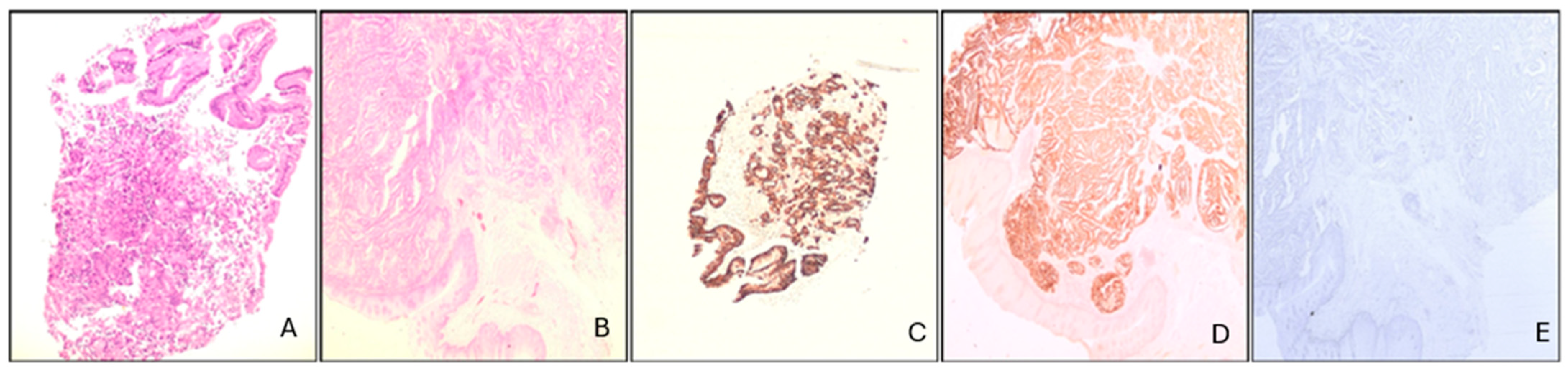
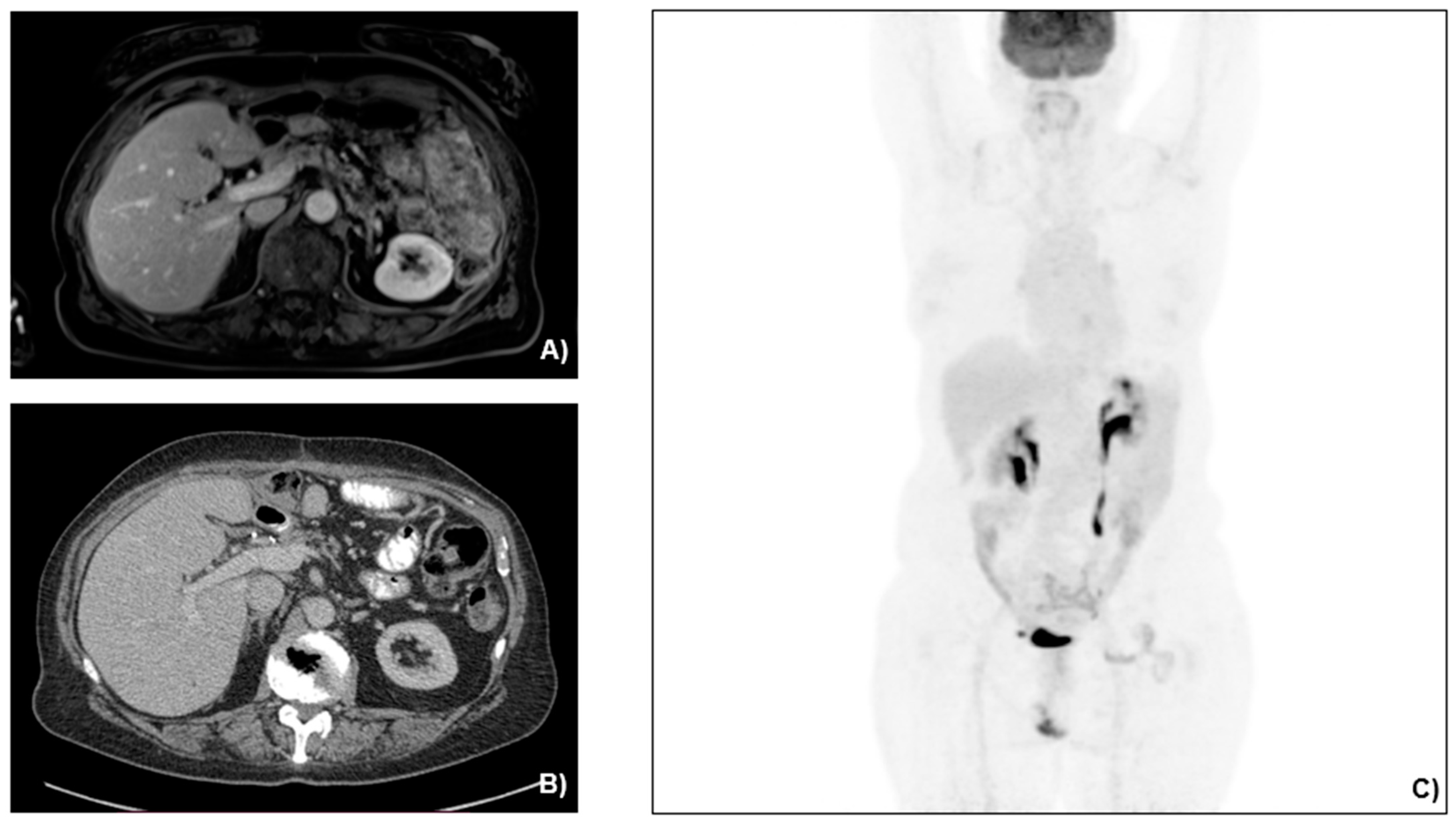
2.2. Case Presentation
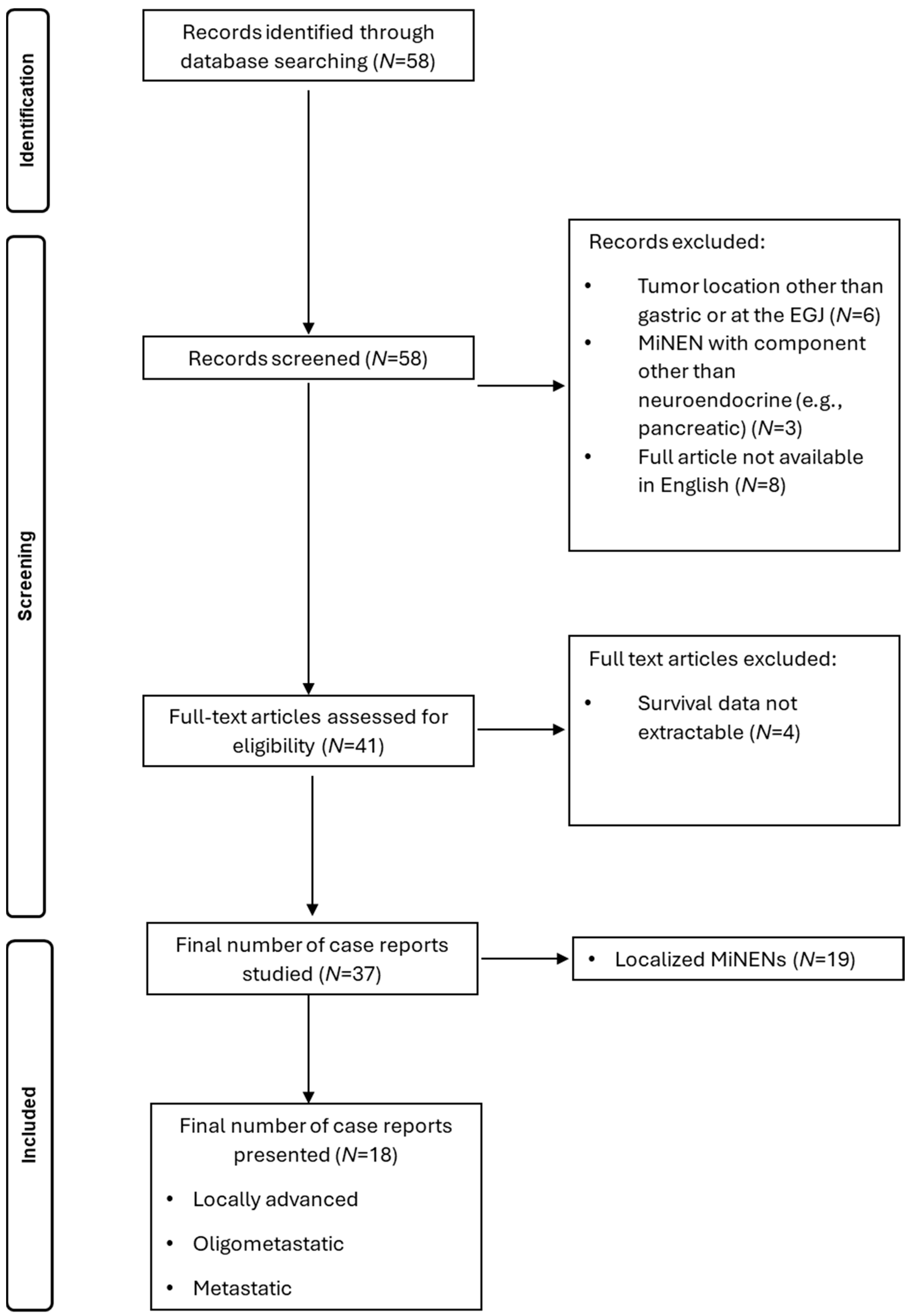
2.3. Search of Literature
3. Results
3.1. Case Management
3.2. Literature Review
| Authors | Locally Advanced | Oligo-Metastatic | Metastatic | Neuroendocrine to Non-Neuroendocrine Ratio | Induction Chemotherapy Regimen | Surgery | Adjuvant Therapy | Other Therapies | Overall Survival |
|---|---|---|---|---|---|---|---|---|---|
| Zheng et al., 2024 [17] | Yes | No | No | 50%/50% | No | Total gastrectomy pT3N1M0 | Delayed due to postoperative condition | Progression with liver metastasis treated with etoposide, cisplatin, toripalumab (6 cycles) | Ongoing at 1 y |
| Lun Tao Woo et al., 2022 Patient 1 [18] | Yes | Left liver lobe | No | 80%/20% | Irinotecan plus cisplatin (4 cycles) | Total gastrectomy and partial left liver resection pT1aN1M0 | Irinotecan plus cisplatin (2 cycles) | No | Ongoing at 3 y |
| Lun Tao Woo et al., 2022 Patient 2 [18] | Yes | No | No | 80%/20% | No | Total gastrectomy pT4aN3aM0 | Irinotecan plus cisplatin (6 cycles) | VATS due to recurrence at 2 y with metastatic lesions at the lower lobe of the right lung | Ongoing at 7 y |
| Deliwala et al., 2021 [32] | Yes | No | Left liver lobe, paraaortic lymph nodes | N/A | Carboplatin plus etoposide (2 cycles) | No | No | Capecitabine plus temozolomide following osseous metastases | 3 m, disease progression |
| Millet et al., 2021 [33] | Yes | No | Liver, retroperitoneal adenopathy, mesenteric implants | N/A | No | No | No | Palliative treatment | Unknown, disease progression |
| Ricco et al., 2020 [19] | Yes | No | No | 60%/40% | Carboplatin plus etoposide (3 cycles) | Total gastrectomy pT4N1M0 | mFOLFOX6 (6 cycles) | Multiple chemotherapy regimens, radiation therapy and antiPD-1 treatment due to disease progression | Ongoing |
| Lin et al., 2019 [20] | Yes | No | No | N/A | No | Total gastrectomy | Oxaliplatin plus capecitabine (4 cycles) | Radiofrequency Ablation due to hepatic metastasis at 4 cycles, paclitaxel plus Cisplatin (6 cycles), Abatinib | Ongoing |
| Golombek et al., 2019 [34] | Yes | No | Liver, distant lymph nodes | N/A | Etoposide plus cisplatin | No | No | No | 9 months, disease progression |
