Non-Surgical Correction of Facial Asymmetry: A Narrative Review of Non-Surgical Modalities and Clinical Case Examples
Abstract
1. Introduction
Literature Search Strategy
2. Clinical Categories of Facial Asymmetry
2.1. Volume-Related Asymmetry
2.2. Soft Tissue Sagging and Skin Laxity
2.3. Dynamic (Muscular) Asymmetry
2.4. Superficial Skin Texture Irregularities
2.5. Composite (Mixed) Asymmetry
3. Non-Surgical Aesthetic Modalities
3.1. Dermal Fillers
3.1.1. Mechanism and Applications
3.1.2. Advantages and Immediate Aesthetic Outcomes
3.1.3. Potential Drawbacks
3.2. Collagen Stimulators (PDO Powder)
3.2.1. Composition and Mode of Action
3.2.2. Clinical Applications and Effectiveness in Asymmetry Correction
3.2.3. Safety Profile and Patient Acceptance
3.3. PDO Thread Lifting
3.3.1. Technique and Mechanistic Basis
3.3.2. Clinical Evidence and Patient Satisfaction
3.3.3. Specific Considerations in Facial Palsy-Related Asymmetry
3.4. Energy-Based Non-Invasive Devices
3.4.1. Types of Devices and Mechanisms of Collagen Remodeling
3.4.2. Clinical Efficacy, Limitations, and Recommended Treatment Protocols
3.5. Extracorporeal Shockwave Therapy
3.5.1. Overview and Types
3.5.2. Mechanism
3.5.3. Clinical Outcomes and Cumulative Effects
4. Advantages of Non-Surgical Facial Asymmetry Correction
4.1. Convenience of Procedure and Immediate Return to Daily Activities
4.2. Minimal Invasiveness and Reduced Discomfort
4.3. Rapid and Progressive Aesthetic Results
4.4. Versatility and Effective Integration into Clinical Practice
5. Discussion and Limitations
5.1. Discussion
5.2. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Guennouni, A.; Aallah, W.H.; Abourak, C.; Oukassem, S.; Ech-Cherif El Kettani, N.; Fikri, M.; Touarsa, F.; Jiddane, M.; Zekri, M.; Bellakhdar, M.; et al. A facial asymmetry revealed: Active mandibular condylar hyperplasia. Radiol. Case Rep. 2025, 20, 2463–2467. [Google Scholar] [CrossRef]
- Shackelford, T.K.; Larsen, R.J. Facial asymmetry as an indicator of psychological, emotional, and physiological distress. J. Pers. Soc. Psychol. 1997, 72, 456–466. [Google Scholar] [CrossRef]
- Bakri, M.M.H.; Vishvnathaiah, S.; Bakmani, H.F.; Hakami, A.J.; Zaidan, M.S.; Dighriri, M.A.; Jad, Y.A.; Hakami, T.M.; Bakri, H.M.H. Prevalence of mandibular asymmetries in the pediatric population of Jazan: A radiographic analytical study. Heliyon 2024, 10, e32362. [Google Scholar] [CrossRef] [PubMed]
- Chojdak-Łukasiewicz, J.; Paradowski, B. Facial Asymmetry: A Narrative Review of the Most Common Neurological Causes. Symmetry 2022, 14, 737. [Google Scholar] [CrossRef]
- Piao, Y.; Kim, S.J.; Yu, H.S.; Cha, J.Y.; Baik, H.S. Five-year investigation of a large orthodontic patient population at a dental hospital in South Korea. Korean J. Orthod. 2016, 46, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.V.V.; Potturi, A.; Rajan, R.; Jhawar, D.; Bharath Bhushan, Y.W.; Pasupuleti, A. Facial Asymmetry-Demystifying the Entity. J. Maxillofac. Oral. Surg. 2023, 22, 749–761. [Google Scholar] [CrossRef] [PubMed]
- La Touche, R.; Losana-Ferrer, A.; Pascual-Vaquerizo, E.; Suso-Martí, L.; Paris-Alemany, A.; Chamorro-Sánchez, J.; Cuenca-Martínez, F. Orofacial sensorimotor behaviour in unilateral chewing: A comparative analysis in asymptomatic population. Physiol. Behav. 2019, 212, 112718. [Google Scholar] [CrossRef]
- Ren, L.; Chen, P.; Musa, M.; Zhao, Y.; Awad, R.; Xiao, Z.; Li, C.; Li, D.; Chen, X. Quantitative and qualitative condylar changes Post-Stabilization splint in patients with temporomandibular disorder and chewing side preference. Sci. Rep. 2025, 15, 10996. [Google Scholar] [CrossRef]
- Heikkinen, E.V.; Vuollo, V.; Heikkinen, T.; Harila, V. Chewing Side Preference, Facial Asymmetry and Related Factors in the Northern Finland Birth Cohort 1986. Acta Odontol. Scand. 2024, 83, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.; Choi, D.; Ko, A.C.; Carter, K.D.; Shriver, E.M. The Effect of Sleep Position Preference on Eyelid and Eyebrow Symmetry. Ophthalmic Plast. Reconstr. Surg. 2022, 38, 266–269. [Google Scholar] [CrossRef]
