Vancomycin Drug Reaction with Eosinophilia and Systemic Symptoms: Meta-Analysis and Pharmacovigilance Study
Abstract
1. Introduction
2. Methods
2.1. Meta-Analysis of Proportions
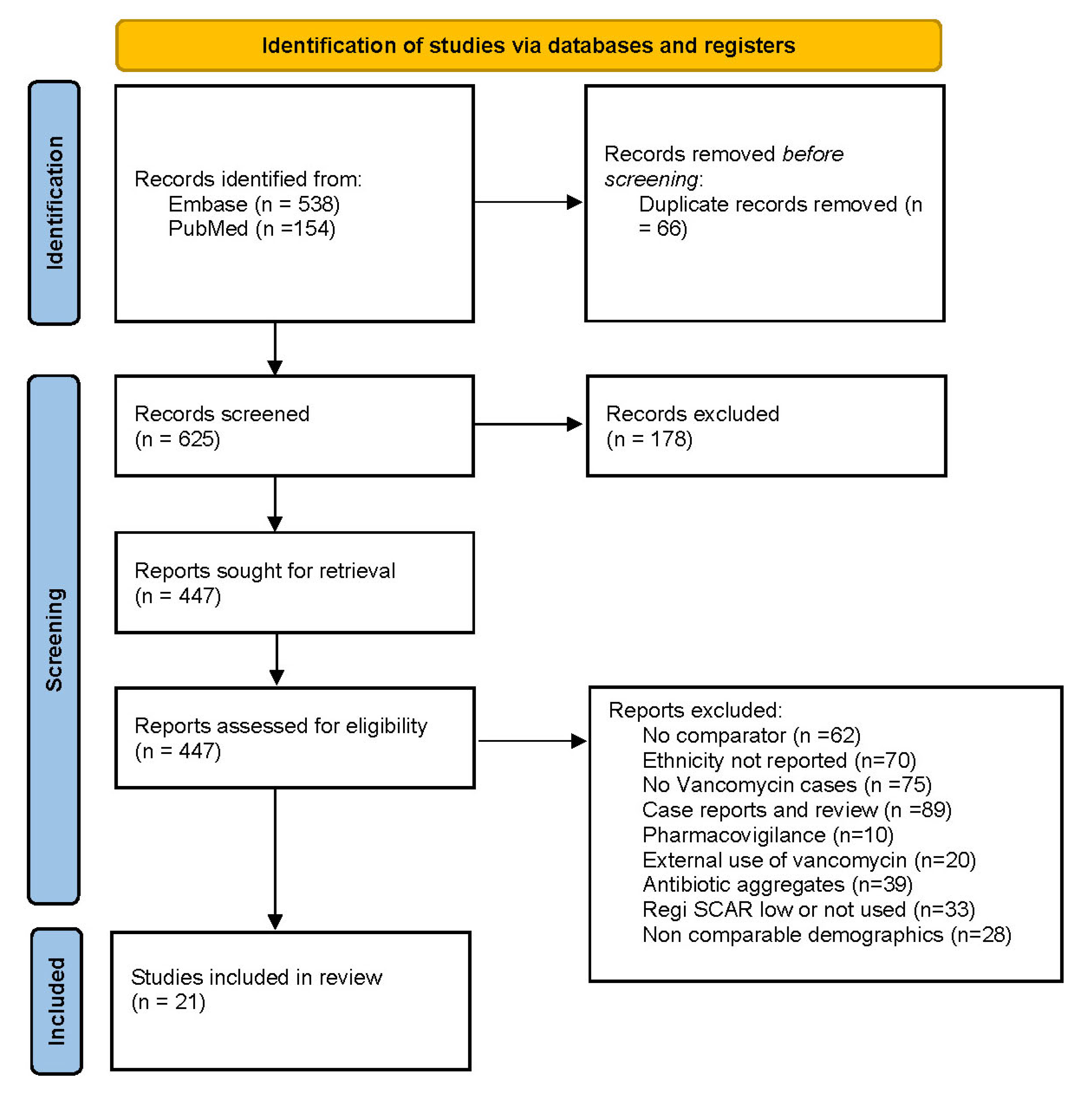
2.1.1. Data Sources and Searches
2.1.2. Study Selection
2.1.3. Data Extraction and Quality Assessment
2.2. Pharmacovigilance Analysis
3. Results
3.1. Characteristics of Observational Studies
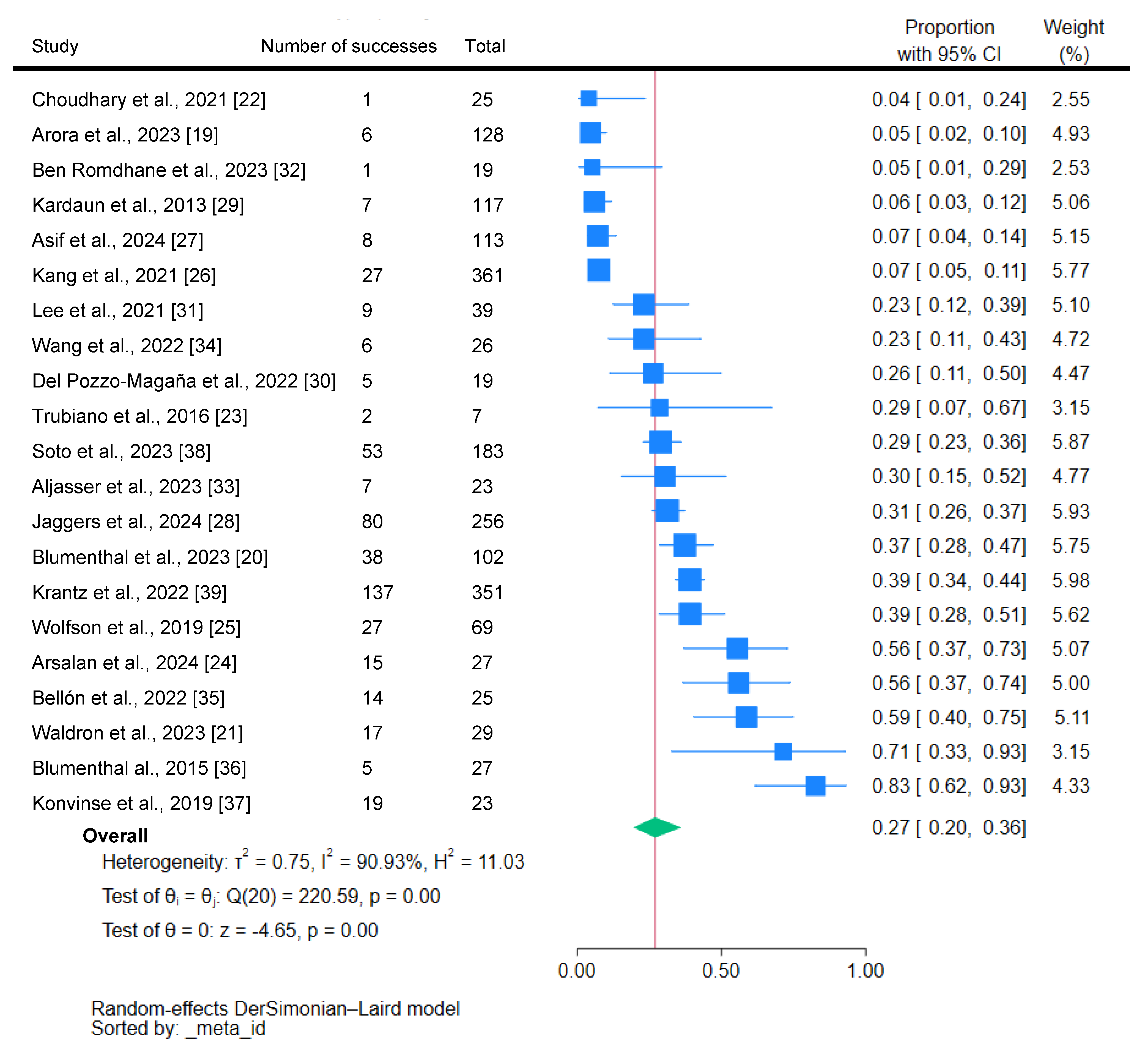
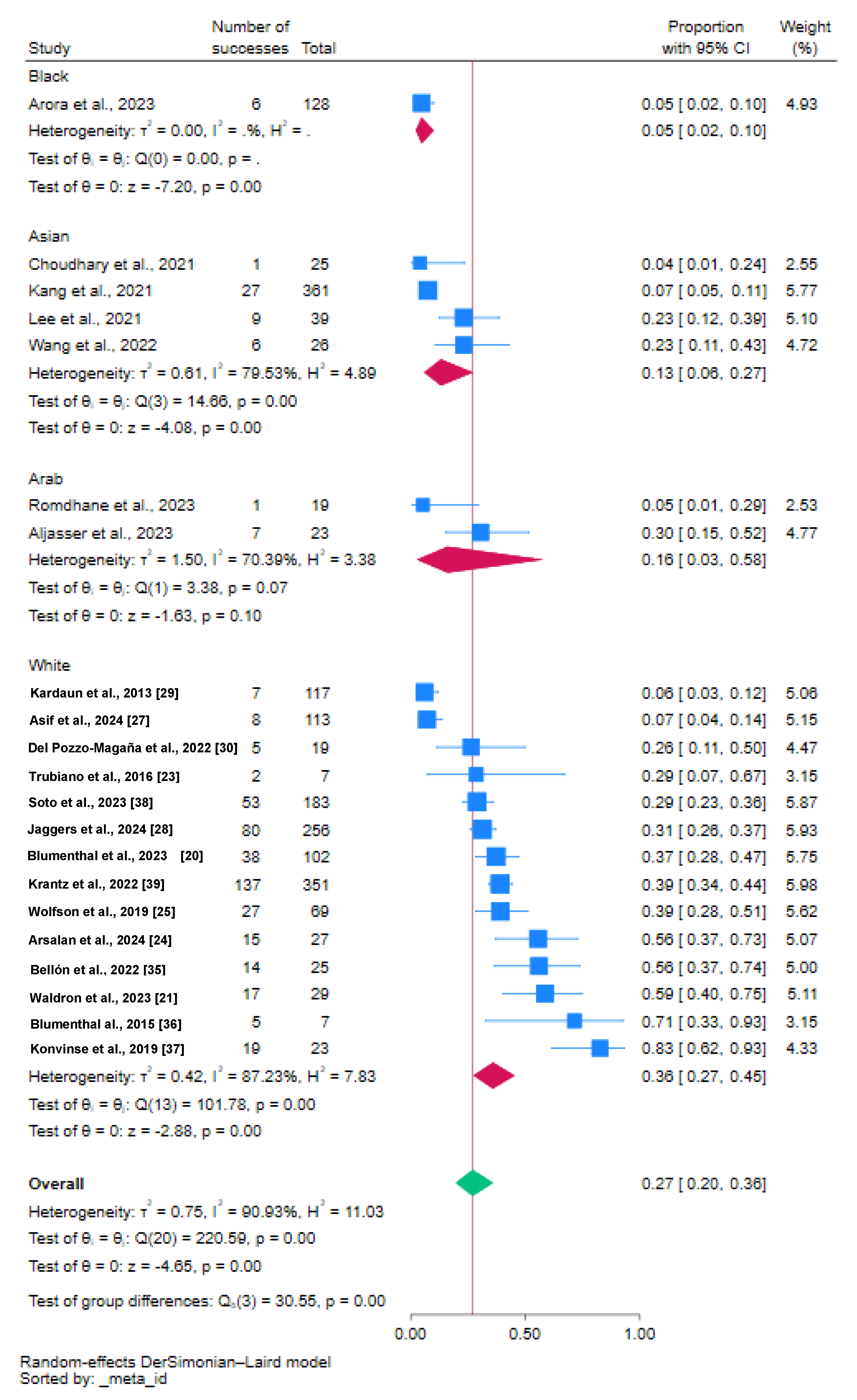
3.2. Proportion of Vancomycin-Induced DRESS
3.2.1. Quality Assessment
3.2.2. Sensitivity Analysis
3.2.3. Publication Bias
3.3. Beta Regression Analysis
3.4. FAERS Disproportionality Analysis
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, H.Y.; Walsh, S.; Creamer, D. Initial presentation of DRESS: Often misdiagnosed as infections. Arch. Dermatol. 2012, 148, 1085–1087. [Google Scholar] [CrossRef] [PubMed]
- England Owen, C.; Stratman, E.J. Failure to recognize and manage patients with DRESS. Arch. Dermatol. 2010, 146, 1379. [Google Scholar] [CrossRef] [PubMed]
- Wattanachai, P.; Amornpinyo, W.; Konyoung, P.; Purimart, D.; Khunarkornsiri, U.; Pattanacheewapull, O.; Tassaneeyakul, W.; Nakkam, N. Association between HLA alleles and beta-lactam antibiotics-related severe cutaneous adverse reactions. Front. Pharmacol. 2023, 14, 1248386. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.; Talmon, A.; Ribak, Y.; Kessler, A.; Martin, Y.; Haran, T.K.; Shamriz, O.; Adini, I.; Tal, Y. Novel targeted inhibition of the IL-5 axis for drug reaction with eosinophilia and systemic symptoms syndrome. Front. Immunol. 2023, 14, 1134178. [Google Scholar] [CrossRef]
- Sharifzadeh, S.; Mohammadpour, A.H.; Tavanaee, A.; Elyasi, S. Antibacterial antibiotic-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: A literature review. Eur. J. Clin. Pharmacol. 2021, 77, 275–289. [Google Scholar] [CrossRef]
- Sim, D.W.; Yu, J.E.; Jeong, J.; Jung, J.W.; Kang, H.R.; Kang, D.Y.; Ye, Y.M.; Jee, Y.K.; Kim, S.; Park, J.W.; et al. Variation of clinical manifestations according to culprit drugs in DRESS syndrome. Pharmacoepidemiol. Drug Saf. 2019, 28, 840–848. [Google Scholar] [CrossRef]