| Tang et al., 2017 Patient 1 [21] | Yes | No | No | 70%/30% | No | Total gastrectomy pT4aN1M0 | Etoposide plus cisplatin | No | Ongoing at 6-month follow up |
| Tang et al., 2017 Patient 2 [21] | Yes | No | No | 30%/70% | No | Total gastrectomy pT4aN1M0 | No treatment due to general weakness | No | 6 m, general weakness |
| Kheiri et al., 2017 [22] | Yes | No | No | N/A | No | Partial gastrectomy T4aN2M0 | mFOLFOX (12 cycles) | No | Ongoing at 2 y follow up |
| Pham et al., 2017 [35] | Yes | No | Liver, pancreas, distant lymph nodes | 80–90%/10–20% | No | No | S-1, CDDP, Trastuzumab (5 cycles) | Yes | 7 m, disease progression |
| Ambesh et al., 2017 [23] | Yes | No | No | 60%/40% | No | Ivor-Lewis Esophagogastrectomy pT3N1M0 | No | No | Unknown |
| Cazzo et al., 2016 [24] | Yes | No | No | 40%/60% | No | Total gastrectomy pT4N0M0 | 5-fluorouracil plus leucovorin (5 cycles) plus radiation therapy | No | Ongoing at 1 y follow up |
| Taguchi et al., 2015 [25] | Yes | No | No | 30%/70% | No | Total gastrectomy | Tegafur plus uracil | No | Ongoing at 1 y and 4 m |
| Gurzu et al., 2015 [26] | Yes | No | No | 40%/60% | No | Total gastrectomy pT4N3b | Patient refused treatment | No | 5 m, disease progression |
| Levi et al., 2014 [27] | Yes | No | No | 30%/70% | No | Total gastrectomy pT4aN0M0 | Cisplatin, doxorubicin, and vincristine | No | Ongoing at 6 m follow up |
| Zhang et al., 2014 [29] | Yes | Left liver lobe | No | N/A | No | Total gastrectomy and left liver resection | Patient refused treatment | No | 2.5 m, disease progression |
| Pericleous et al., 2012 [31] | Yes | No | >3 liver lesions | N/A | No | No | No | Somatostatin analogues | Unknown |
| Miguchi et al., 2012 [28] | Yes | No | No | N/A | No | Total gastrectomy | S-1 | No | Ongoing at 6 m follow up |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MiNEN | Mixed neuroendocrine–non-neuroendocrine neoplasm |
| EGJ | Esophagogastric junction |
| MDT | Multidisciplinary team |
| MANEC | Mixed adenoneuroendocrine carcinoma |
References
- Díaz-López, S.; Jiménez-Castro, J.; Robles-Barraza, C.E.; Ayala-de Miguel, C.; Chaves-Conde, M. Mixed neuroendocrine non-neuroendocrine neoplasms in gastroenteropancreatic tract. World J. Gastrointest. Oncol. 2024, 16, 1166–1179. [Google Scholar] [CrossRef] [PubMed]