- Ko, E.W.; Huang, C.S.; Lin, C.-H.; Chen, Y.-R. Orthodontic Perspective for Face Asymmetry Correction. Symmetry 2022, 14, 1822. [Google Scholar] [CrossRef]
- Kim, S.Y.; Choi, Y.H.; Kim, Y.K. Postoperative malocclusion after maxillofacial fracture management: A retrospective case study. Maxillofac. Plast. Reconstr. Surg. 2018, 40, 27. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Hsieh, E.Y.; Chen, S.H.; Pai, B.C.J.; Tsai, C.Y.; Wang, S.W.; Chou, P.Y. Occlusion-Based Three-Dimensional Craniofacial Anthropometric and Symmetric Evaluation in Preadolescences: A Comparative COHORT Study. J. Clin. Med. 2023, 12, 5017. [Google Scholar] [CrossRef] [PubMed]
- Otel, A.; Montiel-Company, J.M.; Zubizarreta-Macho, Á. Comparative Analysis of Early Class III Malocclusion Treatments—A Systematic Review and Meta-Analysis. Children 2025, 12, 177. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.M.; Patano, A.; Piras, F.; Ruvo, E.D.; Ferrante, L.; Noia, A.D.; Dongiovanni, L.; Palermo, A.; Inchingolo, F.; Inchingolo, A.D.; et al. Orthognathic Surgery and Relapse: A Systematic Review. Bioengineering 2023, 10, 1071. [Google Scholar] [CrossRef]
- Neeraj; Reddy, S.G.; Dixit, A.; Agarwal, P.; Chowdhry, R.; Chug, A. Relapse and temporomandibular joint dysfunction (TMD) as postoperative complication in skeletal class III patients undergoing bimaxillary orthognathic surgery: A systematic review. J. Oral Biol. Craniofac. Res. 2021, 11, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Al-Moghrabi, D.; Salazar, F.C.; Pandis, N.; Fleming, P.S. Compliance with removable orthodontic appliances and adjuncts: A systematic review and meta-analysis. Am. J. Orthod. Dentofac. Orthop. 2017, 152, 17–32. [Google Scholar] [CrossRef]
- Stefanovic, N.L.; Uhac, M.; Brumini, M.; Zigante, M.; Perkovic, V.; Spalj, S. Predictors of patient compliance during Class II division 1 malocclusion functional orthodontic treatment. Angle Orthod. 2021, 91, 502–508. [Google Scholar] [CrossRef]
- Kim, H.J.; Noh, H.K.; Park, H.S. Nonsurgical orthodontic correction of facial asymmetry by condylar remodeling and mandibular repositioning following occlusal cant correction with microimplants: A case report. Angle Orthod. 2023, 93, 111–125. [Google Scholar] [CrossRef]
- Li, K.; Meng, F.; Li, Y.R.; Tian, Y.; Chen, H.; Jia, Q.; Cai, H.; Jiang, H.B. Application of Nonsurgical Modalities in Improving Facial Aging. Int. J. Dent. 2022, 2022, 8332631. [Google Scholar] [CrossRef]
- Mahmood Faris, B.J. The Use of Facial Fillers in Clinical Practice: The Level of Patient Satisfaction and an Overview of Common Clinical Complications. Actas Dermo-Sifiliogr. 2024, 115, 458–465. [Google Scholar] [CrossRef]
- Jia, X.; Feng, Y. Energy-Based Skin Rejuvenation: A Review of Mechanisms and Thermal Effects. J. Cosmet. Dermatol. 2025, 24, e16657. [Google Scholar] [CrossRef]
- Wigley, C.H.; Janssen, T.J.; Mosahebi, A. Shock Wave Therapy in Plastic Surgery: A Review of the Current Indications. Aesthet. Surg. J. 2023, 43, 370–386. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Rai, R.; Kumar, S.; Mitra, B.; Chopra, A.; Singh, G.K.; Mitra, D.; Patil, C.; Sandhu, S. Safety and Efficacy of Restoring Facial Symmetry Using Polydioxanone Thread Face Lift Technique in Patients with Facial Palsy. J. Clin. Aesthet. Dermatol. 2022, 15, 26–29. [Google Scholar] [PubMed]
- Boehm, L.M.; Morgan, A.; Hettinger, P.; Matloub, H.S. Facial Aging: A Quantitative Analysis of Midface Volume Changes over 11 Years. Plast. Reconstr. Surg. 2021, 147, 319–327. [Google Scholar] [CrossRef]
- Mertens, A.; Foyatier, J.L.; Mojallal, A. Quantitative analysis of midface fat compartments mass with ageing and body mass index, anatomical study. Ann. Chir. Plast. Esthét. 2016, 61, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Sarigul Guduk, S.; Cevik Cenkeri, H.; Derin Cicek, E.; Kus, S. Evaluation of aging changes of the superficial fat compartments of the midface over time: A computed tomography study. J. Cosmet. Dermatol. 2022, 21, 1430–1435. [Google Scholar] [CrossRef]