- Nguyen, E.; Yanes, D.; Imadojemu, S.; Kroshinsky, D. Evaluation of Cyclosporine for the Treatment of DRESS Syndrome. JAMA Dermatol. 2020, 156, 704–706. [Google Scholar] [CrossRef]
- Verstegen, R.H.J.; Phillips, E.J.; Juurlink, D.N. First-line therapy in drug reaction with eosinophilia and systemic symptoms (DReSS): Thinking beyond corticosteroids. Front. Med. 2023, 10, 1138464. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Gonzalez-Galarza, F.F.; McCabe, A.; Santos, E.J.; Jones, J.; Takeshita, L.Y.; Ortega-Rivera, N.D.; Del Cid-Pavon, G.M.; Ramsbottom, K.; Ghattaoraya, G.S.; Alfirevic, A.; et al. Allele frequency net database (AFND) 2020 update: Gold-standard data classification, open access genotype data and new query tools. Nucleic Acid Res. 2020, 48, D783–D788. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of intervention. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Brooke, B.S.; Schwartz, T.A.; Pawlik, T.M. MOOSE Reporting Guidelines for Meta-analyses of Observational Studies. JAMA Surg. 2021, 156, 787–788. [Google Scholar] [CrossRef] [PubMed]
- RegiScar Database. Available online: http://www.regiscar.org/Diseases_HSS_DRESS.html (accessed on 30 September 2024).
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- BMJ Best Practice: What Is GRADE? Available online: https://bestpractice.bmj.com/info/us/toolkit/learn-ebm/what-is-grade/ (accessed on 30 September 2024).
- Furuya-Kanamori, L.; Barendregt, J.J.; Doi, S.A.R. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int. J. Evid. Based Healthc. 2018, 16, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Shamim, M.A. Real-life implications of prevalence meta-analyses? Doi plots and prediction intervals are the answer. Lancet Microbe 2023, 4, e490. [Google Scholar] [CrossRef] [PubMed]
- FAERS Database. Available online: https://fis.fda.gov/sense/app/95239e26-e0be-42d9-a960-9a5f7f1c25ee/sheet/7a47a261-d58b-4203-a8aa-6d3021737452/state/analysis (accessed on 1 August 2024).