- Cordier, R. Les Cellules argentaffines dans les tumeurs intestinales. Arch. Int. Med. 1924, 1, 59–63. [Google Scholar]
- La Rosa, S.; Sessa, F.; Uccella, S. Mixed Neuroendocrine-Nonneuroendocrine Neoplasms (MiNENs): Unifying the Concept of a Heterogeneous Group of Neoplasms. Endocr. Pathol. 2016, 27, 284–311. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; The WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Couvelard, A.; Cazes, A.; Cros, J. Updates in histopathological classification and tissue biomarkers of digestive neuroendocrine neoplasms: What the clinician should know. Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101795. [Google Scholar] [CrossRef] [PubMed]
- Scardoni, M.; Vittoria, E.; Volante, M.; Rusev, B.; Bersani, S.; Mafficini, A.; Gottardi, M.; Giandomenico, V.; Malleo, G.; Butturini, G.; et al. Mixed adenoneuroendocrine carcinomas of the gastrointestinal tract: Targeted next-generation sequencing suggests a monoclonal Origin of the Two Components. Neuroendocrinology 2014, 100, 310–316. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, S. Diagnostic, Prognostic, and Predictive Role of Ki67 Proliferative Index in Neuroendocrine and Endocrine Neoplasms: Past, Present, and Future. Endocr. Pathol. 2023, 34, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, V.T.; Hamade, N.; Desai, M.; Rai, T.; Gorrepati, V.S.; Jegadeesan, R.; Sathyamurthy, A.; Sharma, P. Significantly lower annual rates of neoplastic progression in short-compared to long-segment non-dysplastic Barrett’s esophagus: A systematic review and meta-analysis. Endoscopy 2019, 51, 665–672. [Google Scholar] [CrossRef]
- Siewert, J.R.; Stein, H.J. Classification of adenocarcinoma of the oesophagogastric junction. Br. J. Surg. 1998, 85, 1457–1459. [Google Scholar] [CrossRef]
- Daiko, H.; Kato, K. Updates in the 8th edition of the TNM staging system for esophagus and esophagogastric junction cancer. Jpn. J. Clin. Oncol. 2020, 50, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.H.; Goldner, W.S.; Benson, A.B.; Bergsland, E.; Blaszkowsky, L.S.; Brock, P.; Chan, J.; Das, S.; Dickson, P.V.; Fanta, P.; et al. Neuroendocrine and Adrenal Tumors, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 839–868. