Streamlined Management of Basal Cell Carcinoma with Dermoscopy: A Retrospective Case–Control Study
Abstract
1. Introduction
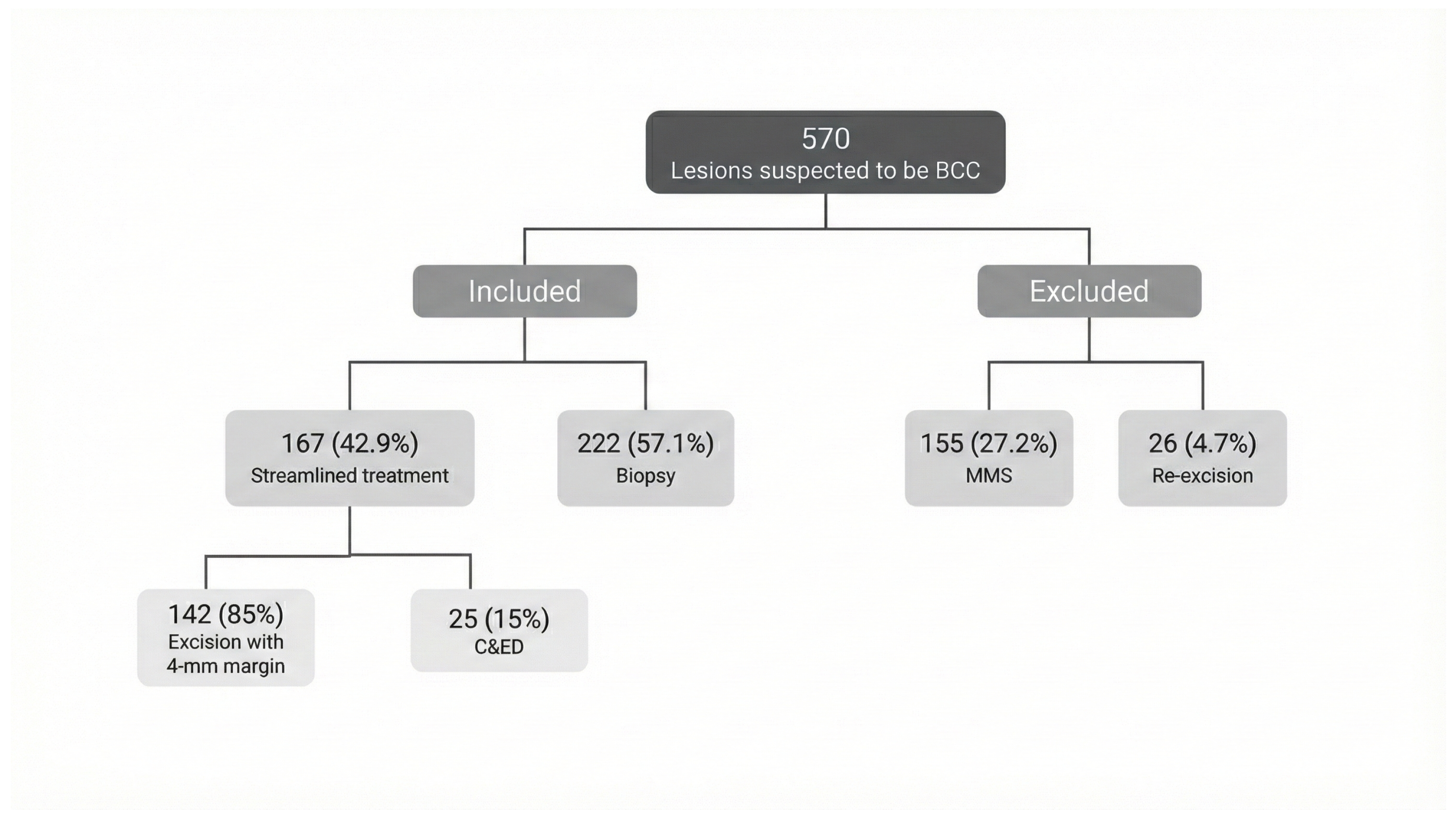
2. Patients and Methods
2.1. Study Design
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Group Characteristics: Biopsy vs. Streamlined Management (Table 1)
| Characteristic | Streamlined Group, n (%) | Biopsy Group, n (%) | p-Value |
|---|---|---|---|
| Total (n) | 167 | 222 | |
| Age (mean, SD) | 68.51 ± 13.98 | 65.9 ± 12.59 | 0.03 |
| Sex (Male) | 112 (67.1) | 107 (48.2) | <0.001 |
| Anatomic zone (H, M, L) | <0.001 | ||
| H | 41 (24.6) | 132 (59.5) | |
| M | 35 (21) | 44 (19.8) | |
| L | 91 (54.5) | 46 (20.7) | |
| Anatomic location | <0.001 | ||
| Head | 59 (35.3) | 155 (69.8) | |
| Neck | 6 (3.6) | 1 (0.5) | |
| Thorax | 9 (5.4) | 10 (4.5) | |
| Abdomen | 7 (4.2) | 4 (1.8) | |
| Back | 38 (22.8) | 11 (5) | |
| Anogenital | 0 | 1 (0.5) | |
| Upper limbs | 27 (16.2) | 12 (5.4) | |
| Lower limbs | 21 (12.6) | 28 (12.6) | |
| Subtype | 0.03 | ||
| Infiltrative | 2 (1.2) | 2 (0.9) | |
| Micronodular | 4 (2.4) | 7 (3.2) | |
| Morpheaform | 1 (0.6) | 11 (5) | |
| Nodular | 73 (43.7) | 64 (28.8) | |
| Superficial | 58 (34.7) | 40 (18) | |
| Mixed | 20 (12) | 21 (9.5) | |
| Other (no BCC) | 9 (5.4) | 77 (34.7) | |
| Aggressive histopathological subtype * | 19 (11.4) | 40 (18) | 0.001 |
| Recurrent BCC | 7 (4.2) | 5 (2.3) | 0.290 |
| Perineural invasion | 0 | 0 | NA |
| Radiotherapy ** | 8 (4.8) | 7 (3.2) | 0.413 |
| Immunosuppression | 3 (1.8) | 7 (3.2) | 0.399 |
3.1.1. Multivariate Analysis Streamlined Management vs. Biopsy
3.1.2. Streamlined Group Sub Analysis: 4 mm Margins vs. C&ED
3.1.3. Multivariate Analysis Streamlined Subgroup
3.2. Outcome Comparison
3.3. Discussion
Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmults, C.D.; Blitzblau, R.; Aasi, S.Z.; Alam, M.; Amini, A.; Bibee, K.; Bordeaux, J.; Chen, P.L.; Contreras, C.M.; DiMaio, D.; et al. Basal Cell Skin Cancer, Version 2.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 1181–1203. [Google Scholar] [CrossRef]
- Work Group; Invited Reviewers; Kim, J.Y.S.; Kozlow, J.H.; Mittal, B.; Moyer, J.; Olencki, T.; Rodgers, P. Guidelines of care for the management of basal cell carcinoma. J. Am. Acad. Dermatol. 2018, 78, 540–559. [Google Scholar] [CrossRef] [PubMed]