- Iblher, N.; Stark, G.B.; Penna, V. The aging perioral region—Do we really know what is happening. J. Nutr. Health Aging 2012, 16, 581–585. [Google Scholar] [CrossRef]
- Wollina, U. Perioral rejuvenation: Restoration of attractiveness in aging females by minimally invasive procedures. Clin. Interv. Aging 2013, 8, 1149–1155. [Google Scholar] [CrossRef]
- Stutman, R.L.; Codner, M.A. Tear Trough Deformity: Review of Anatomy and Treatment Options. Aesthetic Surg. J. 2012, 32, 426–440. [Google Scholar] [CrossRef]
- Tao, B.K.; Butt, F.R.; Dhivagaran, T.; Balas, M.; Nijhawan, N.; Nassrallah, G.; Hussain, A.; Ing, E.B. Periocular Aging Across Populations and Esthetic Considerations: A Narrative Review. J. Clin. Med. 2025, 14, 535. [Google Scholar] [CrossRef]
- Russel, S.M.; Clark, J.M. Periorbital rejuvenation in the clinic: A state-of-the-art review. World J. Otorhinolaryngol. Head Neck Surg. 2023, 9, 242–248. [Google Scholar] [CrossRef]
- Farkas, J.P.; Pessa, J.E.; Hubbard, B.; Rohrich, R.J. The Science and Theory behind Facial Aging. Plast. Reconstr. Surg. Glob. Open 2013, 1, e8–e15. [Google Scholar] [CrossRef]
- Swift, A.; Liew, S.; Weinkle, S.; Garcia, J.K.; Silberberg, M.B. The Facial Aging Process From the "Inside Out". Aesthet. Surg. J. 2021, 41, 1107–1119. [Google Scholar] [CrossRef]
- Minelli, L.; Yang, H.-M.; van der Lei, B.; Mendelson, B. The Surgical Anatomy of the Jowl and the Mandibular Ligament Reassessed. Aesthetic Plast. Surg. 2023, 47, 170–180. [Google Scholar] [CrossRef]
- Kapoor, K.M.; Saputra, D.I.; Porter, C.E.; Colucci, L.; Stone, C.; Brenninkmeijer, E.E.A.; Sloane, J.; Sayed, K.; Winaya, K.K.; Bertossi, D. Treating Aging Changes of Facial Anatomical Layers with Hyaluronic Acid Fillers. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1105–1118. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.R.; Jeong, J.H.; Lee, W.S. Features of facial asymmetry following incomplete recovery from facial paralysis. Yonsei Med. J. 2010, 51, 943–948. [Google Scholar] [CrossRef]
- Guntinas-Lichius, O.; Prengel, J.; Cohen, O.; Mäkitie, A.A.; Vander Poorten, V.; Ronen, O.; Shaha, A.; Ferlito, A. Pathogenesis, diagnosis and therapy of facial synkinesis: A systematic review and clinical practice recommendations by the international head and neck scientific group. Front. Neurol. 2022, 13, 1019554. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.D.; Manoli, I.; Engle, E.C.; Jabs, E.W. A framework for the evaluation of patients with congenital facial weakness. Orphanet J. Rare Dis. 2021, 16, 158. [Google Scholar] [CrossRef]
- Jeong, K.Y.; Min, K.J.; Woo, J.; Yim, S.Y. Craniofacial Asymmetry in Adults With Neglected Congenital Muscular Torticollis. Ann. Rehabil. Med. 2015, 39, 440–450. [Google Scholar] [CrossRef]
- Meghe, S.R.; Khan, A.; Jangid, S.D.; Sarda, B.; Vangala, N.; Saoji, V. Shedding Light on Acne Scars: A Comprehensive Review of CO2 vs. Erbium-Doped Yttrium Aluminium Garnet (Er:YAG) Laser Therapy. Cureus 2024, 16, e57572. [Google Scholar] [CrossRef]
- Meghe, S.; Saoji, V.; Madke, B.; Singh, A. Efficacy of Microneedling and CO2 Laser for Acne Scar Remodelling: A Comprehensive Review. Cureus 2024, 16, e55092. [Google Scholar] [CrossRef]
- Chaudhary, D.C.; Kaur, S.; Bagga, D.S.; Sharma, V.; Deshmukh, A. Rarest muscular imbalance, neutral zone shift and facial asymmetry. Med. J. Armed Forces India 2015, 71, S472–S475. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U.; Kocic, H.; Goldman, A. Hyaluronic Acid in Facial Rehabilitation—A Narrative Review. Cosmetics 2023, 10, 61. [Google Scholar] [CrossRef]
- Juhász, M.L.W.; Levin, M.K.; Marmur, E.S. The Kinetics of Reversible Hyaluronic Acid Filler Injection Treated With Hyaluronidase. Dermatol. Surg. 2017, 43, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Kroumpouzos, G.; Treacy, P. Hyaluronidase for Dermal Filler Complications: Review of Applications and Dosage Recommendations. JMIR Dermatol. 2024, 7, e50403. [Google Scholar] [CrossRef] [PubMed]