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.C.; Huang, C.W.; Karmakar, R.; Mukundan, A.; Chen, T.H.; Chou, C.K.; Yang, K.Y.; Wang, H.C. Precision Imaging for Early Detection of Esophageal Cancer. Bioengineering 2025, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhao, Y.; Zhou, Y.; Tian, Y.; He, Q.; Lin, J.; Hao, H.; Zou, B.; Jiang, L.; Zhao, G.; et al. Comparison of survival and patterns of recurrence in gastric euroendocrine carcinoma, mixed adenoneuroendocrine carcinoma, and adenocarcinoma. JAMA Netw. Open 2021, 4, e2114180. [Google Scholar] [CrossRef] [PubMed]
- Brathwaite, S.; Rock, J.; Yearsley, M.M.; Bekaii-Saab, T.; Wei, L.; Frankel, W.L.; Hays, J.; Wu, C.; Abdel-Misih, S. Mixed Adeno-neuroendocrine Carcinoma: An Aggressive Clinical Entity. Ann. Surg. Oncol. 2016, 23, 2281–2286. [Google Scholar] [CrossRef]
- Liu, L.; Li, Q.; Liu, W.; Qiu, Z.; Wu, Z.; Yu, D.; Deng, W. Gastric mixed neuroendocrine non-neuroendocrine neoplasms. Front. Oncol. 2024, 14, 1335760. [Google Scholar] [CrossRef]
- Chan, H.; Zhang, L.; Choti, M.A.; Kulke, M.; Yao, J.C.; Nakakura, E.K.; Bloomston, M.; Benson, A.B.; Shah, M.H.; Strosberg, J.R.; et al. Recurrence Patterns after Surgical Resection of Gastroenteropancreatic Neuroendocrine Tumors: Analysis from the National Comprehensive Cancer Network Oncology Outcomes Database. Pancreas 2021, 50, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Sun, L.; Ma, J. Favorable response to PD-1 inhibitor plus chemotherapy as first-line treatment for metastatic gastric mixed neuroendocrine-non-neuroendocrine tumor: A case report. Front. Pharmacol. 2024, 15, 1295134. [Google Scholar] [CrossRef] [PubMed]
- Woo, L.T.; Ding, Y.F.; Mao, C.Y.; Qian, J.; Zhang, X.M.; Xu, N. Long-term survival of gastric mixed neuroendocrine-non-neuroendocrine neoplasm: Two case reports. World J. Clin. Cases 2022, 10, 7936–7943. [Google Scholar] [CrossRef] [PubMed]
- Riccò, B.; Salati, M.; Reggiani Bonetti, L.; Dominici, M.; Luppi, G. PD-1 blockade in deficient mismatch repair mixed adenoneuroendocrine carcinoma of the stomach: New hope for an orphan disease. Tumori 2020, 106, NP57–NP62. [Google Scholar] [CrossRef]
