Bone Densitometry Parameters in Females with Ehlers-Danlos Syndrome—Does the Hypermobile Subtype Increase the Risk of Low Bone Mass in Patients with Ehlers-Danlos Syndrome?
Abstract
1. Introduction
2. Material and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scicluna, K.; Formosa, M.M.; Farrugia, R.; Borg, I. Hypermobile Ehlers-Danlos syndrome: A review and a critical appraisal of published genetic research to date. Clin. Genet. 2022, 101, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Concolino, P.; Falhammar, H. CAH-X Syndrome: Genetic and Clinical Profile. Mol. Diagn. Ther. 2022, 26, 293–300. [Google Scholar] [CrossRef]
- Coussens, M.; Banica, T.; Lapauw, B.; De Wandele, I.; Rombaut, L.; Malfait, F.; Calders, P. Bone parameters in hypermobile Ehlers-Danlos syndrome and hypermobility spectrum disorder: A comparative cross-sectional study. Bone 2023, 166, 116583. [Google Scholar] [CrossRef]
- Available online: https://www.orpha.net/ (accessed on 14 September 2024).
- Demmler, J.C.; Atkinson, M.D.; Reinhold, E.J.; Choy, E.; Lyons, R.A.; Brophy, S.T. Diagnosed prevalence of Ehlers-Danlos syndrome and hypermobility spectrum disorder in Wales, UK: A national electronic cohort study and case-control comparison. BMJ Open 2019, 9, e031365. [Google Scholar] [CrossRef] [PubMed]
- Gensemer, C.; Burks, R.; Kautz, S.; Judge, D.P.; Lavallee, M.; Norris, R.A. Hypermobile Ehlers-Danlos syndromes: Complex phenotypes, challenging diagnoses, and poorly understood causes. Dev. Dyn. 2021, 250, 318–344. [Google Scholar] [CrossRef]
- Tinkle, B.; Castori, M.; Berglund, B.; Cohen, H.; Grahame, R.; Kazkaz, H. Hypermobile Ehlers-Danlos syndrome (a.k.a. Ehlers-Danlos syndrome type III and Ehlers-Danlos syndrome hypermobility type): Clinical description and natural history. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 48–69. [Google Scholar] [CrossRef] [PubMed]
- Fukada, T.; Civic, N.; Furuichi, T.; Shimoda, S.; Mishima, K.; Higashiyama, H.; Idaira, Y.; Asada, Y.; Kitamura, H.; Yamasaki, S.; et al. The zinc transporter SLC39A13/ZIP13 is required for connective tissue development; its involvement in BMP/TGF-beta signaling pathways. PLoS ONE 2008, 3, e3642. [Google Scholar] [CrossRef]
- Galli, G.G.; Honnens de Lichtenberg, K.; Carrara, M.; Hans, W.; Wuelling, M.; Mentz, B.; Multhaupt, H.A.; Fog, C.K.; Jensen, K.T.; Rappsilber, J.; et al. Prdm5 regulates collagen gene transcription by association with RNA polymerase II in developing bone. PLoS Genet. 2012, 8, e1002711. [Google Scholar] [CrossRef]