- Arora, N.; Chalasani, N.; Rahnama-Moghadam, S. A Review of Drug-Induced Liver Injury with Rash, Eosinophilia, and Systemic Symptoms (DRESS) Syndrome: Cutaneous Manifestations, Clinical Features, and Management. Clin. Liver Dis. 2024, 23, e0198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blumenthal, K.G.; Alvarez-Arango, S.; Kroshinsky, D.; Lo, Y.C.; Samarakoon, U.; Salem, A.R.; Fu, X.; Bassir, F.; Wang, L.; Jaggers, J.; et al. Drug reaction eosinophilia and systemic symptoms: Clinical phenotypic patterns according to causative drug. J. Am. Acad. Dermatol. 2024, 90, 1240–1242. [Google Scholar] [CrossRef]
- Waldron, J.; James, F.; Vogrin, S.; Chua, K.; Holmes, N.; De-Luca, J.; Goh, M.; Douglas, A.; Trubiano, J. A Shorter Time to DRESS—Redefining beta-lactam associated drug reaction with eosinophilia and systemic symptoms. J. Allergy Clin. Immunol. 2023, 151, AB206. [Google Scholar] [CrossRef]
- Choudhary, R.; Vinay, K.; Srivastava, N.; Bishnoi, A.; Kamat, D.; Parsad, D.; Bhatia, A.; Kumaran, M.S. Clinical, biochemical, and serologic predictors of drug reaction with eosinophilia and systemic symptoms syndrome: A prospective case-control study. J. Am. Acad. Dermatol. 2021, 85, 901–909. [Google Scholar] [CrossRef]
- Trubiano, J.A.; Aung, A.K.; Nguyen, M.; Fehily, S.R.; Graudins, L.; Cleland, H.; Padiglione, A.; Peleg, A.Y. A Comparative Analysis Between Antibiotic- and Nonantibiotic-Associated Delayed Cutaneous Adverse Drug Reactions. J. Allergy Clin. Immunol. Pract. 2016, 4, 1187–1193. [Google Scholar] [CrossRef]
- Arslan, S.Y.; Bal, Z.S.; Ozenen, G.G.; Bilen, N.M.; Avcu, G.; Erci, E.; Kurugol, Z.; Gunay, H.; Tamsel, İ.; Ozkinay, F. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome secondary to antimicrobial therapy in pediatric bone and joint infections. World Allergy Organ. J. 2024, 17, 100850. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, A.R.; Zhou, L.; Li, Y.; Phadke, N.A.; Chow, O.A.; Blumenthal, K.G. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome Identified in the Electronic Health Record Allergy Module. J. Allergy Clin. Immunol. Pract. 2019, 7, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.Y.; Yun, J.; Lee, S.Y.; Koh, Y.I.; Sim, D.W.; Kim, S.; Nam, Y.H.; Park, J.W.; Kim, S.H.; Ye, Y.M.; et al. A Nationwide Study of Severe Cutaneous Adverse Reactions Based on the Multicenter Registry in Korea. J. Allergy Clin. Immunol. Pract. 2021, 9, 929–936.e7. [Google Scholar] [CrossRef] [PubMed]