- Lin, Z.; Chen, J.; Guo, Y. Efficacy of XELOX adjuvant chemotherapy for gastric mixed adenoneuroendocrine carcinoma: A case report. Medicine 2019, 98, e16000. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Zhou, Z.; Chen, J.; Di, M.; Ji, J.; Yuan, W.; Liu, Z.; Wu, L.; Zhang, X.; Li, K.; et al. Correlation of metastasis characteristics with prognosis in gastric mixed adenoneuroendocrine carcinoma: Two case reports. Medicine 2017, 96, e9189. [Google Scholar] [CrossRef]
- Kheiri, B.; Osman, M.; Congdon, D.; Bachuwa, G. A rare case of gastric mixed adenoneuroendocrine carcinoma (MANEC) with gastric Helicobacter pylori -negative mucosa-associated lymphoid tissue (MALT) lymphoma. BMJ Case Rep. 2017, 2017, bcr-2017. [Google Scholar] [CrossRef] [PubMed]
- Ambesh, P.; Weissbrot, J.; Ratner, S.; Sinha, A.; Patti, R.; Balderacchi, J.; Marcelin, M.; Wolf, L.; Kamholz, S. Mixed Adenoneuroendocrine Carcinoma of the Gastroesophageal Junction: A Rare Find. J. Investig. Med. High Impact Case Rep. 2017, 5, 2324709617750180. [Google Scholar] [CrossRef] [PubMed]
- Cazzo, E.; Saito, H.P.d.A.d. Adenocarcinoma neuroendócrino misto do coto gástrico após gastrectomia à Billroth II: Relato de caso e revisão de literatura. Sao Paulo Med. J. 2016, 134, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, J.; Shinozaki, K.; Baba, S.; Kurogi, J.; Nakane, T.; Kinoshita, Y.; Ishii, K.; Ueno, T.; Torimura, T.; Yano, H. A resected case of neuroendocrine carcinoma of the stomach with unusual lymph node metastasis. Med. Mol. Morphol. 2016, 49, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Gurzu, S.; Kadar, Z.; Bara, T.J.; Tamasi, A.; Azamfirei, L.; Jung, I. Mixed adenoneuroendocrine carcinoma of gastrointestinal tract: Report of two cases. World J. Gastroenterol. 2015, 21, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Levi Sandri, G.B.; Carboni, F.; Valle, M.; Visca, P.; Garofalo, A. Mixed adenoneuroendocrine gastric carcinoma: A case report and review of the literature. J. Gastric Cancer 2014, 14, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Miguchi, M.; Iseki, M.; Shimatani, K. Advanced gastric neuroendocrine carcinoma with an adenocarcinoma component. Case Rep. Gastroenterol. 2012, 6, 52–57. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, W.; Ma, H.; Sun, M.; Chen, H.; Zheng, S. Neuroendocrine Liver Metastasis in Gastric Mixed Adenoneuroendocrine Carcinoma with Trilineage Cell Differentiation: A Case Report. Int. J. Clin. Exp. Pathol. 2014, 7, 6333. [Google Scholar] [PubMed]