- Nielsen, R.H.; Couppé, C.; Jensen, J.K.; Olsen, M.R.; Heinemeier, K.M.; Malfait, F.; Symoens, S.; De Paepe, A.; Schjerling, P.; Magnusson, S.P.; et al. Low tendon stiffness and abnormal ultrastructure distinguish classic Ehlers-Danlos syndrome from benign joint hypermobility syndrome in patients. FASEB J. 2014, 28, 4668–4676. [Google Scholar] [CrossRef] [PubMed]
- Rombaut, L.; Malfait, F.; De Wandele, I.; Mahieu, N.; Thijs, Y.; Segers, P.; De Paepe, A.; Calders, P. Muscle-tendon tissue properties in the hypermobility type of Ehlers-Danlos syndrome. Arthritis Care Res. 2012, 64, 766–772. [Google Scholar] [CrossRef]
- Mazziotti, G.; Dordoni, C.; Doga, M.; Galderisi, F.; Venturini, M.; Calzavara-Pinton, P.; Maroldi, R.; Giustina, A.; Colombi, M. High prevalence of radiological vertebral fractures in adult patients with Ehlers-Danlos syndrome. Bone 2016, 84, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Dolan, A.L.; Arden, N.K.; Grahame, R.; Spector, T.D. Assessment of bone in Ehlers Danlos syndrome by ultrasound and densitometry. Ann. Rheum. Dis. 1998, 57, 630–633. [Google Scholar] [CrossRef]
- Eller-Vainicher, C.; Bassotti, A.; Imeraj, A.; Cairoli, E.; Ulivieri, F.M.; Cortini, F.; Dubini, M.; Marinelli, B.; Spada, A.; Chiodini, I. Bone involvement in adult patients affected with Ehlers-Danlos syndrome. Osteoporos. Int. 2016, 27, 2525–2531. [Google Scholar] [CrossRef] [PubMed]
- Yew, K.S.; Kamps-Schmitt, K.A.; Borge, R. Hypermobile Ehlers-Danlos syndrome and Hypermobility Spectrum Disorders. Am. Fam. Physician 2021, 103, 481–492. [Google Scholar]
- Available online: www.ehlers-danlos.com/wp-content/uploads/hEDS-Dx-Criteria-checklist-1.pdf (accessed on 15 September 2024).
- Available online: https://m.facebook.com/StowarzyszenieEhlersDanlosPolska/ (accessed on 15 September 2024).
- Carbone, L.; Tylavsky, F.A.; Bush, A.J.; Koo, W.; Orwoll, E.; Cheng, S. Bone density in Ehlers-Danlos syndrome. Osteoporos. Int. 2000, 11, 388–392. [Google Scholar] [CrossRef]
- Banica, T.; Coussens, M.; Verroken, C.; Calders, P.; de Wandele, I.; Malfait, F. Higher fracture prevalence and smaller bone size in patients with hEDS/HSD-a-prospective cohort study. Osteoporos. Int. 2020, 31, 849–856. [Google Scholar] [CrossRef]
