Exploring the Association Between Dietary Fruit Intake and Endometriosis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Question
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
- Patient: women with and without endometriosis.Exposure: fruit consumption (daily and weekly quantities).Comparator: women without endometriosis.Outcome: incidence of endometriosis.
2.4. Study Selection
2.5. Data Collection
2.6. Quality Assessment
2.7. Quality of Evidence Assessment
2.8. Meta-Analysis
3. Results
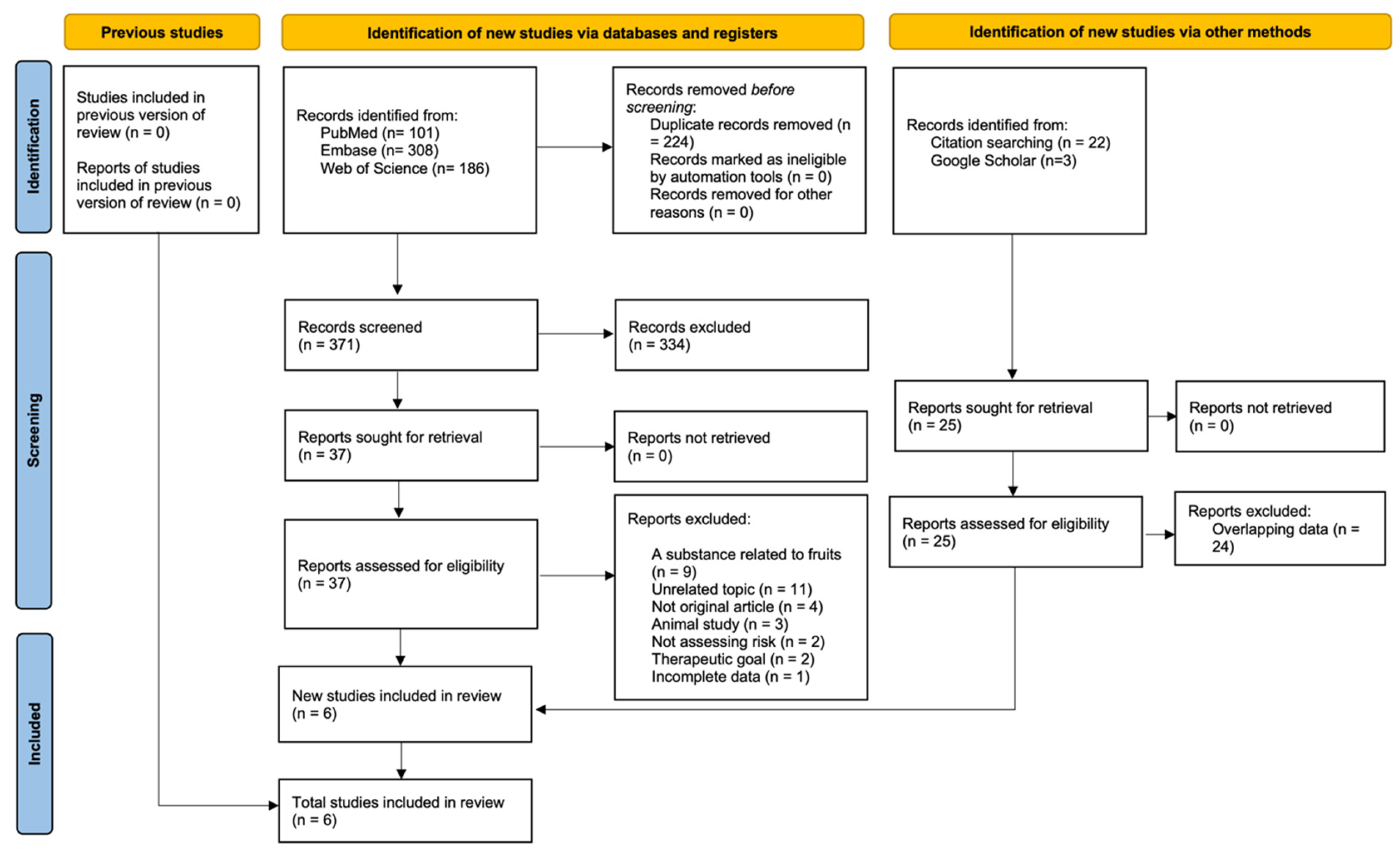
3.1. Search Results
3.2. Study Characteristics
3.3. Review of the Findings
3.4. Quality Assessment
3.5. Quality of Evidence Assessment
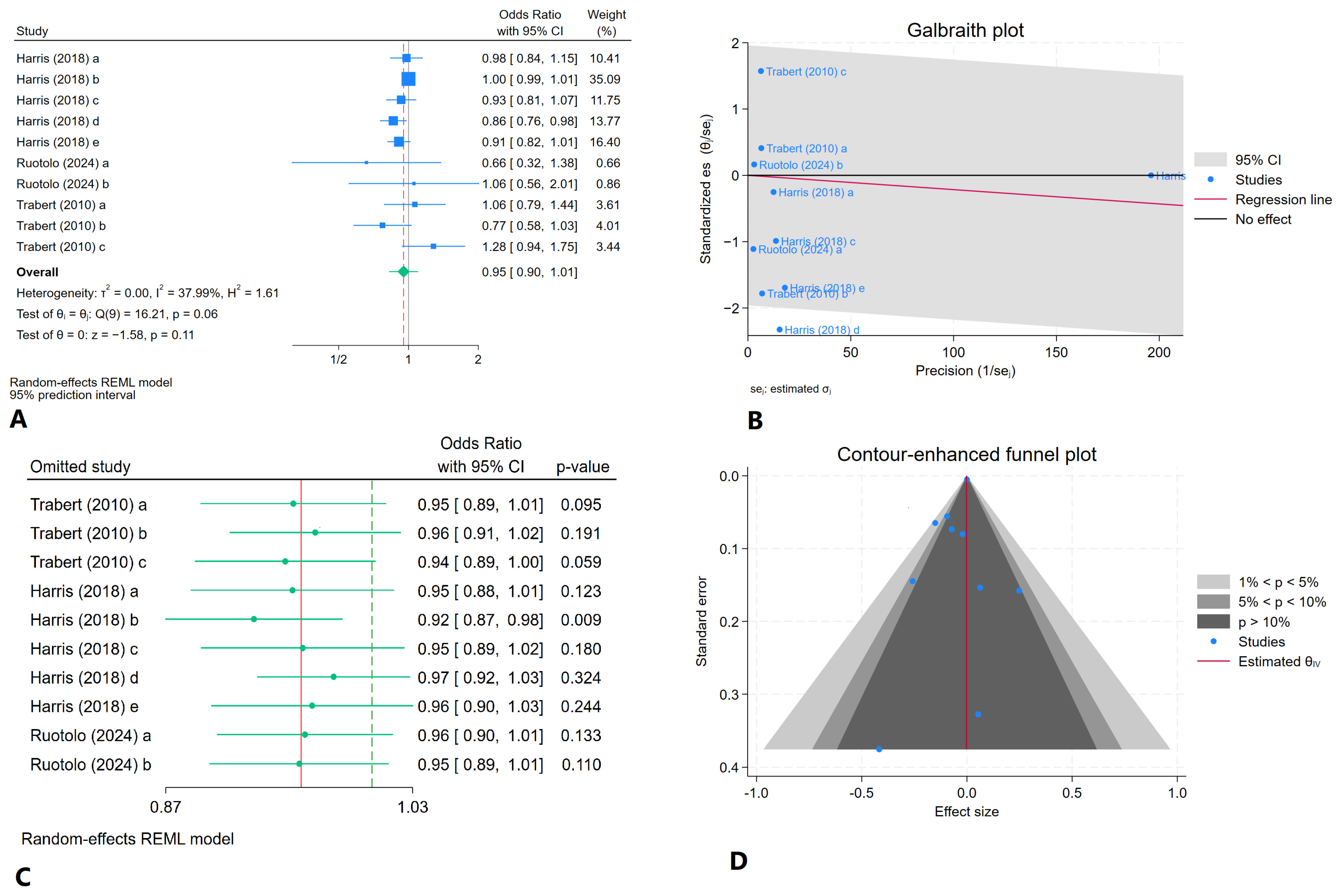
3.6. Meta-Analysis
3.6.1. Comparison of Daily Fruit Consumption in Women with and Without Endometriosis
3.6.2. Comparison of Weekly Fruit Consumption in Women with and Without Endometriosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHEI | Alternative Healthy Eating Index |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| JBI | Joanna Briggs Institute |
| GRADE | Grading of Recommendations, Assessment, Development, and Evaluations |
| OR | Odds Ratio |
| CI | Confidence Interval |
| REML | Restricted Maximum Likelihood |
| NLRP-3 | Nucleotide-binding domain, Leucine-Rich-Repeat-Containing Family, Pyrin Domain-Containing-3 |
| VEGF | Vascular Endothelial Growth Factor |
| TNF-α | Tumor Necrosis Factor alpha |
| NF-kB | Nuclear Factor kappa B |
References
- Parasar, P.; Ozcan, P.; Terry, K.L. Endometriosis: Epidemiology, diagnosis and clinical management. Curr. Obstet. Gynecol. Rep. 2017, 6, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Horne, A.W.; Missmer, S.A. Pathophysiology, diagnosis, and management of endometriosis. BMJ 2022, 379, e070750. [Google Scholar] [CrossRef] [PubMed]
- De Graaff, A.; D’hooghe, T.; Dunselman, G.; Dirksen, C.; Hummelshoj, L.; Consortium, W.E.; Simoens, S.; Bokor, A.; Brandes, I.; Brodszky, V. The significant effect of endometriosis on physical, mental and social wellbeing: Results from an international cross-sectional survey. Hum. Reprod. 2013, 28, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Van Barneveld, E.; Manders, J.; Van Osch, F.H.; Van Poll, M.; Visser, L.; Van Hanegem, N.; Lim, A.C.; Bongers, M.Y.; Leue, C. Depression, anxiety, and correlating factors in endometriosis: A systematic review and meta-analysis. J. Women’s Health 2022, 31, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Lantsberg, D.; Fernando, S.; Cohen, Y.; Rombauts, L. The role of fertility preservation in women with endometriosis: A systematic review. J. Minim. Invasive Gynecol. 2020, 27, 362–372. [Google Scholar] [CrossRef]
