Impact of Right Heart Failure on Outcomes of Transcatheter Aortic Valve Implantation: Insights from the National Inpatient Sample
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
- (1)
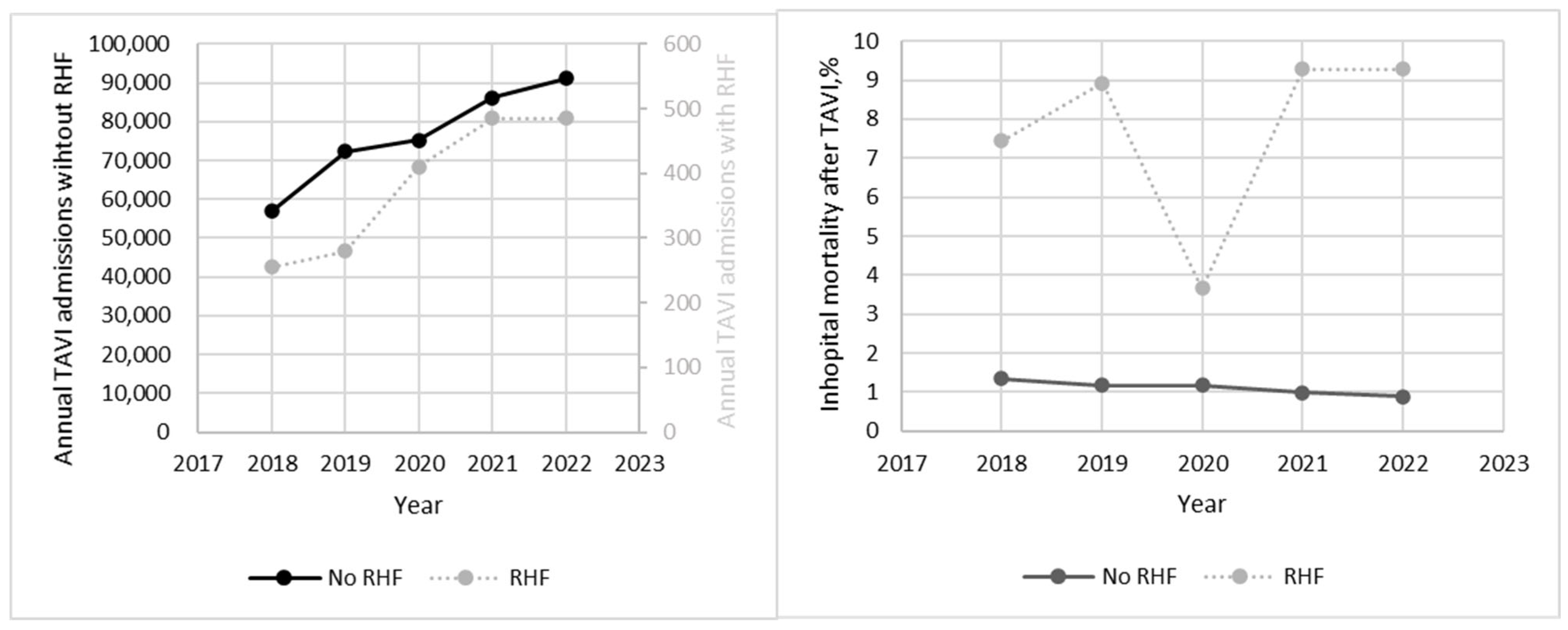
- Patients with RHF who underwent TAVI had a higher burden of comorbidities and had much higher in-hospital mortality (7.57% vs. 1.1%) compared to patients without RHF;
- (2)
- Patients with RHF who underwent TAVI experienced more complications, such as acute kidney injury (37.10% vs. 8.56%), respiratory failure (12.79% vs. 1.91%), and the utilization of mechanical circulatory support (11.48% vs. 0.83%);
- (3)
- Patients with RHF who underwent TAVI had lengthier hospital stays and higher total hospitalization charges.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldsweig, A.M.; Thourani, V.H. Decreasing Prices but Increasing Demand for Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2022, 15, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Fontana, G.P.; Jilaihawi, H.; Kapadia, S.; Pichard, A.D.; Douglas, P.S.; Thourani, V.H.; Babaliaros, V.C.; Webb, J.G.; Herrmann, H.C.; et al. Transcatheter Aortic-Valve Replacement for Inoperable Severe Aortic Stenosis. N. Engl. J. Med. 2012, 366, 1696–1704. [Google Scholar] [CrossRef]
- Thyregod, H.G.H.; Steinbrüchel, D.A.; Ihlemann, N.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrøm, T.; Clemmensen, P.; et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Severe Aortic Valve Stenosis: 1-Year Results From the All-Comers NOTION Randomized Clinical Trial. J. Am. Coll. Cardiol. 2015, 65, 2184–2194. [Google Scholar] [CrossRef]
- Harjai, K.J.; Grines, C.L.; Leon, M.B. Transcatheter Aortic Valve Replacement: 2015 in Review. J. Interv. Cardiol. 2016, 29, 27–46. [Google Scholar] [CrossRef]
- Fan, J.; Liu, X.; Yu, L.; Sun, Y.; Jaiswal, S.; Zhu, Q.; Chen, H.; He, Y.; Wang, L.; Ren, K.; et al. Impact of Tricuspid Regurgitation and Right Ventricular Dysfunction on Outcomes after Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Clin. Cardiol. 2019, 42, 206–212. [Google Scholar] [CrossRef]
- Arnold, S.V.; Reynolds, M.R.; Lei, Y.; Magnuson, E.A.; Kirtane, A.J.; Kodali, S.K.; Zajarias, A.; Thourani, V.H.; Green, P.; Rodés-Cabau, J.; et al. Predictors of Poor Outcomes after Transcatheter Aortic Valve Replacement Results from the PARTNER (Placement of Aortic Transcatheter Valve) Trial. Circulation 2014, 129, 2682–2690. [Google Scholar] [CrossRef]
- Winter, M.P.; Bartko, P.; Hofer, F.; Zbiral, M.; Burger, A.; Ghanim, B.; Kastner, J.; Lang, I.M.; Mascherbauer, J.; Hengstenberg, C.; et al. Evolution of Outcome and Complications in TAVR: A Meta-Analysis of Observational and Randomized Studies. Sci. Rep. 2020, 10, 15568. [Google Scholar] [CrossRef]