- Basalom, S.; Rauch, F. Bone Disease in Patients with Ehlers-Danlos Syndromes. Curr. Osteoporos. Rep. 2020, 18, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Yue, F.; Era, T.; Yamaguchi, T.; Kosho, T. Pathophysiological Investigation of Skeletal Deformities of Musculocontractural Ehlers-Danlos Syndrome Using Induced Pluripotent Stem Cells. Genes 2023, 14, 730. [Google Scholar] [CrossRef]
- Love, A.N.; Palmer, B. Presentation and Management of a Novel Ehlers-Danlos COL5A1 Variant With Birt-Hogg-Dube Syndrome: A Case Study. Cureus 2023, 15, e35866. [Google Scholar] [CrossRef] [PubMed]
- Scheper, M.C.; Nicholson, L.L.; Adams, R.D.; Tofts, L.; Pacey, V. The natural history of children with joint hypermobility syndrome and Ehlers-Danlos hypermobility type: A longitudinal cohort study. Rheumatology 2017, 56, 2073–2083. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, J.V.; Herbland, A.; Hakim, A.; Ninis, N.; Lever, W.; Aziz, Q.; Cairins, M. Exercise beliefs and behaviours of individuals with joint hypermobility syndrome/Ehlers-Danlose syndrome—Hypermobility type. Disabil. Rehabil. 2019, 41, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Robbins, S.M.; Cossette-Levasseur, M.; Kikuchi, K.; Sarjeant, J.; Shiu, Y.G.; Azar, C.; Hazel, E.M. Neuromuscular activation differences during gait in patients with Ehlers-Danlos syndrome and healthy adults. Arthritis Care Res. 2020, 72, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, R.H.; Jensen, J.K.; Voermans, N.C.; Heinemeier, K.M.; Schjerling, P.; Holm, L.; Agergaard, J.; Mackey, A.L.; Andersen, J.L.; Remvig, L.; et al. Skeletal muscle morphology, protein synthesis, and gene expression in Ehlers-Danlose syndrome. J. Appl. Physiol. 2017, 123, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Stern, C.M.; Pepin, M.J.; Stoler, J.M.; Kramer, D.E.; Spencer, S.A.; Stein, C.J. Musculoskeletal conditions in a pediatric population with Ehlers-Danlos syndrome. J. Pediatr. 2017, 181, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Chopra, P.; Tinkle, B.; Hamonet, C.; Brock, I.; Gompel, A.; Bulbena, A.; Francomano, C. Pain management in the Ehlers-Danlos syndromes. Am. J. Med. Genet. Part C Semin. Med. Genet. 2017, 175, 212–219. [Google Scholar] [CrossRef]
- Ericson, W.B.; Wolman, R. Orthopaedic management of the Ehlers-Danlos syndromes. Am. J. Med. Genet. Part C Semin. Med. Genet. 2017, 175, 188–194. [Google Scholar] [CrossRef] [PubMed]