- Reiter, O.; Mimouni, I.; Gdalevich, M.; Marghoob, A.A.; Levi, A.; Hodak, E.; Leshem, Y.A. The diagnostic accuracy of dermoscopy for basal cell carcinoma: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2019, 80, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Lupu, M.; Popa, I.M.; Voiculescu, V.M.; Caruntu, A.; Caruntu, C. A Systematic Review and Meta-Analysis of the Accuracy of in Vivo Reflectance Confocal Microscopy for the Diagnosis of Primary Basal Cell Carcinoma. J. Clin. Med. 2019, 8, 1462. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Elkin, E.B.; Jason Chen, C.S.; Marghoob, A. Traditional versus streamlined management of basal cell carcinoma (BCC): A cost analysis. J. Am. Acad. Dermatol. 2015, 73, 791–798. [Google Scholar] [CrossRef]
- Abramson, A.K.; Krasny, M.J.; Goldman, G.D. Tangential shave removal of basal cell carcinoma. Dermatol. Surg. 2013, 39, 387–392. [Google Scholar] [CrossRef]
- McLaughlin, S.J.P.; Kenealy, J.; Locke, M.B. Effect of a See and Treat clinic on skin cancer treatment time. ANZ J. Surg. 2018, 88, 474–479. [Google Scholar] [CrossRef]
- Cameron, M.C.; Lee, E.; Hibler, B.P.; Giordano, C.N.; Barker, C.A.; Mori, S.; Cordova, M.; Nehal, K.S.; Rossi, A.M. Basal cell carcinoma: Contemporary approaches to diagnosis, treatment, and prevention. J. Am. Acad. Dermatol. 2019, 80, 321–339. [Google Scholar] [CrossRef]
- Reiter, O.; Mimouni, I.; Dusza, S.; Halpern, A.C.; Leshem, Y.A.; Marghoob, A.A. Dermoscopic features of basal cell carcinoma and its subtypes: A systematic review. J. Am. Acad. Dermatol. 2021, 85, 653–664. [Google Scholar] [CrossRef]
- Ceder, H.; Backman, E.; Marghoob, A.; Navarrete-Dechent, C.; Polesie, S.; Reiter, O.; Paoli, J. Importance of Both Clinical and Dermoscopic Findings in Predicting High-Risk Histopathological Subtype in Facial Basal Cell Carcinomas. Dermatol. Pract. Concept. 2024, 14, e2024212. [Google Scholar] [CrossRef]