- Cabral, L.R.B.; Teixeira, L.N.; Gimenez, R.P.; Demasi, A.P.D.; de Brito Junior, R.B.; de Araújo, V.C.; Martinez, E.F. Effect of Hyaluronic Acid and Poly-L-Lactic Acid Dermal Fillers on Collagen Synthesis: An in vitro and in vivo Study. Clin. Cosmet. Investig. Dermatol. 2020, 13, 701–710. [Google Scholar] [CrossRef]
- Attenello, N.H.; Maas, C.S. Injectable fillers: Review of material and properties. Facial Plast. Surg. 2015, 31, 29–34. [Google Scholar] [CrossRef]
- Fundarò, S.P.; Salti, G.; Malgapo, D.M.H.; Innocenti, S. The Rheology and Physicochemical Characteristics of Hyaluronic Acid Fillers: Their Clinical Implications. Int. J. Mol. Sci. 2022, 23, 10518. [Google Scholar] [CrossRef] [PubMed]
- da Costa, A.; Biccigo, D.G.Z.; de Souza Weimann, E.T.; Mercadante, L.M.; Oliveira, P.R.G.; Prebianchi, S.B.; Abdalla, B.M.Z. Durability of Three Different Types of Hyaluronic Acid Fillers in Skin: Are There Differences Among Biphasic, Monophasic Monodensified, and Monophasic Polydensified Products? Aesthetic Surg. J. 2017, 37, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Salti, G.; Siquier-Dameto, G.; Rharbaoui, S.; Hernandez Malgapo, D.M.; Innocenti, S.; Manni, M. An Interim 6-Month Analysis of the Dermatologic Effects and Midface Volume Correction With XTR CL Filler in a Prospective, Single-Center Study. Dermatol. Surg. 2023, 49, 943–948. [Google Scholar] [CrossRef]
- Owczarczyk-Saczonek, A.; Zdanowska, N.; Wygonowska, E.; Placek, W. The Immunogenicity of Hyaluronic Fillers and Its Consequences. Clin. Cosmet. Investig. Dermatol. 2021, 14, 921–934. [Google Scholar] [CrossRef]
- Kaufman-Janette, J.; Taylor, S.C.; Cox, S.E.; Weinkle, S.H.; Smith, S.; Kinney, B.M. Efficacy and safety of a new resilient hyaluronic acid dermal filler, in the correction of moderate-to-severe nasolabial folds: A 64-week, prospective, multicenter, controlled, randomized, double-blind and within-subject study. J. Cosmet. Dermatol. 2019, 18, 1244–1253. [Google Scholar] [CrossRef]
- Jacovella, P.F. Use of calcium hydroxylapatite (Radiesse) for facial augmentation. Clin. Interv. Aging 2008, 3, 161–174. [Google Scholar] [CrossRef]
- Radilla-Flores, M.d.C.; Márquez-Gutiérrez, E.A.; Vélez-Palafox, M.; Castrejón-Vázquez, M.I.; Chávez-Flores, O.C.; Chopin-Doroteo, M.; González-Torres, M. Feasibility of calcium hydroxyapatite (Radiesse®) for improving the biomechanical properties of facial burn scars: A pilot study. JPRAS Open 2025, 44, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Loghem, J.V.; Yutskovskaya, Y.A.; Philip Werschler, W. Calcium hydroxylapatite: Over a decade of clinical experience. J. Clin. Aesthet. Dermatol. 2015, 8, 38–49. [Google Scholar]
- Su, D.; Yang, W.; He, T.; Wu, J.; Zou, M.; Liu, X.; Li, R.; Wang, S.; Lai, C.; Wang, J. Clinical applications of a novel poly-L-lactic acid microsphere and hyaluronic acid suspension for facial depression filling and rejuvenation. J. Cosmet. Dermatol. 2024, 23, 3508–3516. [Google Scholar] [CrossRef] [PubMed]
- Avelar, L.E.; Nabhani, S.; Wüst, S. Unveiling the Mechanism: Injectable Poly-L-Lactic Acid’s Evolving Role-Insights From Recent Studies. J. Cosmet. Dermatol. 2025, 24, e16635. [Google Scholar] [CrossRef]
- Ao, Y.J.; Yi, Y.; Wu, G.H. Application of PLLA (Poly-L-Lactic acid) for rejuvenation and reproduction of facial cutaneous tissue in aesthetics: A review. Medicine 2024, 103, e37506. [Google Scholar] [CrossRef] [PubMed]
- Ditre, C.M. Facial aesthetic correction with injectable poly-L-lactic Acid following removal of malar cheek implants. J. Clin. Aesthet. Dermatol. 2009, 2, 32–35. [Google Scholar] [PubMed]
- Rivkin, A. PMMA-collagen Gel in Nonsurgical Rhinoplasty Defects: A Methodological Overview and 15-year Experience. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4477. [Google Scholar] [CrossRef]
- Limongi, R.M.; Tao, J.; Borba, A.; Pereira, F.; Pimentel, A.R.; Akaishi, P.; Velasco e Cruz, A.A. Complications and Management of Polymethylmethacrylate (PMMA) Injections to the Midface. Aesthetic Surg. J. 2015, 36, 132–135. [Google Scholar] [CrossRef]