- Konstam, M.A.; Kiernan, M.S.; Bernstein, D.; Bozkurt, B.; Jacob, M.; Kapur, N.K.; Kociol, R.D.; Lewis, E.F.; Mehra, M.R.; Pagani, F.D.; et al. Evaluation and Management of Right-Sided Heart Failure: A Scientific Statement from the American Heart Association. Circulation 2018, 137, e578–e622. [Google Scholar] [CrossRef]
- Higuchi, S.; Mochizuki, Y.; Omoto, T.; Matsumoto, H.; Masuda, T.; Maruta, K.; Aoki, A.; Shinke, T. Clinical Impact of the Right Ventricular Impairment in Patients Following Transcatheter Aortic Valve Replacement. Sci. Rep. 2024, 14, 1776. [Google Scholar] [CrossRef]
- Koifman, E.; Didier, R.; Patel, N.; Jerusalem, Z.; Kiramijyan, S.; Ben-Dor, I.; Negi, S.I.; Wang, Z.; Goldstein, S.A.; Lipinski, M.J.; et al. Impact of Right Ventricular Function on Outcome of Severe Aortic Stenosis Patients Undergoing Transcatheter Aortic Valve Replacement. Am. Heart J. 2017, 184, 141–147. [Google Scholar] [CrossRef]
- Ito, S.; Pislaru, S.V.; Soo, W.M.; Huang, R.; Greason, K.L.; Mathew, V.; Sandhu, G.S.; Eleid, M.F.; Suri, R.M.; Oh, J.K.; et al. Impact of Right Ventricular Size and Function on Survival Following Transcatheter Aortic Valve Replacement. Int. J. Cardiol. 2016, 221, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.A.; Rozenbaum, Z.; Ghantous, E.; Kramarz, J.; Biner, S.; Ghermezi, M.; Shimiaie, J.; Finkelstein, A.; Banai, S.; Aviram, G.; et al. Impact of Right Ventricular Dysfunction and Tricuspid Regurgitation on Outcomes in Patients Undergoing Transcatheter Aortic Valve Replacement. J. Am. Soc. Echocardiogr. 2017, 30, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Griese, D.P.; Kerber, S.; Barth, S.; Diegeler, A.; Babin-Ebell, J.; Reents, W. Impact of Right and Left Ventricular Systolic Dysfunction on Perioperative Outcome and Long-Term Survival after Transcatheter Aortic Valve Replacement. J. Interv. Cardiol. 2017, 30, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Kutsal, D.A.; Terzi, S. Factors Associated with Acute Kidney Injury in Patients Undergoing Transcatheter Aortic Valve Implantation: Short-Term Outcomes and Impact of Right Heart Failure. N. Clin. Istanb. 2024, 11, 133. [Google Scholar] [CrossRef]
- HCUP Databases Healthcare Cost and Utilization Project (HCUP). 2006–2019. Agency for Healthcare Research and Quality, Rockville, MD. Available online: https://hcup-us.ahrq.gov/nisoverview.jsp (accessed on 23 January 2025).
- Dugoff, E.H.; Schuler, M.; Stuart, E.A. Generalizing Observational Study Results: Applying Propensity Score Methods to Complex Surveys. Health Serv. Res. 2014, 49, 284–303. [Google Scholar] [CrossRef]