- Peris, K.; Fargnoli, M.C.; Kaufmann, R.; Arenberger, P.; Bastholt, L.; Seguin, N.B.; Bataille, V.; Brochez, L.; Del Marmol, V.; Dummer, R.; et al. European consensus-based interdisciplinary guideline for diagnosis and treatment of basal cell carcinoma—Update 2023. Eur. J. Cancer 2023, 192, 113254. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-L.; Mukundan, A.; Karmakar, R.; Avala, P.; Chang, W.-Y.; Wang, H.-C. Hyperspectral Imaging for Enhanced Skin Cancer Classification Using Machine Learning. Bioengineering 2025, 12, 755. [Google Scholar] [CrossRef] [PubMed]
- Fünfer, K.; Mozaffari, M.; Mayer, O.; Schlingmann, S.; Welzel, J.; Schuh, S. One-Stop Shop: Diagnosis and Treatment of Basal Cell Carcinoma in One Step. J. Clin. Med. 2024, 13, 3830. [Google Scholar] [CrossRef] [PubMed]
- Kadouch, D.J.; Leeflang, M.M.; Elshot, Y.S.; Longo, C.; Ulrich, M.; van der Wal, A.C.; Wolkerstorfer, A.; Bekkenk, M.W.; de Rie, M.A. Diagnostic accuracy of confocal microscopy imaging vs. punch biopsy for diagnosing and subtyping basal cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1641–1648. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Z.R.; Zhou, Y.; Liu, M.X.; Wang, X.Q.; Wang, D.G. The effect of dermoscopy in assisting on defining surgical margins of basal cell carcinoma. Dermatol. Ther. 2022, 35, e15711. [Google Scholar] [CrossRef]
- Hoorens, I.; Batteauw, A.; Van Maele, G.; Lapiere, K.; Boone, B.; Ongenae, K. Mohs micrographic surgery for basal cell carcinoma: Evaluation of the indication criteria and predictive factors for extensive subclinical spread. Br. J. Dermatol. 2016, 174, 847–852. [Google Scholar] [CrossRef]
- Mosterd, K.; Krekels, G.A.; Nieman, F.H.; Ostertag, J.U.; Essers, B.A.; Dirksen, C.D.; Steijlen, P.M.; Vermeulen, A.; Neumann, H.A.M.; Kelleners-Smeets, N.W. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: A prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008, 9, 1149–1156. [Google Scholar] [CrossRef]
- Nasr, I.; McGrath, E.J.; Harwood, C.A.; Botting, J.; Buckley, P.; Budny, P.G.; Fairbrother, P.; Fife, K.; Gupta, G.; Hashme, M.; et al. British Association of Dermatologists’ Clinical Standards Unit. British Association of Dermatologists guidelines for the management of adults with basal cell carcinoma 2021. Br. J. Dermatol. 2021, 185, 899–920. [Google Scholar] [CrossRef]