- Asif, B.A.; Koh, C.; Phillips, E.J.; Gu, J.; Li, Y.J.; Barnhart, H.; Chalasani, N.; Fontana, R.J.; Hayashi, P.H.; Navarro, V.J.; et al. Vancomycin-Induced Liver Injury, DRESS, and HLA-A∗32:01. J. Allergy Clin. Immunol. Pract. 2024, 12, 168–174.e2. [Google Scholar] [CrossRef]
- Jaggers, J.; Samarakoon, U.; King, A.; Kroshinsky, D.; Bassir, F.; Salem, A.; Phillips, E.; Wang, L.; Zhou, L.; Blumenthal, K.G. Drug reaction with eosinophilia and systemic symptoms: Medication adherence and quality of life in survivors. J. Allergy Clin. Immunol. Pract. 2024, 12, 239–241.e1. [Google Scholar] [CrossRef]
- Kardaun, S.H.; Sekula, P.; Valeyrie-Allanore, L.; Liss, Y.; Chu, C.Y.; Creamer, D.; Sidoroff, A.; Naldi, L.; Mockenhaupt, M.; Roujeau, J.C.; et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): An original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br. J. Dermatol. 2013, 169, 1071–1080. [Google Scholar] [CrossRef]
- Del Pozzo-Magaña, B.R.; Rieder, M.J.; Garcia-Bournissen, F.; Lazo-Langner, A. Drug reaction with eosinophilia and systemic symptoms (DRESS): A tertiary care centre retrospective study. Br. J. Clin. Pharmacol. 2022, 88, 4134–4141. [Google Scholar] [CrossRef]
- Lee, K.H.; Kang, D.Y.; Kim, H.H.; Kim, Y.J.; Kim, H.J.; Kim, J.H.; Song, E.Y.; Yun, J.; Kang, H.R. Reducing severe cutaneous adverse and type B adverse drug reactions using pre-stored human leukocyte antigen genotypes. Clin. Transl. Allergy 2022, 12, e12098. [Google Scholar] [CrossRef]
- Ben Romdhane, H.; Fadhel, N.B.; Chadli, Z.; Chaabane, A.; Benzarti, W.; Fredj, N.B.; Aouam, K. Drug reaction with eosinophilia and systemic symptoms in a pediatric population: Interest of skin tests. Contact Dermatitis 2023, 89, 488–495. [Google Scholar] [CrossRef]
- AlJasser, M.I. Severe Cutaneous Adverse Drug Reactions at a Tertiary Care Center in Saudi Arabia. Dermatol. Res. Pract. 2023, 2023, 8928198. [Google Scholar] [CrossRef]