- Rashidian, P. An update on oncofertility in prepubertal females. J. Gynecol. Obstet. Hum. Reprod. 2024, 53, 102742. [Google Scholar] [CrossRef]
- Wang, P.-H.; Yang, S.-T.; Chang, W.-H.; Liu, C.-H.; Lee, F.-K.; Lee, W.-L. Endometriosis: Part I. basic concept. Taiwan J. Obstet. Gynecol. 2022, 61, 927–934. [Google Scholar] [CrossRef]
- Dursun, P.; Doğan, N.U.; Ayhan, A. Oncofertility for gynecologic and non-gynecologic cancers: Fertility sparing in young women of reproductive age. Crit. Rev. Oncol./Hematol. 2014, 92, 258–267. [Google Scholar] [CrossRef]
- Ye, L.; Whitaker, L.H.; Mawson, R.L.; Hickey, M. Easily Missed? BMJ 2022, 379, e068950. [Google Scholar] [CrossRef]
- Viganò, D.; Zara, F.; Usai, P. Irritable bowel syndrome and endometriosis: New insights for old diseases. Dig. Liver Dis. 2018, 50, 213–219. [Google Scholar] [CrossRef]
- Zhou, F.; Li, C.; Zhang, S.-Y. NLRP3 inflammasome: A new therapeutic target for high-risk reproductive disorders? Chin. Med. J. 2021, 134, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim Abd El-Fadil Sehsah, F.; Taha Abd El-Fattah, A.; Mohammed Saeed, A. The role of antioxidant supplementation in reducing the endometriosis related chronic pelvic pain in women. Al-Azhar Med. J. 2022, 51, 121–134. [Google Scholar]
- Sinha, A.; Gupta, S. The role of antioxidant supplementation in endometriosis therapy. J. Gynecol. Women’s Health 2017, 3, 555601. [Google Scholar] [CrossRef]
- Mier-Cabrera, J.; Aburto-Soto, T.; Burrola-Méndez, S.; Jiménez-Zamudio, L.; Tolentino, M.C.; Casanueva, E.; Hernández-Guerrero, C. Women with endometriosis improved their peripheral antioxidant markers after the application of a high antioxidant diet. Reprod. Biol. Endocrinol. 2009, 7, 54. [Google Scholar] [CrossRef]
- Harris, H.; Eke, A.; Chavarro, J.; Missmer, S. Fruit and vegetable consumption and risk of endometriosis. Hum. Reprod. 2018, 33, 715–727. [Google Scholar] [CrossRef]
- Dougan, M.M.; Fest, S.; Cushing-Haugen, K.; Farland, L.V.; Chavarro, J.; Harris, H.R.; Missmer, S.A. A prospective study of dietary patterns and the incidence of endometriosis diagnosis. Am. J. Obstet. Gynecol. 2024, 231, 443.e1–443.e10. [Google Scholar] [CrossRef]