- Park, T.H.; Seo, S.W.; Kim, J.K.; Chang, C.H. Clinical experience with polymethylmethacrylate microsphere filler complications. Aesthetic Plast. Surg. 2012, 36, 421–426. [Google Scholar] [CrossRef]
- Clark, N.W.; Pan, D.R.; Barrett, D.M. Facial fillers: Relevant anatomy, injection techniques, and complications. World J. Otorhinolaryngol. Head Neck Surg. 2023, 9, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Funt, D.; Pavicic, T. Dermal fillers in aesthetics: An overview of adverse events and treatment approaches. Clin. Cosmet. Investig. Dermatol. 2013, 6, 295–316. [Google Scholar] [CrossRef]
- Biesman, B.S.; Green, J.B.; George, R.; Jacob, C.; Palm, M.; Jones, D.H.; Grunebaum, L.; Beer, K.; Cho, Y.; Joseph, J.H.; et al. A Multicenter, Randomized, Evaluator-Blinded Study to Examine the Safety and Effectiveness of Hyaluronic Acid Filler in the Correction of Infraorbital Hollows. Aesthet. Surg. J. 2024, 44, 1001–1013. [Google Scholar] [CrossRef]
- Bhojani-Lynch, T.; Deckers, A.; Ohanes, O.; Poupard, K.; Maffert, P. A Prospective, Observational Registry Study to Evaluate Effectiveness and Safety of Hyaluronic Acid-Based Dermal Fillers in Routine Practice: Interim Analysis Results with One Year of Subject Follow-Up. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Kim, Y.J. Foreign body granulomas after the use of dermal fillers: Pathophysiology, clinical appearance, histologic features, and treatment. Arch. Plast. Surg. 2015, 42, 232–239. [Google Scholar] [CrossRef]
- Hong, G.W.; Hu, H.; Chang, K.; Park, Y.; Lee, K.W.A.; Chan, L.K.W.; Yi, K.H. Review of the Adverse Effects Associated with Dermal Filler Treatments: Part I Nodules, Granuloma, and Migration. Diagnostics 2024, 14, 1640. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahawi, S.; Ehsani, A.; Jozdani, T.; Rahimnia, A.; Ehsani, A.H.; Razavi, Z.; Emadi, S.N. The Demographics of Patients With Dermal Filler Complications. J. Cosmet. Dermatol. 2025, 24, e70043. [Google Scholar] [CrossRef]
- Dierckx, S.; Patrizi, M.; Merino, M.; González, S.; Mullor, J.L.; Nergiz-Unal, R. Collagen peptides affect collagen synthesis and the expression of collagen, elastin, and versican genes in cultured human dermal fibroblasts. Front. Med. 2024, 11, 1397517. [Google Scholar] [CrossRef]
- Bernardo, R.T.R.; Oliveira, R.C.G.; Freitas, K.M.S.; Albergaria-Barbosa, J.R.; Rizzatti-Barbosa, C.M. Effect of poly-L-lactic acid and polydioxanone biostimulators on type I and III collagen biosynthesis. Skin Res. Technol. 2024, 30, e13681. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.M.; Kim, B.Y.; Hye Suh, D.; Lee, S.J.; Moon, H.R.; Ryu, H.J. The efficacy of powdered polydioxanone in terms of collagen production compared with poly-L-lactic acid in a murine model. J. Cosmet. Dermatol. 2019, 18, 1893–1898. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Kang, S.M.; Gu, Y.J.; Zhang, X.R.; Yon, D.K.; Shin, B.H.; Ham, J.R.; Lee, W.K.; Jeong, J.G.; Kwon, H.J.; et al. Bio-characteristics and Efficacy Analysis of Biodegradable Poly Dioxanone Dermal Filler in a Mouse Model and Humans. In Vivo 2023, 37, 1093–1102. [Google Scholar] [CrossRef]
- Sedush, N.G.; Kalinin, K.T.; Azarkevich, P.N.; Gorskaya, A.A. Physicochemical Characteristics and Hydrolytic Degradation of Polylactic Acid Dermal Fillers: A Comparative Study. Cosmetics 2023, 10, 110. [Google Scholar] [CrossRef]
- Bolke, L.; Schlippe, G.; Gerß, J.; Voss, W. A Collagen Supplement Improves Skin Hydration, Elasticity, Roughness, and Density: Results of a Randomized, Placebo-Controlled, Blind Study. Nutrients 2019, 11, 2494. [Google Scholar] [CrossRef]
- Trehan, A.; Anand, R.; Chaudhary, G.; Garg, H.; Verma, M.K. Efficacy and Safety of Skin Radiance Collagen on Skin and Hair Matrix: A Placebo-Controlled Clinical Trial in Healthy Human Subjects. Clin. Cosmet. Investig. Dermatol. 2024, 17, 581–591. [Google Scholar] [CrossRef]