- Wang, C.W.; Lin, W.C.; Chen, W.T.; Chen, C.B.; Lu, C.W.; Hou, H.H.; Hui, R.C.; Wu, J.; Chang, C.J.; Chang, Y.C.; et al. Associations of HLA-A and HLA-B with vancomycin-induced drug reaction with eosinophilia and systemic symptoms in the Han-Chinese population. Front. Pharmacol. 2022, 13, 954596. [Google Scholar] [CrossRef] [PubMed]
- Bellón, T.; Lerma, V.; Guijarro, J.; Ramírez, E.; Martínez, C.; Escudero, C.; Fiandor, A.M.; Barranco, R.; de Barrio, M.; de Abajo, F.; et al. LTT and HLA testing as diagnostic tools in Spanish vancomycin-induced DRESS cases: A case-control study. Front. Pharmacol. 2022, 13, 959321. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, K.G.; Youngster, I.; Rabideau, D.J.; Parker, R.A.; Manning, K.S.; Walensky, R.P.; Nelson, S.B. Peripheral blood eosinophilia and hypersensitivity reactions among patients receiving outpatient parenteral antibiotics. J. Allergy Clin. Immunol. 2015, 136, 1288–1294.e1. [Google Scholar] [CrossRef] [PubMed]
- Konvinse, K.C.; Trubiano, J.A.; Pavlos, R.; James, I.; Shaffer, C.M.; Bejan, C.A.; Schutte, R.J.; Ostrov, D.A.; Pilkinton, M.A.; Rosenbach, M.; et al. HLA-A*32:01 is strongly associated with vancomycin-induced drug reaction with eosinophilia and systemic symptoms. J. Allergy Clin. Immunol. 2019, 144, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.L.; Zhou, L.; Phillips, E.; Blackley, S.; Young, M.; Banerji, A.; Blumenthal, K. Documentation of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) in the Allergy and Problem List of patients with a prior history of DRESS. J. Allergy Clin. Immunol. 2023, 153, AB157. [Google Scholar] [CrossRef]
- Krantz, M.; Wei, W.-Q.; Phillips, E. Deep phenotyping algorithms for drug reaction with eosinophilia and systemic symptoms facilitate the identification of cases within the electronic health record. J. Allergy Clin. Immunol. 2024, 153, AB235. [Google Scholar] [CrossRef]
- Book: Holt, P.; Lambton, A.; Lewis, B. The Cambridge History of Islam; Cambridge University Press: Cambridge, UK, 1977. [Google Scholar]