- Arab, A.; Karimi, E.; Vingrys, K.; Kelishadi, M.R.; Mehrabani, S.; Askari, G. Food groups and nutrients consumption and risk of endometriosis: A systematic review and meta-analysis of observational studies. Nutr. J. 2022, 21, 58. [Google Scholar] [CrossRef]
- Zaragoza-Martí, A.; Cabrera-González, K.; Martín-Manchado, L.; Moya-Yeste, A.M.; Sánchez-Sansegundo, M.; Hurtado-Sánchez, J.A. The importance of nutrition in the prevention of endometriosis—Systematic review. Nutr. Hosp. 2024, 41, 906–915. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Brooke, B.S.; Schwartz, T.A.; Pawlik, T.M. MOOSE Reporting Guidelines for Meta-analyses of Observational Studies. JAMA Surg. 2021, 156, 787–788. [Google Scholar] [CrossRef]
- Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar]
- Morgan, R.L.; Whaley, P.; Thayer, K.A.; Schünemann, H.J. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ. Int. 2018, 121, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K. Systematic reviews of etiology and risk. In Joanna Briggs Institute Reviewer’s Manual; The Joanna Briggs Institute Adelaide: Adelaide, Australia, 2017; Volume 5, pp. 217–269. [Google Scholar]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Duval, S.; Tweedie, R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar]
- Ashrafi, M.; Jahangiri, N.; Sadatmahalleh, S.J.; Aliani, F.; Akhoond, M. Diet and the risk of endometriosis in Iranian women: A case-control study. Int. J. Fertil. Steril. 2020, 14, 193–200. [Google Scholar] [CrossRef]
- Khan, J.H.; Rahman, S.; Nasreen, C.T.; Alam, J.; Fatema, P. Risk profiles associated with endometriosis among infertile women. Bangladesh J. Obstet. Gynecol. 2020, 33, 131–139. [Google Scholar] [CrossRef]
- Parazzini, F.; Chiaffarino, F.; Surace, M.; Chatenoud, L.; Cipriani, S.; Chiantera, V.; Benzi, G.; Fedele, L. Selected food intake and risk of endometriosis. Hum. Reprod. 2004, 19, 1755–1759. [Google Scholar] [CrossRef]
- Trabert, B.; Peters, U.; De Roos, A.J.; Scholes, D.; Holt, V.L. Diet and risk of endometriosis in a population-based case-control study. Br. J. Nutr. 2010, 105, 459–467. [Google Scholar] [CrossRef]
- Ruotolo, A.; Vannuccini, S.; Capezzuoli, T.; Pampaloni, F.; Cecere, S.; Gallucci, E.; Petraglia, F. Diet characteristics in patients with endometriosis. J. Endometr. Uterine Disord. 2025, 9, 100094. [Google Scholar] [CrossRef]