- Hong, G.W.; Kim, S.B.; Park, S.Y.; Wan, J.; Yi, K.H. Thread Lifting Materials: A Review of Its Difference in Terms of Technical and Mechanical Perspective. Clin. Cosmet. Investig. Dermatol. 2024, 17, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Giang, N.N.; Kim, H.J.; Chien, P.N.; Kwon, H.J.; Ham, J.R.; Lee, W.K.; Gu, Y.J.; Zhou, S.Y.; Zhang, X.R.; Nam, S.Y.; et al. An evaluation of the effectiveness of ‘ULTRACOL 200’ in enhancing nasolabial fold wrinkles through cutaneous repair. Skin Res. Technol. 2024, 30, e13679. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Wang, S.; He, T.; Wang, J. Experimental investigation of biostimulatory effects after polydioxanone thread insertion in a pig model. J. Cosmet. Dermatol. 2024, 23, 658–665. [Google Scholar] [CrossRef]
- Sulyman, O.; Cristel, R.; Gandhi, N.; Kola, E.; Borst, S.G.; Caughlin, B.; Dayan, S. Non-surgical rhinoplasty using polydioxanone threads. J. Cosmet. Dermatol. 2024, 23, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Unal, M.; İslamoğlu, G.K.; Ürün Unal, G.; Köylü, N. Experiences of barbed polydioxanone (PDO) cog thread for facial rejuvenation and our technique to prevent thread migration. J. Dermatol. Treat. 2021, 32, 227–230. [Google Scholar] [CrossRef]
- Ali, Y.H. Two years’ outcome of thread lifting with absorbable barbed PDO threads: Innovative score for objective and subjective assessment. J. Cosmet. Laser Ther. 2018, 20, 41–49. [Google Scholar] [CrossRef]
- Niu, Z.; Han, Y.; Jin, R.; Li, Y.; Liu, J.; Li, N.; Li, W.; Li, D.; Chen, Y.; Han, Y. Complications Following Facial Thread-Lifting. Chin. J. Plast. Reconstr. Surg. 2020, 2, 204–211. [Google Scholar] [CrossRef]
- Contreras, C.; Ariza-Donado, A.; Ariza-Fontalvo, A. Using PDO threads: A scarcely studied rejuvenation technique. Case report and systematic review. J. Cosmet. Dermatol. 2023, 22, 2158–2165. [Google Scholar] [CrossRef] [PubMed]
- Zhukova, O.; Dydykin, S.; Kubíková, E.; Markova, N.; Vasil’ev, Y.; Kapitonova, M. A New Complex Minimally Invasive Thread Lift Method for One-Time Three-Step Fixation of the Face and Neck Soft Tissues. Arch. Plast. Surg. 2022, 49, 296–303. [Google Scholar] [CrossRef]
- Burko, P.; Sulamanidze, G.; Nikishin, D. Efficacy of Lifting Threads Composed of Poly(L-Lactide-Co-ε-Caprolactone) Copolymers Coated With Hyaluronic Acid: A Long-Term Study on Biorevitalizing Properties in Skin Remodeling. J. Cosmet. Dermatol. 2025, 24, e70077. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.W.; Shin, B.H.; Heo, C.Y.; Shim, J.H. Efficacy study of the new polycaprolactone thread compared with other commercialized threads in a murine model. J. Cosmet. Dermatol. 2021, 20, 2743–2749. [Google Scholar] [CrossRef]
- Hügül, H.; Özkoca, D.; Kutlubay, Z. Thread lifting: Does patient satisfaction change according to age, type of threads used, number of threads used and treatment area? J. Cosmet. Dermatol. 2022, 21, 1936–1939. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.O.; Karypidis, D. Validation of Non-surgical Facial Lifting with PDO Thread using a 3D system. Adv. Oral Maxillofac. Surg. 2023, 10, 100411. [Google Scholar] [CrossRef]
- Ehlinger-David, A.; Gorj, M.; Braccini, F.; Loreto, F.; Grand-Vincent, A.; Garcia, P.; Taieb, M.; Benadiba, L.; Catoni, I.; Mathey, E.R.; et al. A prospective multicenter clinical trial evaluating the efficacy and safety of a hyaluronic acid-based filler with Tri-Hyal technology in the treatment of lips and the perioral area. J. Cosmet. Dermatol. 2023, 22, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.H.; Park, S.Y. Facial Thread Lifting Complications. J. Cosmet. Dermatol. 2025, 24, e16745. [Google Scholar] [CrossRef]
- Alster, T.S.; Lupton, J.R. Nonablative cutaneous remodeling using radiofrequency devices. Clin. Dermatol. 2007, 25, 487–491. [Google Scholar] [CrossRef]
- Byun, J.W.; Kang, Y.R.; Park, S.; Hong, W. Efficacy of radiofrequency combined with single-dot ultrasound efficacy for skin rejuvenation: A non-randomized split-face trial with blinded response evaluation. Skin Res. Technol. 2023, 29, e13452. [Google Scholar] [CrossRef]
- Dayan, E.; Theodorou, S. Not all Radiofrequency Devices Are Created Equal: A Thermal Assessment. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4077. [Google Scholar] [CrossRef]