- El Moncer, W.; Esteban, E.; Bahri, R.; Gayà-Vidal, M.; Carreras-Torres, R.; Athanasiadis, G.; Moral, P.; Chaabani, H. Mixed origin of the current Tunisian population from the analysis of Alu and Alu/STR compound systems. J. Hum. Genet. 2010, 55, 827–833. [Google Scholar] [CrossRef]
- Jansen, M.A.; van den Heuvel, D.; Bouthoorn, S.H.; Jaddoe, V.W.; Hooijkaas, H.; Raat, H.; Fraaij, P.L.; van Zelm, M.C.; Moll, H.A. Determinants of Ethnic Differences in Cytomegalovirus, Epstein-Barr Virus, and Herpes Simplex Virus Type 1 Seroprevalence in Childhood. J. Pediatr. 2016, 170, 126–134.e6. [Google Scholar] [CrossRef]
- Mallal, S.; Phillips, E.; Carosi, G.; Molina, J.M.; Workman, C.; Tomazic, J.; Jägel-Guedes, E.; Rugina, S.; Kozyrev, O.; Cid, J.F.; et al. HLA-B*5701 screening for hyper-sensitivity to abacavir. N. Engl. J. Med. 2008, 358, 568–579. [Google Scholar] [CrossRef]
- Randolph, H.E.; Fiege, J.K.; Thielen, B.K.; Mickelson, C.K.; Shiratori, M.; Barroso-Batista, J.; Langlois, R.A.; Barreiro, L.B. Genetic ancestry effects on the response to viral infection are pervasive but cell type specific. Science 2021, 374, 1127–1133. [Google Scholar] [CrossRef]
- Martin, M.A.; Hoffman, J.M.; Freimuth, R.R.; Klein, T.E.; Dong, B.J.; Pirmohamed, M.; Hicks, J.K.; Wilkinson, M.R.; Haas, D.W.; Kroetz, D.L.; et al. Clinical Pharmacogenetics Implementation Consortium Guidelines for HLA-B Genotype and Abacavir Dosing: 2014 update. Clin. Pharmacol. Ther. 2014, 95, 499–500. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Lin, J.J.; Lu, C.S.; Ong, C.T.; Hsieh, P.F.; Yang, C.C.; Tai, C.T.; Wu, S.L.; Lu, C.H.; Hsu, Y.C.; et al. Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N. Engl. J. Med. 2011, 364, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Park, W.B.; Choe, P.G.; Song, K.H.; Lee, S.; Jang, H.C.; Jeon, J.H.; Park, S.W.; Park, M.H.; Oh, M.D.; Choe, K.W. Should HLA-B*5701 screening be performed in every ethnic group before starting abacavir? Clin. Infect. Dis. 2009, 48, 365–367. [Google Scholar] [CrossRef] [PubMed][Green Version]
- UpToDate. Available online: https://www.uptodate.com/drug-interactions/?source=responsive_home#di-analyze (accessed on 31 September 2024).