- Samaneh, Y.; ShahidehJahanian, S.; Azadeh, M.; Anoshirvan, K. The association of food consumption and nutrient intake with endometriosis risk in Iranian women: A case-control study. Int. J. Reprod. Biomed. 2019, 17, 661–670. [Google Scholar] [CrossRef]
- Garcia-Reyes, J.F.; Gilbert-Lopez, B.; Molina-Diaz, A.; Fernandez-Alba, A.R. Determination of pesticide residues in fruit-based soft drinks. Anal. Chem. 2008, 80, 8966–8974. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.; Winter, C. Comparison of pesticide exposure from consumption of domestic and imported fruits and vegetables. Food Chem. Toxicol. 2009, 47, 335–338. [Google Scholar] [CrossRef]
- Morinaga, H.; Yanase, T.; Nomura, M.; Okabe, T.; Goto, K.; Harada, N.; Nawata, H. A benzimidazole fungicide, benomyl, and its metabolite, carbendazim, induce aromatase activity in a human ovarian granulose-like tumor cell line (KGN). Endocrinology 2004, 145, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- Rehnberg, G.L.; Cooper, R.L.; Goldman, J.M.; Gray, L.E.; Hein, J.F.; McElroy, W.K. Serum and testicular testosterone and androgen binding protein profiles following subchronic treatment with carbendazim. Toxicol. Appl. Pharmacol. 1989, 101, 55–61. [Google Scholar] [CrossRef]
- Cummings, A.M.; Metcalf, J.L. Effects of estrogen, progesterone, and methoxychlor on surgically induced endometriosis in rats. Fundam. Appl. Toxicol. 1995, 27, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Lee, J. Sorbitol, Rubus fruit, and misconception. Food Chem. 2015, 166, 616–622. [Google Scholar] [CrossRef]
- Van Dam, R.; Seidell, J. Carbohydrate intake and obesity. Eur. J. Clin. Nutr. 2007, 61, S75–S99. [Google Scholar] [CrossRef]
- Bosy-Westphal, A.; Müller, M.J. Impact of carbohydrates on weight regain. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 389–394. [Google Scholar] [CrossRef]
- Buyken, A.E.; Goletzke, J.; Joslowski, G.; Felbick, A.; Cheng, G.; Herder, C.; Brand-Miller, J.C. Association between carbohydrate quality and inflammatory markers: Systematic review of observational and interventional studies. Am. J. Clin. Nutr. 2014, 99, 813–833. [Google Scholar] [CrossRef]
- Zhang, A.M.; Wellberg, E.A.; Kopp, J.L.; Johnson, J.D. Hyperinsulinemia in obesity, inflammation, and cancer. Diabetes Metab. J. 2021, 45, 285–311. [Google Scholar] [CrossRef]