- Lang, B.M.; Balermpas, P.; Bauer, A.; Blum, A.; Brölsch, G.F.; Dirschka, T.; Follmann, M.; Frank, J.; Frerich, B.; Fritz, K.; et al. S2k Guidelines for Cutaneous Basal Cell Carcinoma - Part 2: Treatment, Prevention and Follow-up. J. Dtsch. Dermatol. Ges. 2019, 17, 214–230. [Google Scholar] [CrossRef]
- Brown, A.C.; Brindley, L.; Hunt, W.T.N.; Earp, E.M.; Veitch, D.; Mortimer, N.J.; Salmon, P.J.; Wernham, A. A review of the evidence for Mohs micrographic surgery. Part 2: Basal cell carcinoma. Clin. Exp. Dermatol. 2022, 47, 1794–1804. [Google Scholar] [CrossRef]
- Van Loo, E.; Mosterd, K.; Krekels, G.A.; Roozeboom, M.H.; Ostertag, J.U.; Dirksen, C.D.; Steijlen, P.M.; Neumann, H.M.; Nelemans, P.J.; Kelleners-Smeets, N.W. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: A randomised clinical trial with 10 year follow-up. Eur. J. Cancer 2014, 50, 3011–3020. [Google Scholar] [CrossRef] [PubMed]
- Van Coile, L.; Verhaeghe, E.; Ongenae, K.; Destrooper, L.; Mohamadi, Z.; Brochez, L.; Hoorens, I. The therapeutic dilemma of basal cell carcinoma in older adults: A review of the current literature. J. Geriatr. Oncol. 2023, 14, 101475. [Google Scholar] [CrossRef] [PubMed]
- Van Coile, L.; Meertens, A.; Shen, A.; Waalboer-Spuij, R.; Vossaert, K.; Verhaeghe, E.; Brochez, L.; Hoorens, I. The impact of basal cell carcinoma on the quality-of-life in older patients. Sci. Rep. 2024, 14, 21739. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.; Imran, D.; Moncrieff, M.; O’Neill, T.J.; Sassoon, E.M. The see-and-treat clinic in plastic surgery: An efficient, cost-effective, and training-friendly setup. Plast. Reconstr. Surg. 2004, 113, 1060–1063. [Google Scholar] [CrossRef]
- Nightingale, J.; Travers, L.; Campbell, J.; Huang, J.; Green, M.; Warren, T.; Fitzgerald, G. Outpatient surgical management of non-melanoma skin cancers of the head and neck in a regional center: An analysis of costs and outcomes. ANZ J. Surg. 2021, 91, 139–144. [Google Scholar] [CrossRef]
- Gorman, M.; Coelho, J.; Gujral, S.; McKay, A. One-Stop Clinic Utilization in Plastic Surgery: Our Local Experience and the Results of a UK-Wide National Survey. Plast. Surg. Int. 2015, 747961. [Google Scholar] [CrossRef]