- Aluru, J.S.; Barsouk, A.; Saginala, K.; Rawla, P.; Barsouk, A. Valvular Heart Disease Epidemiology. Med. Sci. 2022, 10, 32. [Google Scholar] [CrossRef]
- Otto, C.M. Timing of Aortic Valve Surgery. Heart 2000, 84, 211–218. [Google Scholar] [CrossRef]
- Iung, B.; Cachier, A.; Baron, G.; Messika-Zeitoun, D.; Delahaye, F.; Tornos, P.; Gohlke-Bärwolf, C.; Boersma, E.; Ravaud, P.; Vahanian, A. Decision-Making in Elderly Patients with Severe Aortic Stenosis: Why Are so Many Denied Surgery? Eur. Heart J. 2005, 26, 2714–2720. [Google Scholar] [CrossRef]
- Boskovski, M.T.; Nguyen, T.C.; McCabe, J.M.; Kaneko, T. Outcomes of Transcatheter Aortic Valve Replacement in Patients With Severe Aortic Stenosis: A Review of a Disruptive Technology in Aortic Valve Surgery. JAMA Surg. 2020, 155, 69–77. [Google Scholar] [CrossRef]
- Arora, S.; Strassle, P.D.; Qamar, A.M.D.; Kolte, D.; Pandey, A.; Paladugu, M.B.; Borhade, M.B.; Ramm, C.J.; Bhatt, D.L.; Vavalle, J.P. Trends in Inpatient Complications after Transcatheter and Surgical Aortic Valve Replacement in the Transcatheter Aortic Valve Replacement Era. Circ. Cardiovasc. Interv. 2018, 11, e007517. [Google Scholar] [CrossRef]
- Amabile, N.; Ramadan, R.; Ghostine, S.; Cheng, S.; Azmoun, A.; Raoux, F.; To, N.T.; Haddouche, Y.; Troussier, X.; Nottin, R.; et al. Early and Mid-Term Cardiovascular Outcomes Following TAVI: Impact of Pre-Procedural Transvalvular Gradient. Int. J. Cardiol. 2013, 167, 687–692. [Google Scholar] [CrossRef]
- Cribier, A. Invention and Uptake of TAVI over the First 20 Years. Nat. Rev. Cardiol. 2022, 19, 427–428. [Google Scholar] [CrossRef]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Muñoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e1159–e1195. [Google Scholar] [CrossRef]
- Lillo, R.; Graziani, F.; Ingrasciotta, G.; Przbybylek, B.; Iannaccone, G.; Locorotondo, G.; Pedicino, D.; Aurigemma, C.; Romagnoli, E.; Trani, C.; et al. Right Ventricle Systolic Function and Right Ventricle-Pulmonary Artery Coupling in Patients with Severe Aortic Stenosis and the Early Impact of TAVI. Int. J. Cardiovasc. Imaging 2022, 38, 1761–1770. [Google Scholar] [CrossRef]
- Grevious, S.N.; Fernandes, M.F.; Annor, A.K.; Ibrahim, M.; Saint Croix, G.R.; de Marchena, E.; Cohen, M.G.; Alfonso, C.E. Prognostic Assessment of Right Ventricular Systolic Dysfunction on Post–Transcatheter Aortic Valve Replacement Short-Term Outcomes: Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2020, 9, 14463. [Google Scholar] [CrossRef]