- el-Domyati, M.; el-Ammawi, T.S.; Medhat, W.; Moawad, O.; Brennan, D.; Mahoney, M.G.; Uitto, J. Radiofrequency facial rejuvenation: Evidence-based effect. J. Am. Acad. Dermatol. 2011, 64, 524–535. [Google Scholar] [CrossRef]
- Oh, S.; Rhee, D.Y.; Batsukh, S.; Son, K.H.; Byun, K. High-Intensity Focused Ultrasound Increases Collagen and Elastin Fiber Synthesis by Modulating Caveolin-1 in Aging Skin. Cells 2023, 12, 2275. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, E.; Kim, J.; Ro, Y.; Ko, J. High-Intensity Focused Ultrasound for the Treatment of Wrinkles and Skin Laxity in Seven Different Facial Areas. Ann. Dermatol. 2015, 27, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, X.; Li, H.; Fu, C.; Jiang, L.; Hu, Y.; Huang, J.; Chen, J.; Zeng, Q. Dynamic panoramic presentation of skin function after fractional CO(2) laser treatment. iScience 2023, 26, 107559. [Google Scholar] [CrossRef]
- Preissig, J.; Hamilton, K.; Markus, R. Current Laser Resurfacing Technologies: A Review that Delves Beneath the Surface. Semin. Plast. Surg. 2012, 26, 109–116. [Google Scholar] [CrossRef]
- Shin, J.; Sung, Y.; Jin, S.; Hwang, C.-L.; Kim, H.; Hong, D.; Jung, K.E.; Seo, Y.-J.; Lee, Y. Efficacy and Safety of Monopolar Radiofrequency for Tightening the Skin of Aged Faces. Cosmetics 2024, 11, 71. [Google Scholar] [CrossRef]
- Werschler, W.P.; Werschler, P.S. Long-term Efficacy of Micro-focused Ultrasound with Visualization for Lifting and Tightening Lax Facial and Neck Skin Using a Customized Vectoring Treatment Method. J. Clin. Aesthet. Dermatol. 2016, 9, 27–33. [Google Scholar]
- Meaike, J.D.; Agrawal, N.; Chang, D.; Lee, E.I.; Nigro, M.G. Noninvasive Facial Rejuvenation. Part 3: Physician-Directed-Lasers, Chemical Peels, and Other Noninvasive Modalities. Semin. Plast. Surg. 2016, 30, 143–150. [Google Scholar] [CrossRef]
- Piet, A.; Jablonski, L.; Daniel Onwuchekwa, J.I.; Unkel, S.; Weber, C.; Grzegorzek, M.; Ehlers, J.P.; Gaus, O.; Neumann, T. Non-Invasive Wearable Devices for Monitoring Vital Signs in Patients with Type 2 Diabetes Mellitus: A Systematic Review. Bioengineering 2023, 10, 1321. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wu, W. Multimodal non-invasive non-pharmacological therapies for chronic pain: Mechanisms and progress. BMC Med. 2023, 21, 372. [Google Scholar]
- Mysore, V.; Deepthi, M.; Chandrashekar, B.S.; Shah, S.D.; Gold, M.H.; Shivani, S.R.; Kanumuru, P.; Anirudh, P. Standard operating protocol for utilizing energy-based devices in aesthetic practice. J. Cosmet. Dermatol. 2024, 23, 3809–3820. [Google Scholar] [CrossRef] [PubMed]
- Haykal, D. Emerging and Pioneering AI Technologies in Aesthetic Dermatology: Sketching a Path Toward Personalized, Predictive, and Proactive Care. Cosmetics 2024, 11, 206. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Shi, L.; Wang, P.; Gao, F.; Sun, W. Effectiveness of Focused Shockwave Therapy versus Radial Shockwave Therapy for Noncalcific Rotator Cuff Tendinopathies: A Randomized Clinical Trial. Biomed. Res. Int. 2021, 2021, 6687094. [Google Scholar] [CrossRef] [PubMed]
- Ko, N.Y.; Chang, C.N.; Cheng, C.H.; Yu, H.K.; Hu, G.C. Comparative Effectiveness of Focused Extracorporeal versus Radial Extracorporeal Shockwave Therapy for Knee Osteoarthritis-Randomized Controlled Study. Int. J. Environ. Res. Public Health 2022, 19, 9001. [Google Scholar] [CrossRef] [PubMed]
- Alshihri, A.; Kämmerer, P.W.; Heimes, D.; Niu, W.; Alnassar, T.; Spector, M. Extracorporeal Shock Wave Stimulates Angiogenesis and Collagen Production in Facial Soft Tissue. J. Surg. Res. 2020, 245, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Simplicio, C.L.; Purita, J.; Murrell, W.; Santos, G.S.; Dos Santos, R.G.; Lana, J. Extracorporeal shock wave therapy mechanisms in musculoskeletal regenerative medicine. J. Clin. Orthop. Trauma 2020, 11, S309–S318. [Google Scholar] [CrossRef]
- Lv, F.; Li, Z.; Jing, Y.; Sun, L.; Li, Z.; Duan, H. The effects and underlying mechanism of extracorporeal shockwave therapy on fracture healing. Front. Endocrinol. 2023, 14, 1188297. [Google Scholar] [CrossRef]