- Navarrete-Dechent, C.; Rajadhyaksha, M.; Nehal, K.S. Perioperative Noninvasive Optical Imaging: A Changing Paradigm for Management of Keratinocyte Carcinomas. J. Investig. Dermatol. 2020, 140, 1895–1898. [Google Scholar] [CrossRef]
- Villani, A.; Fabbrocini, G.; Costa, C.; Scalvenzi, M. Reflectance Confocal Microscopy Identification of Subclinical Basal Cell Carcinoma after Vismodegib Treatment: Report of a Case. Dermatol. Ther. 2021, 11, 1071–1074. [Google Scholar] [CrossRef]
- Cappilli, S.; Mannino, M.; Palmisano, G.; Bocchino, E.; Piccerillo, A.; Paradisi, A.; Di Stefani, A.; Peris, K. Locally advanced basal cell carcinoma treated with sonidegib: In vivo monitoring with line-field confocal optical coherence tomography. Ski. Health Dis. 2025, 5, 37–40. [Google Scholar] [CrossRef]
- Venturi, F.; Rapparini, L.; Sgarzani, R.; Scotti, B.; Campione, E.; Dika, E. Cytoreductive approach with hedgehog inhibitors followed by reflectance confocal microscopy assisted Mohs surgery for morpheiform basal cell carcinoma. JEADV 2025, 39, e32–e34. [Google Scholar] [CrossRef]
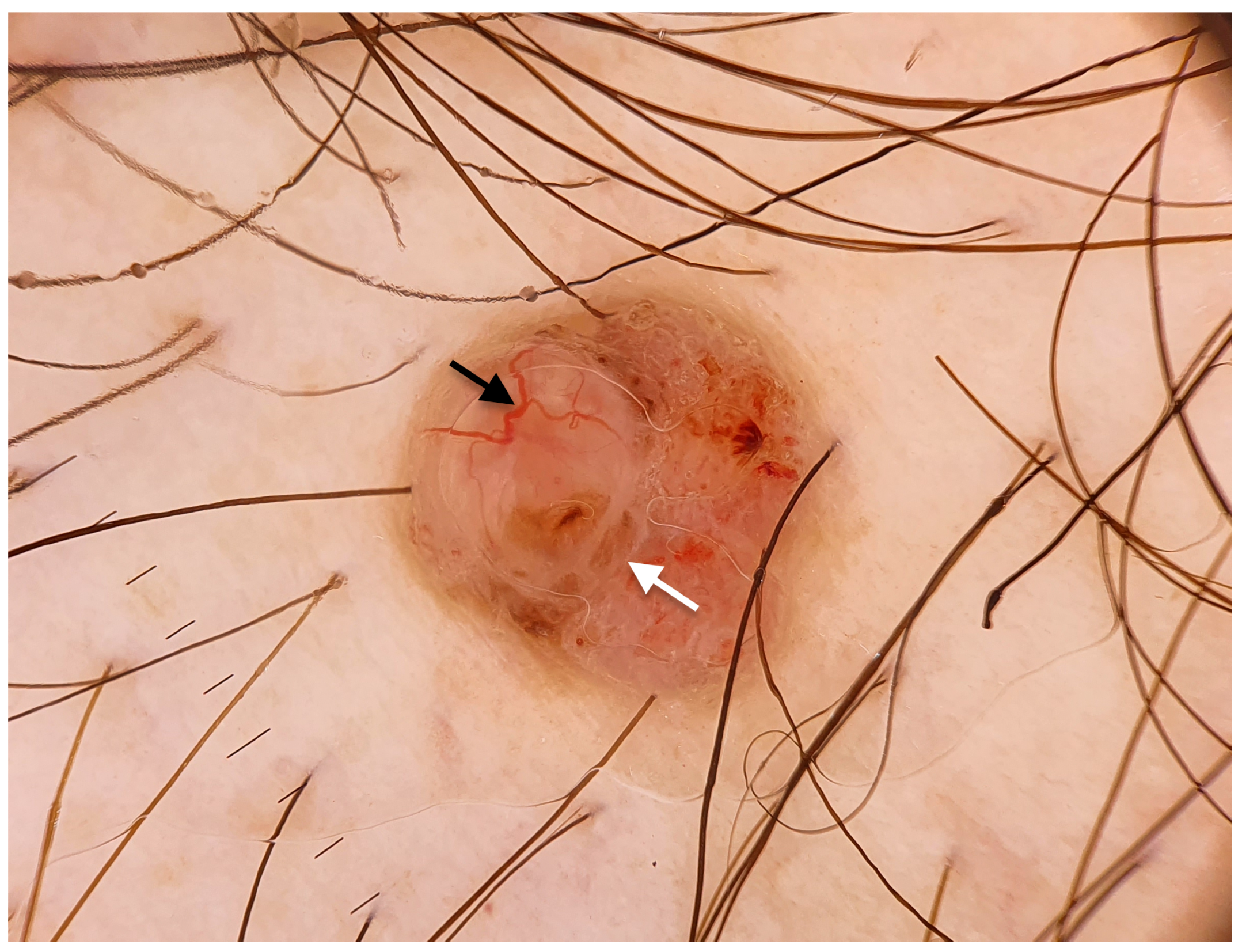
| Characteristic | Excision with 4 mm Margins, n (%) | C&ED, n (%) | p-Value |
|---|---|---|---|
| Total (n) | 142 (85) | 25 (15) | |
| Age (mean, SD) | 68.91 ± 14.6 | 66.28 ± 9.85 | 0.388 |
| Sex (Male) | 89 (62.7) | 23 (92) | 0.004 |
| Anatomic zone (H, M, L) | 0.033 | ||
| H | 40 (28.2) | 1 (4) | |
| M | 29 (20.4) | 6 (24) | |
| L | 73 (51.4) | 18 (72) | |
| Anatomic location | 0.001 | ||
| Head | 58 (40.8) | 1 (4) | |
| Neck | 6 (4.2) | 0 | |
| Thorax | 5 (3.5) | 4 (16) | |
| Abdomen | 7 (4.9) | 0 | |
| Back | 30 (21.1) | 8 (32) | |
| Anogenital | 0 | 0 | |
| Upper limbs | 21 (14.8) | 6 (24) | |
| Lower limbs | 15 (10.6) | 6 (24) | |
| Subtype | 0.008 | ||
| Infiltrative | 1 (0.7) | 1 (4) | |
| Micronodular | 5 (3.5) | 0 | |
| Morpheaform | 1 (0.7) | 0 | |
| Nodular | 68 (47.9) | 5 (20) | |
| Superficial | 42 (29.6) | 16 (64) | |
| Mixed | 17 (12) | 1 (4) | |
| Other (no BCC) | 7 (4.9) | 2 (8) | |
| Aggressive histopathologic subtype | 17 (12) | 2 (8) | 0.588 |
| Recurrent BCC | 7 (4.9) | 0 | 0.309 |
| Perineural invasion | 0 | 0 | NA |
| Radiotherapy ** | 8 (5.6) | 0 | 0.609 |
| Immunosuppression | 3 (2.1) | 0 | 0.607 |
| Characteristic | Streamlined Group | Biopsy Group | p-Value |