| Study | Overview | Country | Age (Y) | Female Sex (%) | Ethnicity | Haplotype and Frequency | Sample Size | Limitations |
|---|---|---|---|---|---|---|---|---|
| Arora et al., 2023 [19] | Drug-Induced Liver Injury Network [DILIN], events defined as DRESS with DILI. | US | 42.3 | 59 | Black | NR (HLA-A*32:01, 3.8%, 0.02) | 128 | No concomitant antibiotics or comorbidities were reported. |
| Blumenthal et al., 2023 [20] | Research letter electronic health records. | Europe | 52 | 63 | Caucasians | NR (HLA-A*32:01, 0.019–0.072) | 102 | No concomitant antibiotics or comorbidities were reported. Specific populations in Europe were not stated. |
| Waldron et al., 2023 [21] | Abstract two prospective multicenter cohorts. | Australia | NR | NR | Caucasians | HLA-A*32:01 (0.0340) | 29 | Lack of demographic data. |
| Choudhary et al., 2021 [22] | Observational prospective tertiary care center. | India | 44.3 | NR | Asian | NR (HLA-A*32:01, 7.8%, 0.0390) | 25 | Lack of demographic data. |
| Trubiano et al., 2016 [23] | Retrospective cohort study inpatient study. | Australia | 57 | 40 | Caucasians | NR (HLA-A*32:01, 0.0340) | 84 | Median no. of concomitant antibiotics was 3. |
| Arsalan et al., 2024 [24] | A retrospective study was conducted at a tertiary-level university hospital. | Turkey | 10 | 34 | Caucasians | NR (HLA-A*32:01, 0.0490) | 27 | Vancomycin and meropenem or Ceftazidime. |
| Wolfson et al., 2019 [25] | Registry of severe cutaneous adverse reactions. Partners Healthcare System (PHS), Boston. | Europe and US | 60 | 55 | Caucasians | NR (HLA-A*32:01, 0.036) | 69 | 55% included Vancomycin alone. |
| Kang et al., 2021 [26] | A nationwide registry by the Korean SCAR consortium. | Korea | 63 | 50 | Asian | NR (HLA-A*32:01, 0.0061) | 361 | HLA-A*32:01, the genetic risk marker of vancomycin-induced DRESS, is rare (0.3%) in Koreans, but other genetic risk factors were not stated. |
| Asif et al., 2024 [27] | Observational, DILIN National Institutes of Health. | US | 60 | 33 | Caucasians | HLA-A*32:01, 78% of cases, (0.022) | 113 | 3 cases were judged to be highly likely due to vancomycin-carried HLA-A*32:01 and 4 of the 6 cases (67%) were judged to be probably due to vancomycin. Allele frequency was inferred by the author’s location. |
| Jaggers et al., 2024 [28] | Clinical communication. Health-related quality of life questionnaire | US | 46 | 40 | Caucasians | NR (HLA-A*32:01, 4.4%, 0.022) | 256 | 29% were non-responders. Concomitant medications are not stated. |
| Kardaun et al., 2013 [29] | Prospective multinational registry of severe cutaneous adverse reactions (SCAR). | Austria, England, France, Germany, Israel, Italy, Taiwan, and The Netherlands | 48 | 20 | Caucasians | NR (HLA-A*32:01, 5.5–13.8%, 0.027–0.050) | 117 | The number of cases in Taiwanese was not stated, but the majority were European. The median concomitant medications were 4. The number of suspect drugs was substantially reduced after elimination due to the time course. |
| Del Pozzo-Magaña et al., 2022 [30] | Retrospective study of all cases of DRESS admitted to the Institution. | Canada | 59 | 48 | Caucasians | NR (HLA-A*32:01, 5.6%, 0.0278) | 19 | One patient used concomitant ceftazidime with vancomycin. |
| Lee et al., 2021 [31] | Retrospective study of medical records in patients with HLA results using SUPREME®, a clinical data warehouse of the Seoul National University Hospital (SNUH). | Korea | 44 | 37 | Asian | HLA-A*32:01, 1%, (0.0061) | 11,998 | DRESS inferred but not explicitly stated. |
| Ben Romdhane et al., 2023 [32] | Retrospective analysis of all cases of DRESS is diagnosed in pediatric patients (age ≤ 18 years). | Tunisia | 7 | 100 | Arab | NR (HLA-A*32:01, 3%, 0.026) | 19 | Concomitant with Ceftriaxone. |
| Aljasser et al., 2023 [33] | A cross-sectional study conducted at King Abdulaziz Medical City, Riyadh. | Saudi Arabia | 41 | 47.8 | Arab | NR (HLA-A*32:01, 4.7%. 0.024) | 23 | There was one case of SJS-TEN and DRESS-TEN overlap. Concomitant medications are not stated. |