- Kroese, T.E.; Bronzwaer, S.; van Rossum, P.S.; Schoppman, S.F.; Deseyne, P.R.; van Cutsem, E.; Haustermans, K.; Nafteux, P.; Thomas, M.; Obermannova, R.; et al. European clinical practice guidelines for the definition, diagnosis, and treatment of oligometastatic esophagogastric cancer (OMEC-4). Eur. J. Cancer 2024, 20, 114062. [Google Scholar] [CrossRef] [PubMed]
- Pericleous, M.; Toumpanakis, C.; Lumgair, H.; Caplin, M.E.; Morgan-Rowe, L.; Clark, I.; Luong, T.V. Gastric mixed adenoneuroendocrine carcinoma with a trilineage cell differentiation: Case report and review of the literature. Case Rep. Oncol. 2012, 5, 313–319. [Google Scholar] [CrossRef]
- Deliwala, S.S.; Ponnapalli, A.; Gakhal, I.; Modi, V.; Haykal, T.; Bachuwa, G.; Chawla, S. When Variants Collide: An Unusual Presentation of Metastatic Gastric Mixed Neuroendocrine-Non-Neuroendocrine Neoplasm. ACG Case Rep. J. 2021, 8, e00569. [Google Scholar] [CrossRef] [PubMed]
- Millet, C.; Farokhian, A.; Mekheal, N.; Singh, B.; Baddoura, W. Massive Mixed Adenoneuroendocrine Carcinoma: A Case Report. Cureus 2021, 13, e15928. [Google Scholar] [CrossRef] [PubMed]
- Golombek, T.; Henker, R.; Rehak, M.; Quäschling, U.; Lordick, F.; Knödler, M. A Rare Case of Mixed Adenoneuroendocrine Carcinoma (MANEC) of the Gastroesophageal Junction with HER2/neu Overexpression and Distinct Orbital and Optic Nerve Toxicity after Intravenous Administration of Cisplatin. Oncol. Res. Treat. 2019, 42, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.D.; Mori, I.; Osamura, R.Y. A Case Report: Gastric Mixed Neuroendocrine-Nonneuroendocrine Neoplasm with Aggressive Neuroendocrine Component. Case Rep. Pathol. 2017, 201, 9871687. [Google Scholar] [CrossRef]
- Yoon, S.E.; Kim, J.H.; Lee, S.J.; Lee, J.; Park, S.H.; Park, J.O.; Lim, H.Y.; Kang, W.K.; Park, Y.S.; Kim, S.T. The impact of primary tumor site on outcomes of treatment with etoposide and cisplatin in grade 3 gastroenteropancreatic neuroendocrine carcinoma. J. Cancer 2019, 10, 3140–3144. [Google Scholar] [CrossRef] [PubMed]