- Hossain, R.; Chelala, L.; Sleilaty, G.; Amin, S.; Vairavamurthy, J.; Chen, R.; Gupta, A.; Jeudy, J.; White, C. Preprocedure Ct Findings of Right Heart Failure as a Predictor of Mortality after Transcatheter Aortic Valve Replacement. Am. J. Roentgenol. 2021, 216, 57–65. [Google Scholar] [CrossRef]
- Koschutnik, M.; Dannenberg, V.; Nitsche, C.; Donà, C.; Siller-Matula, J.M.; Winter, M.P.; Andreas, M.; Zafar, A.; Bartko, P.E.; Beitzke, D.; et al. Right Ventricular Function and Outcome in Patients Undergoing Transcatheter Aortic Valve Replacement. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 1295–1303. [Google Scholar] [CrossRef]
- Ullah, W.; Zahid, S.; Hamzeh, I.; Birnbaum, Y.; Virani, S.S.; Alam, M. Trends and Predictors of Transcatheter Aortic Valve Implantation Related In-Hospital Mortality (From the National Inpatient Sample Database). Am. J. Cardiol. 2021, 143, 97–103. [Google Scholar] [CrossRef]
- Lindman, B.R.; Maniar, H.S.; Jaber, W.A.; Lerakis, S.; Mack, M.J.; Suri, R.M.; Thourani, V.H.; Babaliaros, V.; Kereiakes, D.J.; Whisenant, B.; et al. Effect of Tricuspid Regurgitation and the Right Heart on Survival after Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2015, 8, e002073. [Google Scholar] [CrossRef] [PubMed]
- Bursi, F.; McNallan, S.M.; Redfield, M.M.; Nkomo, V.T.; Lam, C.S.P.; Weston, S.A.; Jiang, R.; Roger, V.L. Pulmonary Pressures and Death in Heart Failure: A Community Study. J. Am. Coll. Cardiol. 2012, 59, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Sinning, J.M.; Hammerstingl, C.; Chin, D.; Ghanem, A.; Schueler, R.; Sedaghat, A.; Bence, J.; Spyt, T.; Werner, N.; Kovac, J.; et al. Decrease of Pulmonary Hypertension Impacts on Prognosis after Transcatheter Aortic Valve Replacement. EuroIntervention 2014, 9, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Pibarot, P.; Iung, B.; Cavalcante, J.L. Risk Stratification in Patients With Aortic Stenosis: Pay More Attention to the Right-Side Unit! JACC Cardiovasc. Interv. 2019, 12, 2169–2172. [Google Scholar] [CrossRef]
| Baseline Characteristics, n (%) | Total | No RHF | RHF | p Value |
|---|---|---|---|---|
| n = 383,860 | n = 381,945 | n = 1915 | ||
| Female | 166,160 (43%) | 165,465 (43%) | 695 (36%) | <0.01 |
| Age, years, median (IQR) | 79 (73–85) | 79 (73–85) | 77 (68–84) | <0.01 |
| White race | 327,025 (87%) | 325,465 (87%) | 1560 (86%) | 0.35 |
| Charlson Comorbidity Index | <0.01 | |||
| 1.00 | 78,440 (20%) | 78,195 (20%) | 245 (13%) | |
| 2.00 | 78,435 (20%) | 78,115 (20%) | 320 (17%) | |
| 3.00 or more | 196,860 (51%) | 195,510 (51%) | 1350 (71%) | |
| Median household income for patient’s ZIP | 0.26 | |||
| 0–25th percentile | 80,460 (21%) | 80,100 (21%) | 360 (19%) | |
| 26th to 50th percentile (median) | 97,895 (26%) | 97,335 (26%) | 560 (30%) | |