- Maybin, J.A.; Critchley, H.O.; Jabbour, H.N. Inflammatory pathways in endometrial disorders. Mol. Cell. Endocrinol. 2011, 335, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Arangia, A.; Marino, Y.; Fusco, R.; Siracusa, R.; Cordaro, M.; D’Amico, R.; Macrì, F.; Raffone, E.; Impellizzeri, D.; Cuzzocrea, S.; et al. Fisetin, a Natural Polyphenol, Ameliorates Endometriosis Modulating Mast Cells Derived NLRP-3 Inflammasome Pathway and Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 5076. [Google Scholar] [CrossRef] [PubMed]
- Khodarahmian, M.; Amidi, F.; Moini, A.; Kashani, L.; Salahi, E.; Danaii-mehrabad, S.; Nashtaei, M.S.; Mojtahedi, M.F.; Esfandyari, S.; Sobhani, A. A randomized exploratory trial to assess the effects of resveratrol on VEGF and TNF-α 2 expression in endometriosis women. J. Reprod. Immunol. 2021, 143, 103248. [Google Scholar] [CrossRef] [PubMed]
- Li, L.P.; Luo, Y.; Huang, C.; Wang, X.R.; Huang, T.T.; Zou, Y.Y.; Huang, S.H.; Liu, Y.Q.; Yang, B.C. In Vitro Inhibitory Effects of Maqian Essential Oil against Ectopic Endometrial Stromal Cells and LPS-Induced Endometrial Epithelial Cells. Chem. Biodivers. 2022, 19, e202200756. [Google Scholar] [CrossRef]
- Song, J.; Ham, J.; Park, S.; Park, S.J.; Kim, H.S.; Song, G.; Lim, W. Alpinumisoflavone Activates Disruption of Calcium Homeostasis, Mitochondria and Autophagosome to Suppress Development of Endometriosis. Antioxidants 2023, 12, 1324. [Google Scholar] [CrossRef]
- Taniguchi, F.; Tagashira, Y.; Suou, K.; Iwabe, T.; Harada, T. Apigenin inhibits TNFα-induced cell proliferation in endometriotic stromal cells. Fertil. Steril. 2009, 92, S11. [Google Scholar] [CrossRef]
- Woo, J.H.; Jang, D.S.; Choi, J.H. Luteolin promotes apoptosis of endometriotic cells and inhibits the alternative activation of endometriosis-associated macrophages. Biomol. Ther. 2021, 29, 678–684. [Google Scholar] [CrossRef]
- Youseflu, S.; Jahanian Sadatmahalleh, S.H.; Mottaghi, A.; Kazemnejad, A. Dietary Phytoestrogen Intake and The Risk of Endometriosis in Iranian Women: A Case-Control Study. Int. J. Fertil. Steril. 2020, 13, 296–300. [Google Scholar] [CrossRef]
- Youseflu, S.; Sadatmahalleh, S.J.; Mottaghi, A.; Kazemnejad, A. Evaluation of the role of dietary flavonoid intake in the risk of endometriosis among Iranian women. Iran. J. Obstet. Gynecol. Infertil. 2019, 22, 68–75. [Google Scholar]