- McKeon, K.E.; London, D.A.; Osei, D.A.; Gelberman, R.H.; Goldfarb, C.h.A.; Boyer, M.I.; Calfee, R.P. Ligamentous hyperlaxity and dorsal wrist ganglions. J. Hand Surg. Am. 2013, 38, 2138–2143. [Google Scholar] [CrossRef] [PubMed]
- Castori, M.; Camerota, F.; Celletti, C.; Danese Ch Santilli, V.; Saraceni, V.M.; Grammatico, P. Natural history and manifestations of the hypermobility type Ehlers-Danlos syndrome: A pilot study on 21 patients. Am. J. Med. Genet. Part A 2010, 152A, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Malfait, F.; Francomano, C.; Byers, P.; Francomano, C.; Tinkle, B.; Malfait, F. The 2017 international classification of the Ehlers-Danlos syndromes. Am. J. Med. Genet. Part C Semin. Med. Genet. 2017, 175, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Hakim, A.J.; Grahame, R. A simple questionnaire to detect hypermobility: An adjunct to the assessment of patients with diffuse musculoskeletal pain. Int. J. Clin. Pract. 2003, 57, 163–166. [Google Scholar] [CrossRef] [PubMed]
- De Wandele, I.; Rombaut, L.; Malfait, F.; De Backer, T.; De Paepe, A.; Calders, P. Clinical heterogeneity in patients with the hypermobility type of Ehlers-Danlos syndrome. Res. Dev. Disabil. 2013, 34, 873–881. [Google Scholar] [CrossRef] [PubMed]
- McGillis, L.; Mittal, N.; Mina, D.S.; So, J.; Soowamber, M.; Weinrib, A.; Soever, L.; Rozenberg, D.; Liu, L.; Tse, Y.; et al. Utilization of the 2017 diagnostic criteria for hEDS by the Toronto GoodHope Ehlers-Danlos syndrome clinic: A retrospective review. Am. J. Med. Genet. Part A 2020, 182, 484–492. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Group 1 (N = 9) | Group 2 (N = 21) | Significance of the Difference (p-Value) |
|---|---|---|---|
| Left femur neck BMD [g/cm2] | 0.919 ± 0.133 | 0.947 ± 0.118 | 0.824 |
| Left femur neck age-matched BMD [%] | 92.875 ± 13.432 | 94 ± 11.369 | 0.979 |
| Left femur upper neck BMD [g/cm2] | 0.75 ± 0.2 | 0.814 ± 0.144 | 0.572 |
| Left femur upper neck age-matched BMD [%] | 92.333 ± 24.799 | 99.476 ± 17.394 | 0.734 |
| Left femur lower neck BMD [g/cm2] | 1.066 ± 0.139 | 1.075 ± 0.127 | 0.928 |
| Left femur Ward’s triangle BMD [g/cm2] | 0.781 ± 0.119 | 0.795 ± 0.148 | 0.751 |
| Left femur Ward’s triangle age-matched BMD [%] | 88.111 ± 10.925 | 88.286 ± 15.153 | 0.964 |
| Left femur trochanter BMD [g/cm2] | 0.726 ± 0.126 | 0.725 ± 0.125 | 0.982 |
| Left femur trochanter age-matched BMD [%] | 86.222 ± 12.143 | 85.333 ± 12.055 | 0.803 |
| Left femur shaft BMD [g/cm2] | 1.146 ± 0.138 | 12.055 ± 0.167 | 0.635 |