| 51st to 75th percentile | 100,850 (27%) | 100,330 (27%) | 520 (28%) | |
| 76th to 100th percentile | 99,595 (26%) | 99,150 (26%) | 445 (24%) | |
| Insurance | <0.01 | |||
| Medicare | 333,120 (89%) | 331,615 (89%) | 1505 (81%) | |
| Medicaid | 6085 (2%) | 5990 (2%) | 95 (5%) | |
| Private insurance | 33,870 (9%) | 33,650 (9%) | 220 (12%) | |
| Self-pay | 1735 (0%) | 1690 (0%) | 45 (2%) | |
| Region of hospital | <0.01 | |||
| Northeast | 84,420 (22%) | 84,135 (22%) | 285 (15%) | |
| Midwest | 90,025 (23%) | 89,430 (23%) | 595 (31%) | |
| South | 130,850 (34%) | 130,245 (34%) | 605 (32%) | |
| West | 78,630 (20%) | 78,200 (20%) | 430 (22%) | |
| Hospital bed size | 0.03 | |||
| Small | 32,340 (8%) | 32,225 (8%) | 115 (6%) | |
| Medium | 86,585 (23%) | 86,240 (23%) | 345 (18%) | |
| Large | 265,000 (69%) | 263,545 (69%) | 1455 (76%) | |
| Location/teaching status of hospital | 0.22 | |||
| Rural | 6150 (2%) | 6105 (2%) | 45 (2%) | |
| Urban nonteaching | 34,525 (9%) | 34,395 (9%) | 130 (7%) | |
| Urban teaching | 343,250 (89%) | 341,510 (89%) | 1740 (91%) | |
| Elective | 322,430 (84%) | 321,465 (84%) | 965 (51%) | <0.01 |
| Comorbidities | ||||
| Hypertension | 345,740 (90%) | 344,090 (90%) | 1650 (86%) | 0.01 |
| Diabetes mellitus | 144,460 (38%) | 143,700 (38%) | 760 (40%) | 0.38 |
| Chronic kidney disease | 124,315 (32%) | 123,385 (32%) | 930 (49%) | <0.01 |
| Peripheral vascular disease | 76,625 (20%) | 76,205 (20%) | 420 (22%) | 0.35 |
| Coronary artery disease | 259,615 (68%) | 258,365 (68%) | 1250 (65%) | 0.32 |
| Chronic lung disease | 94,970 (25%) | 94,425 (25%) | 545 (28%) | 0.09 |
| Chronic liver disease | 15,190 (4%) | 14,920 (4%) | 270 (14%) | <0.01 |
| Obesity | 85,115 (22%) | 84,650 (22%) | 465 (24%) | 0.31 |
| Smoker | 134,250 (35%) | 133,825 (35%) | 425 (22%) | <0.01 |
| Coagulopathy | 37,665 (10%) | 37,115 (10%) | 550 (29%) | <0.01 |
| Atrial fibrillation | 107,005 (28%) | 6270 (28%) | 735 (38%) | <0.01 |
| Outcome, n (%) | Total | No RHF | RHF | OR | 95% CI | p Value |
|---|---|---|---|---|---|---|
| Mortality | 4305 (1.12%) | 4160 (1.11%) | 145 (7.57%) | 7.44 | 5.05–10.96 | <0.01 |
| Permanent pacemaker implantation | 31,260 (8.14%) | 31,075 (8.13%) | 185 (9.66%) | 1.21 | 0.83–1.76 | 0.33 |
| Mechanical circulatory support * | 3395 (0.88%) | 3175 (0.83%) | 220 (11.48%) | 15.49 | 11.09–21.63 | <0.01 |
| Myocardial or pericardial complications ** | 4950 (1.29%) | 4910 (1.28%) | 40 (2.08%) | 1.64 | 0.81–3.30 | 0.17 |
| Respiratory failure | 7530 (2.00%) | 7285 (1.91%) | 245 (12.79%) | 7.55 | 5.50–10.36 | <0.01 |
| Acute kidney injury | 33,405 (8.70%) | 32,695 (8.56%) | 710 (37.10%) | 6.30 | 5.16–7.68 | <0.01 |