- Mihal, M.; Roychoudhury, S.; Sirotkin, A.V.; Kolesarova, A. Sea buckthorn, its bioactive constituents, and mechanism of action: Potential application in female reproduction. Front. Endocrinol. 2023, 14, 1244300. [Google Scholar] [CrossRef]
- Erten, O.U.; Ensari, T.A.; Dilbaz, B.; Cakiroglu, H.; Altinbas, S.K.; Çaydere, M.; Goktolga, U. Vitamin C is effective for the prevention and regression of endometriotic implants in an experimentally induced rat model of endometriosis. Taiwan. J. Obstet. Gynecol. 2016, 55, 251–257. [Google Scholar] [CrossRef]
- Sauqi, H.; Santoso, B.; Widjiati, W.; Hendarto, H. Red fruit (Pandanus conoideus) inhibits the development of endometriosis lesions through downregulation of NF-kB and VEGF expression. Rawal Med. J. 2020, 45, 985–989. [Google Scholar]
- Park, S.; Lim, W.; Song, G. Delphinidin induces antiproliferation and apoptosis of endometrial cells by regulating cytosolic calcium levels and mitochondrial membrane potential depolarization. J. Cell. Biochem. 2019, 120, 5072–5084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Huang, T.T.; Li, L.P.; Liu, D.P.; Luo, Y.; Lu, W.; Huang, N.; Ma, P.P.; Liu, Y.Q.; Zhang, P.; et al. ITRAQ-based proteomics analysis of human ectopic endometrial stromal cells treated by Maqian essential oil. BMC Complement. Med. Ther. 2023, 23, 427. [Google Scholar] [CrossRef]
- Schwartz, N.R.M.; Afeiche, M.C.; Terry, K.L.; Farland, L.V.; Chavarro, J.E.; Missmer, S.A.; Harris, H.R. Glycemic Index, Glycemic Load, Fiber, and Gluten Intake and Risk of Laparoscopically Confirmed Endometriosis in Premenopausal Women. J. Nutr. 2022, 152, 2088–2096. [Google Scholar] [CrossRef]
- Bogusz, A.; Górnicka, M. Low Diet Quality and Nutritional Knowledge in Women with Endometriosis: A Pilot Study. Healthcare 2024, 12, 673. [Google Scholar] [CrossRef]
- Mazza, E.; Troiano, E.; Mazza, S.; Ferro, Y.; Abbinante, A.; Agneta, M.T.; Montalcini, T.; Pujia, A. The impact of endometriosis on dietary choices and activities of everyday life: A cross-sectional study. Front. Nutr. 2023, 10, 1273976. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, Q.; Chen, L. Dietary factors and risk for endometriosis: A Mendelian randomization analysis. Res. Sq. 2024. [Google Scholar] [CrossRef]

| Study | Country | Design | Confirmation of Endometriosis | Mean Age ± SD (Range); y | Number of Women with Endometriosis | Number of Women Without Endometriosis | Fruit Consumption in Woman with Endometriosis | Fruit Consumption in Woman Without Endometriosis | Main Findings on Fruit Consumption and Endometriosis Risk | Study Quality (JBI Tool) |