| Left femur total BMD [g/cm2] | 0.956 ± 0.122 | 0.948 ± 0.136 | 0.965 |
| Left femur total age-matched BMD [%] | 95.333 ± 9.152 | 95.048 ± 12.002 | 0.909 |
| L1–L4 BMD [g/cm2] | 1.218 ± 0.212 | 1.261 ± 0.181 | 0.476 |
| L1–L4 age-matched BMD [%] | 101.333 ± 14.361 | 104.1 ± 10.004 | 0.570 |
| L1 BMD [g/cm2] | 1.121 ± 0.176 | 1.189 ± 0.218 | 0.469 |
| L1 age-matched BMD [%] | 97.444 ± 12.4 | 101.55 ± 10.928 | 0.369 |
| L2 BMD [g/cm2] | 1.199 ± 0.252 | 1.262 ± 0.175 | 0.222 |
| L3 age-matched BMD [%] | 98 ± 16.256 | 103 ± 11.272 | 0.257 |
| L3 BMD [g/cm2] | 1.261 ± 0.233 | 1.324 ± 0.184 | 0.175 |
| L3 age-matched BMD [%] | 103.111 ± 15.004 | 107.45 ± 9.506 | 0.310 |
| L4 BMD [g/cm2] | 1.262 ± 0.233 | 1.259 ± 0.181 | 0.928 |
| L4 age-matched BMD [%] | 103.667 ± 17.493 | 102.45 ± 10.899 | 0.887 |
| L1–L2 BMD [g/cm2] | 1.162 ± 0.212 | 1.227 ± 0.189 | 0.319 |
| L1–L2 age-matched BMD [%] | 97.778 ± 14.087 | 102.5 ± 11.009 | 0.408 |
| L1–L3 BMD [g/cm2] | 1.197 ± 0.217 | 1.262 ± 0.186 | 0.205 |
| L1–L3 age-matched BMD [%] | 100.444 ± 14.293 | 105 ± 10.126 | 0.321 |
| L2–L3 BMD [g/cm2] | 1.232 ± 0.239 | 1.294 ± 0.176 | 0.124 |
| L2–L3 age-matched BMD [%] | 100.778 ± 15.352 | 105.35 ± 9.99 | 0.228 |
| L2–L4 BMD [g/cm2] | 1.245 ± 0.228 | 1.28 ± 0.175 | 0.469 |
| L2–L4 age-matched BMD [%] | 101.889 ± 15.536 | 104.25 ± 10.02 | 0.637 |
| L3–L4 BMD [g/cm2] | 1.263 ± 0.225 | 1.289 ± 0.18 | 0.556 |
| L3–L4 age-matched BMD [%] | 103.667 ± 15.716 | 104.65 ± 9.832 | 0.869 |
| Characteristics | Group 1 (N = 9) | Group 2 (N = 21) | Significance of the Difference (p-Value) |
|---|---|---|---|
| Age [years] | 39.8 ± 8.3 | 33.5 ± 9.0 | 0.045 |
| Body weight [kg] | 74 ± 20.3 | 68 ± 17.4 | 0.283 |
| BMI [kg/m2] | 26.7 ± 6.6 | 25.3 ± 6.6 | 0.348 |
| Total calcium [mmol/L] | 2.403 ± 0.106 | 2.399 ± 0.074 | 0.756 |
| Inorganic phosphate [mg/dL] | 3.642 ± 0.958 | 3.406 ± 0.443 | 0.449 |
| PTH [pg/mL] | 50.522 ± 16.4 | 39.552 ± 13.679 | 0.064 |
| 25-OH vitamin D [ng/mL] | 22.489 ± 10.144 | 34.076 ± 18.011 | 0.022 |
| Alkaline phosphatase [µg/L] | 10.7 ± 2.46 | 9.029 ± 2.672 | 0.077 |
| CTx [ng/mL] | 0.379 ± 0.209 | 0.395 ± 0.181 | 0.789 |
| Osteocalcin [ng/mL] | 19.4 ± 7.191 | 21.081 ± 7.597 | 0.449 |
| Sodium [mmol/L] | 140.889 ± 2.759 | 141.619 ± 2.085 | 0.563 |
| Potassium [mmol/L] | 4.766 ± 0.301 | 4.599 ± 0.403 | 0.193 |
| TSH [µIU/mL] | 1.946 ± 0.975 | 2.187 ± 1.526 | 0.929 |