| Vascular complications *** | 5450 (1.42%) | 5415 (1.41%) | 35 (1.83%) | 1.29 | 0.62–2.70 | 0.49 |
| Blood transfusion | 19,620 (5.11%) | 19,425 (5.08%) | 195 (10.20%) | 2.12 | 1.51–2.97 | <0.01 |
| Venous thromboembolism | 2030 (0.50%) | 1980 (0.50%) | 50 (2.60%) | 5.15 | 2.73–9.70 | <0.01 |
| Length of stay, days, median (IQR) | 2 (1–3) | 2 (1–3) | 7 (2–15) | - | <0.01 | |
| Total charges, $, median (IQR) | 180,740 (133,808– 264,586) | 180,501 (133,702–264,007) | 257,239 (171,467–411,012) | - | <0.01 |
| Outcome, n (%) | OR | 95% CI | p Value |
|---|---|---|---|
| Mortality | 4.11 | 2.25–7.52 | <0.01 |
| Permanent pacemaker implantation | 0.90 | 0.51–1.58 | 0.711 |
| Mechanical circulatory support * | 5.92 | 3.61–9.72 | <0.01 |
| Myocardial or pericardial complications ** | 1.12 | 0.44–2.83 | 0.811 |
| Respiratory failure | 4.26 | 2.36–7.71 | <0.01 |
| Acute kidney injury | 2.10 | 1.54–2.86 | <0.01 |
| Vascular complications *** | 0.84 | 0.20–3.47 | 0.813 |
| Blood transfusion | 1.00 | 0.59–1.68 | 0.995 |
| Venous thromboembolism | 1.78 | 0.80–3.97 | 0.157 |
| Length of stay, days | - | <0.01 | |
| Total charges, $ | - | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kansakar, S.; Qureshi, W.T.; Sharma, N.R.; Shrestha, D.B.; Shtembari, J.; Shetty, V.; Moskovits, N.; Dahal, K.; Mattumpuram, J.; Katz, D.H. Impact of Right Heart Failure on Outcomes of Transcatheter Aortic Valve Implantation: Insights from the National Inpatient Sample. J. Clin. Med. 2025, 14, 841. https://doi.org/10.3390/jcm14030841
Kansakar S, Qureshi WT, Sharma NR, Shrestha DB, Shtembari J, Shetty V, Moskovits N, Dahal K, Mattumpuram J, Katz DH. Impact of Right Heart Failure on Outcomes of Transcatheter Aortic Valve Implantation: Insights from the National Inpatient Sample. Journal of Clinical Medicine. 2025; 14(3):841. https://doi.org/10.3390/jcm14030841
Chicago/Turabian StyleKansakar, Sajog, Waqas T. Qureshi, Nava Raj Sharma, Dhan Bahadur Shrestha, Jurgen Shtembari, Vijay Shetty, Norbert Moskovits, Khagendra Dahal, Jishanth Mattumpuram, and Daniel H. Katz. 2025. "Impact of Right Heart Failure on Outcomes of Transcatheter Aortic Valve Implantation: Insights from the National Inpatient Sample" Journal of Clinical Medicine 14, no. 3: 841. https://doi.org/10.3390/jcm14030841
APA StyleKansakar, S., Qureshi, W. T., Sharma, N. R., Shrestha, D. B., Shtembari, J., Shetty, V., Moskovits, N., Dahal, K., Mattumpuram, J., & Katz, D. H. (2025). Impact of Right Heart Failure on Outcomes of Transcatheter Aortic Valve Implantation: Insights from the National Inpatient Sample. Journal of Clinical Medicine, 14(3), 841. https://doi.org/10.3390/jcm14030841