| Wang et al., 2022 [34] | Retrospectively enrolled from the SCAR consortium. | Taiwan | 57 | 19 | Asian | HLA-A*32:01, 7.7%, (0.0040), HLA-B*40:06, 11.5%, (0.02), HLA-B*67:01, 7.7%, (0.0060), HLA-B*07:05, 3.8%, (0.001) | 26 | 6 out of 26 subjects were concomitantly receiving other medicines (including amoxicillin, ceftriaxone, teicoplanin, valproic acid, diclofenac, and esomeprazole) when prescribed with vancomycin. |
| Bellón et al., 2022 [35] | Case–control study of Spanish registry PIELenRed. | Europe-Spain | 44 | 36 | Caucasians | HLA-A*32:01, 4%, (0.040) | 25 | Latency was inferred from non-events. Demographic characteristics were lacking, but 50% of DRESS vancomycin cases carried HLA-A*32:01 compared with 4%, OR: 13.33 (1.36–130.30) |
| Blumenthal et al., 2015 [36] | Cohort study of inpatients Massachusetts General Hospital (Boston, Massachusetts). | US | 64 | 48 | Caucasians | NR (HLA-A*32:01, 0.036) | 210 | Ethnicity was inferred from the cohort used. The proportion of ethnic origins was not stated. |
| Konvinse et al., 2019 [37] | Retrospective study of patients detected by using Vanderbilt’s BioVU repository, a deidentified electronic health record (EHR) database linked to a DNA biobank. | Australia | 51 | 38 | Caucasians | HLA-A*32:01, 6.8%, (0.034) | 54,249 | The study reported cases of vancomycin DRESS in HLA-A*32:01 carriers vs. no carriers. Two patients were on vancomycin alone. |
| Soto et al., 2023 [38] | Abstract of a retrospective study at Mass Bringham. | US | 50 | 58 | Caucasians | NR (HLA-A*32:01, 0.036) | 183 | 66% were Caucasians; the other 34% ethnicity was not stated. |
| Krantz et al., 2022 [39] | Retrospective DRESS cases from the Synthetic Derivative (SD) of Vanderbilt University Medical Center (VUMC). | Australia | 48.5 | 56 | Caucasians | NR (HLA-A*32:01, 0.026) | 351 | Ethnicity was not reported but was inferred from the location of the hospital. |
| Comparator | Crude Reporting Odds Ratio (95%CI) | Adjusted Reporting Odds Ratio (95%CI) | Effect of Concomitant Antibiotics (95%CI) | Female Sex (95%CI) | p-Value |
|---|---|---|---|---|---|
| Composite Other Antimicrobials | 2.52 (2.39–2.66) | 2.40 (2.27–2.55) | 1.77 (1.72–1.83) | 0.83 (0.79–0.88) | <0.0001 |
| Sulfonamides | 1.98 (1.85–2.10) | 1.68 (1.57–1.80) | 1.66 (1.59–1.73) | 0.74 (0.69–0.77) | <0.0001 |
| Third Generation Cephalosporins | 1.79 (1.64–1.94) | 2.99 (2.72–3.28) | 1.81 (1.74–1.89) | 0.97 (0.89–1.04) | <0.0001 |
| Quinolones | 4.47 (4.11–4.86) | 3.67 (3.35–4.02) | 1.73 (1.66–1.81) | 0.91 (0.84–0.98) | <0.0001 |
| Piperacillin/tazobactam | 1.05 (0.98–1.13) | 0.95 (0.88–1.02) | 1.89 (1.81–1.97) | 0.99 (0.92–1.06) | 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboukaoud, M.; Adi, Y.; Abu-Shakra, M.; Morhi, Y.; Agbaria, R. Vancomycin Drug Reaction with Eosinophilia and Systemic Symptoms: Meta-Analysis and Pharmacovigilance Study. J. Clin. Med. 2025, 14, 930. https://doi.org/10.3390/jcm14030930
Aboukaoud M, Adi Y, Abu-Shakra M, Morhi Y, Agbaria R. Vancomycin Drug Reaction with Eosinophilia and Systemic Symptoms: Meta-Analysis and Pharmacovigilance Study. Journal of Clinical Medicine. 2025; 14(3):930. https://doi.org/10.3390/jcm14030930
Chicago/Turabian StyleAboukaoud, Mohammed, Yotam Adi, Mahmoud Abu-Shakra, Yocheved Morhi, and Riad Agbaria. 2025. "Vancomycin Drug Reaction with Eosinophilia and Systemic Symptoms: Meta-Analysis and Pharmacovigilance Study" Journal of Clinical Medicine 14, no. 3: 930. https://doi.org/10.3390/jcm14030930
APA StyleAboukaoud, M., Adi, Y., Abu-Shakra, M., Morhi, Y., & Agbaria, R. (2025). Vancomycin Drug Reaction with Eosinophilia and Systemic Symptoms: Meta-Analysis and Pharmacovigilance Study. Journal of Clinical Medicine, 14(3), 930. https://doi.org/10.3390/jcm14030930