- Morizane, C.; Machida, N.; Honma, Y.; Okusaka, T.; Boku, N.; Kato, K.; Nomura, S.; Hiraoka, N.; Sekine, S.; Taniguchi, H.; et al. Effectiveness of Etoposide and Cisplatin vs Irinotecan and Cisplatin Therapy for Patients with Advanced Neuroendocrine Carcinoma of the Digestive System: The TOPIC-NEC Phase 3 Randomized Clinical Trial. JAMA Oncol. 2022, 8, 1447–1455. [Google Scholar] [CrossRef]
- Jepsen, D.N.M.; Fiehn, A.-M.K.; Garbyal, R.S.; Engel, U.; Holm, J.; Federspiel, B. Immunohistochemical Staining With Neuroendocrine Markers is Essential in the Diagnosis of Neuroendocrine Neoplasms of the Esophagogastric Junction. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 454–461. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotiropoulou, A.; Avgoustidou, M.; Milionis, V.; Papadimitriou, I.; Vergadis, C.; Schizas, D.; Arkadopoulos, N.; Lyros, O. Oligometastatic Mixed Neuroendocrine Adenocarcinoma of the Esophago-Gastric Junction: A Case of Successful Multidisciplinary Management, the Lessons Learnt and Review of the Literature. J. Clin. Med. 2025, 14, 1503. https://doi.org/10.3390/jcm14051503
Sotiropoulou A, Avgoustidou M, Milionis V, Papadimitriou I, Vergadis C, Schizas D, Arkadopoulos N, Lyros O. Oligometastatic Mixed Neuroendocrine Adenocarcinoma of the Esophago-Gastric Junction: A Case of Successful Multidisciplinary Management, the Lessons Learnt and Review of the Literature. Journal of Clinical Medicine. 2025; 14(5):1503. https://doi.org/10.3390/jcm14051503
Chicago/Turabian StyleSotiropoulou, Anastasia, Maria Avgoustidou, Vassilis Milionis, Ioannis Papadimitriou, Chrysovalantis Vergadis, Dimitrios Schizas, Nikolaos Arkadopoulos, and Orestis Lyros. 2025. "Oligometastatic Mixed Neuroendocrine Adenocarcinoma of the Esophago-Gastric Junction: A Case of Successful Multidisciplinary Management, the Lessons Learnt and Review of the Literature" Journal of Clinical Medicine 14, no. 5: 1503. https://doi.org/10.3390/jcm14051503
APA StyleSotiropoulou, A., Avgoustidou, M., Milionis, V., Papadimitriou, I., Vergadis, C., Schizas, D., Arkadopoulos, N., & Lyros, O. (2025). Oligometastatic Mixed Neuroendocrine Adenocarcinoma of the Esophago-Gastric Junction: A Case of Successful Multidisciplinary Management, the Lessons Learnt and Review of the Literature. Journal of Clinical Medicine, 14(5), 1503. https://doi.org/10.3390/jcm14051503