|---|---|---|---|
| n = 167 (n; %) | n = 222 (n; %) | ||
| Negative margins | 139 (97.9) * | NA | |
| Diagnostic error | 9 (5.4) | 59 (26.6) | <0.0001 |
| SCC | 3 (33.3) | 12 (20.3) | |
| AK | 0 | 11 (18.6) | |
| Adnexal Tumor | 1 (11.1) | 7 (11.9) | |
| Folliculitis | 0 | 6 (10.2) | |
| Seborrheic Keratosis | 1 (11.1) | 5 (8.4) | |
| LPLK | 0 | 4 (6.8) | |
| Dermal nevus | 1 (11.1) | 4 (6.8) | |
| Unspecific/Normal skin | 0 | 3 (5.1) | |
| Sebaceous Hyperplasia | 1 (11.1) | 2 (3.4) | |
| Telangiectatic Granuloma | 0 | 2 (3.4) | |
| Cyst | 0 | 1 (1.7) | |
| Melanoma | 0 | 1 (1.7) | |
| Foreign body | 0 | 1 (1.7) | |
| Atypical nevus | 1 (11.1) | 0 | |
| Sebaceous nevus | 1 (11.1) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donoso, F.; Aguero, R.; Caussade, M.-C.; Peirano, D.; Hidalgo, L.; Villagrán, S.; De Amesti, P.; Meza, V.; Hasenberg, J.; Droppelmann, K.; et al. Streamlined Management of Basal Cell Carcinoma with Dermoscopy: A Retrospective Case–Control Study. J. Clin. Med. 2025, 14, 8945. https://doi.org/10.3390/jcm14248945
Donoso F, Aguero R, Caussade M-C, Peirano D, Hidalgo L, Villagrán S, De Amesti P, Meza V, Hasenberg J, Droppelmann K, et al. Streamlined Management of Basal Cell Carcinoma with Dermoscopy: A Retrospective Case–Control Study. Journal of Clinical Medicine. 2025; 14(24):8945. https://doi.org/10.3390/jcm14248945
Chicago/Turabian StyleDonoso, Francisca, Rosario Aguero, Marie-Chantal Caussade, Dominga Peirano, Leonel Hidalgo, Sofía Villagrán, Pascal De Amesti, Víctor Meza, Josefina Hasenberg, Katherine Droppelmann, and et al. 2025. "Streamlined Management of Basal Cell Carcinoma with Dermoscopy: A Retrospective Case–Control Study" Journal of Clinical Medicine 14, no. 24: 8945. https://doi.org/10.3390/jcm14248945
APA StyleDonoso, F., Aguero, R., Caussade, M.-C., Peirano, D., Hidalgo, L., Villagrán, S., De Amesti, P., Meza, V., Hasenberg, J., Droppelmann, K., Abarzúa-Araya, Á., Camilo Castro-Ayala, J., Paoli, J., Uribe, P., & Navarrete-Dechent, C. (2025). Streamlined Management of Basal Cell Carcinoma with Dermoscopy: A Retrospective Case–Control Study. Journal of Clinical Medicine, 14(24), 8945. https://doi.org/10.3390/jcm14248945