| DHEA-S [µg/dL] | 194.278 ± 85.494 | 217.086 ± 103.734 | 0.624 |
| Cortisol [µg/dL] | 14.756 ± 4.231 | 15.243 ± 6.63 | 0.859 |
| History of fractures | 4 (44.44%) | 13 (61.905%) | 0.476 |
| Variable | Odds Ratio, OR (95% Cl) | Significance of the Difference (p-Value) |
|---|---|---|
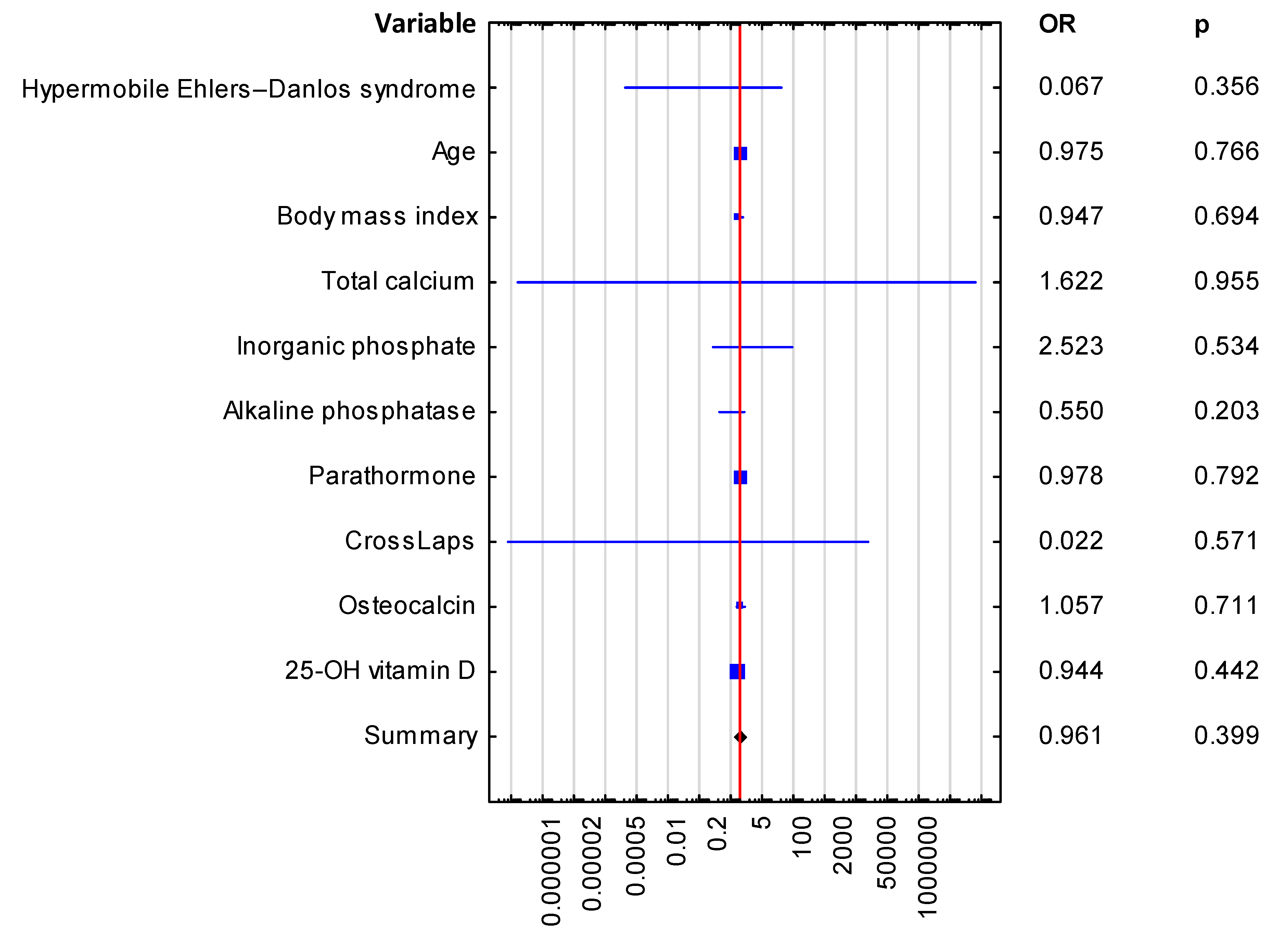
| Hypermobile Ehlers-Danlos syndrome | 0.067 (0.0–20.927) | 0.356 |
| Age | 0.975 (0.823–1.155) | 0.766 |
| Body mass index | 0.947 (0.724–1.24) | 0.694 |
| Total calcium | 1.622 (0.0–32,902,246.74) | 0.955 |
| Inorganic phosphate | 2.523 (0.137–46.529) | 0.534 |
| Alkaline phosphatase | 0.550 (0.219–1.38) | 0.203 |
| Parathormone | 0.978 (0.828–1.155) | 0.792 |
| CrossLaps (CTx) | 0.022 (0.0–12,177.508) | 0.571 |
| Osteocalcin | 1.057 (0.789–1.417) | 0.711 |
| 25-OH vitamin D | 0.944 (0.816–1.093) | 0.442 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kałuża, B.; Rychlik, I.; Domański, J.; Żuk-Łapan, A.; Babula, E.; Poprawa, I.; Podstawka, J.; Kowalów, E.; Franek, E. Bone Densitometry Parameters in Females with Ehlers-Danlos Syndrome—Does the Hypermobile Subtype Increase the Risk of Low Bone Mass in Patients with Ehlers-Danlos Syndrome? J. Clin. Med. 2025, 14, 941. https://doi.org/10.3390/jcm14030941
Kałuża B, Rychlik I, Domański J, Żuk-Łapan A, Babula E, Poprawa I, Podstawka J, Kowalów E, Franek E. Bone Densitometry Parameters in Females with Ehlers-Danlos Syndrome—Does the Hypermobile Subtype Increase the Risk of Low Bone Mass in Patients with Ehlers-Danlos Syndrome? Journal of Clinical Medicine. 2025; 14(3):941. https://doi.org/10.3390/jcm14030941
Chicago/Turabian StyleKałuża, Bernadetta, Ivan Rychlik, Jan Domański, Aleksandra Żuk-Łapan, Emilia Babula, Iga Poprawa, Jakub Podstawka, Ewa Kowalów, and Edward Franek. 2025. "Bone Densitometry Parameters in Females with Ehlers-Danlos Syndrome—Does the Hypermobile Subtype Increase the Risk of Low Bone Mass in Patients with Ehlers-Danlos Syndrome?" Journal of Clinical Medicine 14, no. 3: 941. https://doi.org/10.3390/jcm14030941
APA StyleKałuża, B., Rychlik, I., Domański, J., Żuk-Łapan, A., Babula, E., Poprawa, I., Podstawka, J., Kowalów, E., & Franek, E. (2025). Bone Densitometry Parameters in Females with Ehlers-Danlos Syndrome—Does the Hypermobile Subtype Increase the Risk of Low Bone Mass in Patients with Ehlers-Danlos Syndrome? Journal of Clinical Medicine, 14(3), 941. https://doi.org/10.3390/jcm14030941