|---|---|---|---|---|---|---|---|---|---|---|
| Parazzini (2004) [29] | Italy | CC | Laparoscopy | case: (20–65) control: (20–61) | 503 | 504 |
|
| Inverse association | LOW ROB |
| Trabert (2010) [30] | USA | CC | N/A | (18–49) | 284 | 660 |
|
| Positive association | Moderate ROB |
| Harris (2018) [15] | USA | PC | Laparoscopy | N/A | 2609 | - |
| - | Inverse association | Moderate ROB |
| Ashrafi (2020) [27] | Iran | CC | Laparoscopy | case: 31.50 ± 5.52 control: 29.35 ± 7.00 | 207 | 206 |
|
| Inverse association | LOW ROB |
| Khan (2020) [28] | Bangladesh | CC | Laparoscopy and/or laparotomy and/or sonography | N/A | 6 | 13 |
|
| No association | LOW ROB |
| Ruotolo (2024) [31] | Italy | CS | Transvaginal sonography and/or MRI | 33 ± 4.5 | 80 | 80 |
|
| No association | LOW ROB |
| Quality Assessment | Quality | ||||||
|---|---|---|---|---|---|---|---|
| No of Studies | Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | |
| Daily | |||||||
| 10 | observational studies | serious | no serious inconsistency | no serious indirectness | no serious imprecision | none | VERY LOW |
| Weekly | |||||||
| 10 | observational studies | serious | serious | no serious indirectness | no serious imprecision | none | VERY LOW |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashidian, P.; Amini-Salehi, E.; Karami, S.; Nezhat, C.; Nezhat, F. Exploring the Association Between Dietary Fruit Intake and Endometriosis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 1246. https://doi.org/10.3390/jcm14041246
Rashidian P, Amini-Salehi E, Karami S, Nezhat C, Nezhat F. Exploring the Association Between Dietary Fruit Intake and Endometriosis: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(4):1246. https://doi.org/10.3390/jcm14041246
Chicago/Turabian StyleRashidian, Pegah, Ehsan Amini-Salehi, Shaghayegh Karami, Camran Nezhat, and Farr Nezhat. 2025. "Exploring the Association Between Dietary Fruit Intake and Endometriosis: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 4: 1246. https://doi.org/10.3390/jcm14041246
APA StyleRashidian, P., Amini-Salehi, E., Karami, S., Nezhat, C., & Nezhat, F. (2025). Exploring the Association Between Dietary Fruit Intake and Endometriosis: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(4), 1246. https://doi.org/10.3390/jcm14041246