- Sukubo, N.G.; Tibalt, E.; Respizzi, S.; Locati, M.; d’Agostino, M.C. Effect of shock waves on macrophages: A possible role in tissue regeneration and remodeling. Int. J. Surg. 2015, 24, 124–130. [Google Scholar] [CrossRef]
- Kou, D.; Chen, Q.; Wang, Y.; Xu, G.; Lei, M.; Tang, X.; Ni, H.; Zhang, F. The application of extracorporeal shock wave therapy on stem cells therapy to treat various diseases. Stem Cell Res. Ther. 2024, 15, 271. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lyu, K.; Lu, J.; Jiang, L.; Zhu, B.; Liu, X.; Li, Y.; Liu, X.; Long, L.; Wang, X.; et al. Biological response of extracorporeal shock wave therapy to tendinopathy in vivo (review). Front. Vet. Sci. 2022, 9, 851894. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Cho, S.B. Clinical Efficacy and Safety of Low-Energy Extracorporeal Shock Wave Therapy for Various Conditions of Deep Dermal and Subdermal Fibrosis. Skin Res. Technol. 2024, 30, e70082. [Google Scholar] [CrossRef]
- de Lima Morais, T.M.; Meyer, P.F.; de Vasconcellos, L.S.; e Silva, J.C.; e Andrade, I.F.; de Farias, V.A.F.; da Silva, I.C.; Araújo, R.M.F.G.; da Silva, R.M.V.; Pacheco, E.F.; et al. Effects of the extracorporeal shock wave therapy on the skin: An experimental study. Lasers Med. Sci. 2019, 34, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Tanaka, Y. Facial Tightening Effects, Following Focused and Radial Acoustic Wave Therapy Assessment, Using a Three-Dimensional Digital Imaging. Lasers Surg. Med. 2021, 53, 630–639. [Google Scholar] [CrossRef]
- Knobloch, K.; Kraemer, R. Extracorporeal shock wave therapy (ESWT) for the treatment of cellulite—A current metaanalysis. Int. J. Surg. 2015, 24, 210–217. [Google Scholar] [CrossRef]
- Goel, A.; Rai, K. Non-surgical facelift-by PDO threads and dermal filler: A case report. J. Cosmet. Dermatol. 2022, 21, 4241–4244. [Google Scholar] [CrossRef]
- Levy, L.L.; Emer, J.J. Complications of minimally invasive cosmetic procedures: Prevention and management. J. Cutan. Aesthet. Surg. 2012, 5, 121–132. [Google Scholar] [PubMed]
- Rahbin, S.; Sunnergren, O.; McBride, E.; Darabi, H.; Alinasab, B. Does More Invasive Surgery Result in Higher Patient Satisfaction? A Long-Term Follow-Up of 136 Zygomaticomaxillary Complex Fractures. Craniomaxillofac. Trauma Reconstr. 2024, 17, Np271–Np280. [Google Scholar] [CrossRef] [PubMed]
- Harb, A.; Abdul-Razzak, A. Nonsurgical Correction of Surgical Rhinoplasty Complications with Hyaluronic Acid Fillers: A Retrospective Review of 2088 Cases. Plast. Reconstr. Surg. Glob. Open 2024, 12, e6126. [Google Scholar] [CrossRef]
- Rękas-Dudziak, A.; Męcińska-Jundziłł, K.; Walkowiak, K.; Witmanowski, H. The use of local anaesthetics in dermatology, aesthetic medicine and plastic surgery: Review of the literature. Postep. Dermatol. Alergol. 2023, 40, 22–27. [Google Scholar] [CrossRef]
- Smith, L.; Cockerham, K. Hyaluronic acid dermal fillers: Can adjunctive lidocaine improve patient satisfaction without decreasing efficacy or duration? Patient Prefer. Adherence 2011, 5, 133–139. [Google Scholar]
- Alenazi, N.F.; AlBattal, N.Z.; Albalawi, I.A.S.; Saleh, N.A.; Alnaim, M.F.; Mahmoud, A.Z.B.; Vasilescu, D.C. Rate and Predictors of Satisfaction after Noninvasive Facial Cosmetic Procedures: A National Study in Saudi Arabia. Plast. Reconstr. Surg. Glob. Open 2024, 12, e5607. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.L.; Rivkin, A.; Dayan, S.; Shamban, A.; Werschler, W.P.; Teller, C.F.; Kaminer, M.S.; Sykes, J.M.; Weinkle, S.H.; Garcia, J.K. Multimodal Facial Aesthetic Treatment on the Appearance of Aging, Social Confidence, and Psychological Well-being: HARMONY Study. Aesthet. Surg. J. 2022, 42, Np115–Np124. [Google Scholar] [CrossRef]
- Nestor, M.S. Facial Lift and Patient Satisfaction Following Treatment with Absorbable Suspension Sutures: 12-Month Data from a Prospective, Masked, Controlled Clinical Study. J. Clin. Aesthet. Dermatol. 2019, 12, 18–26. [Google Scholar] [PubMed]
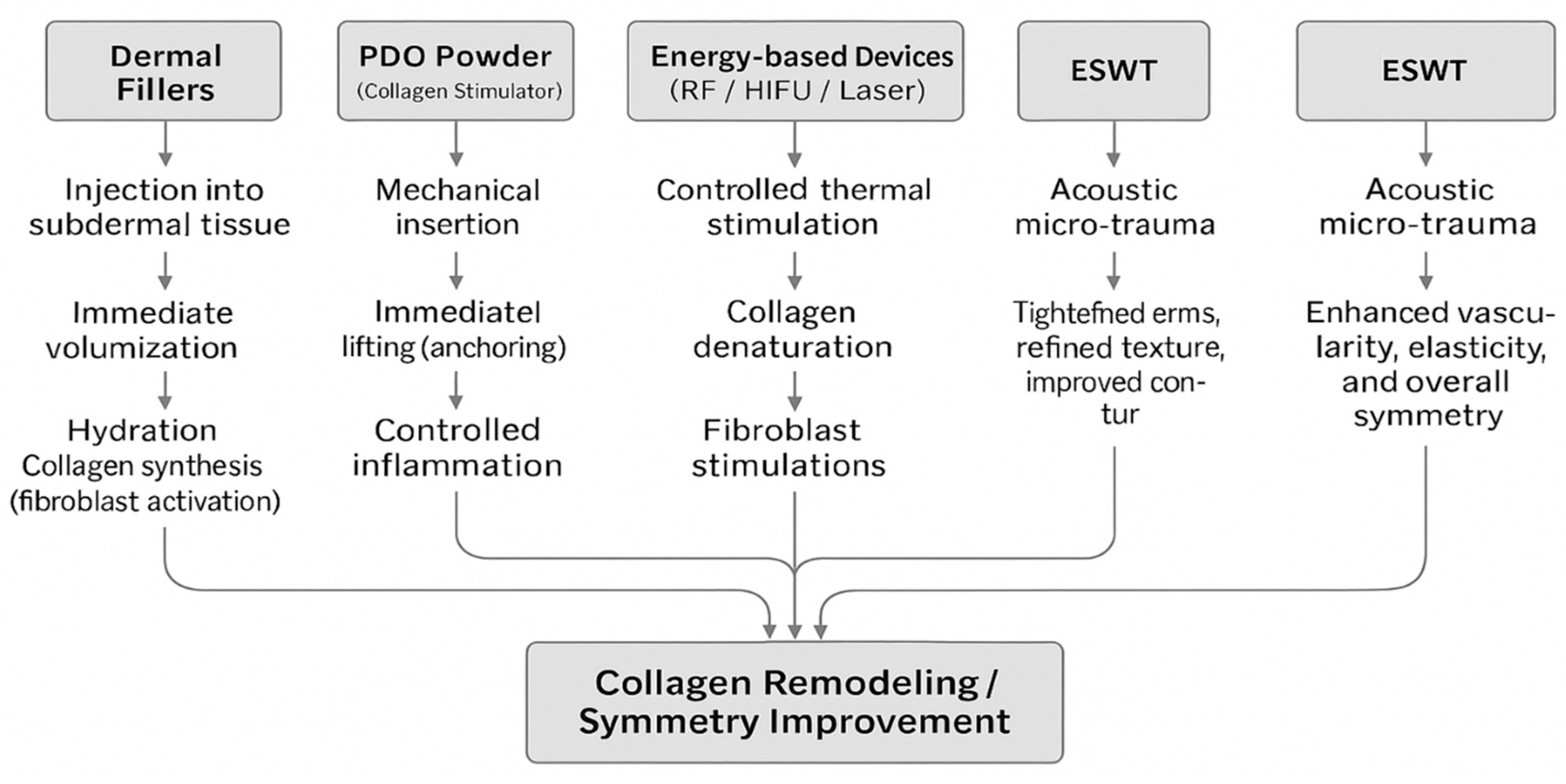
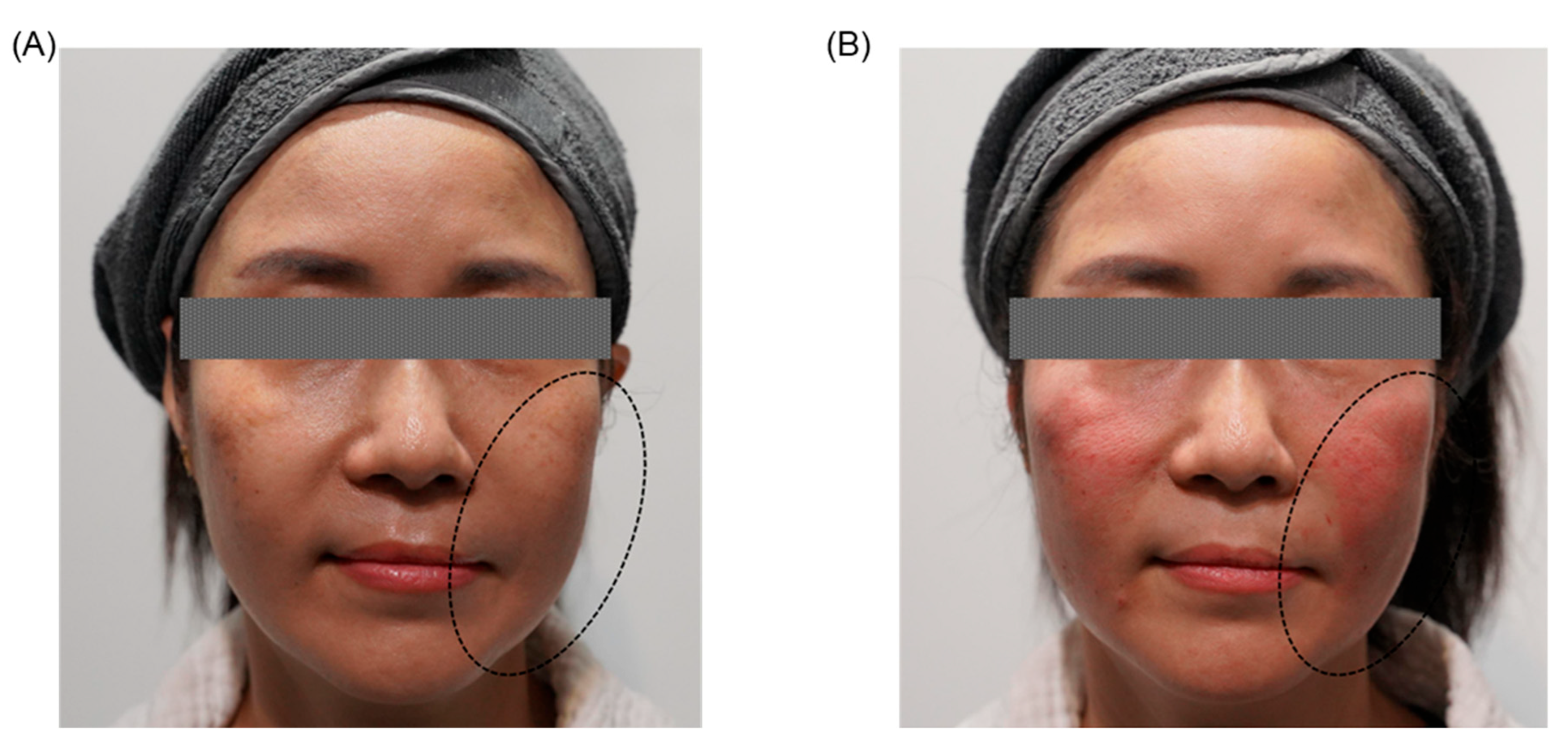
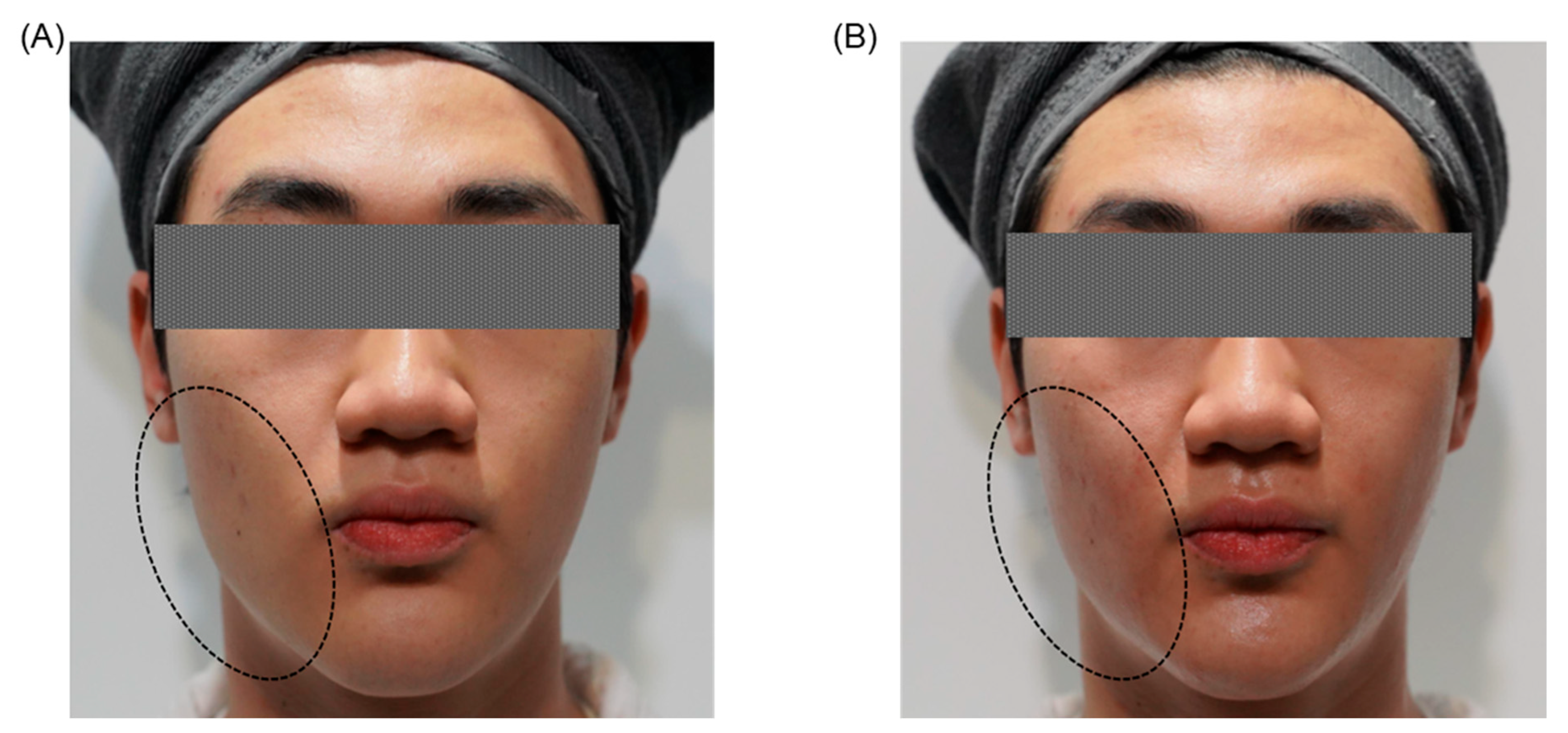
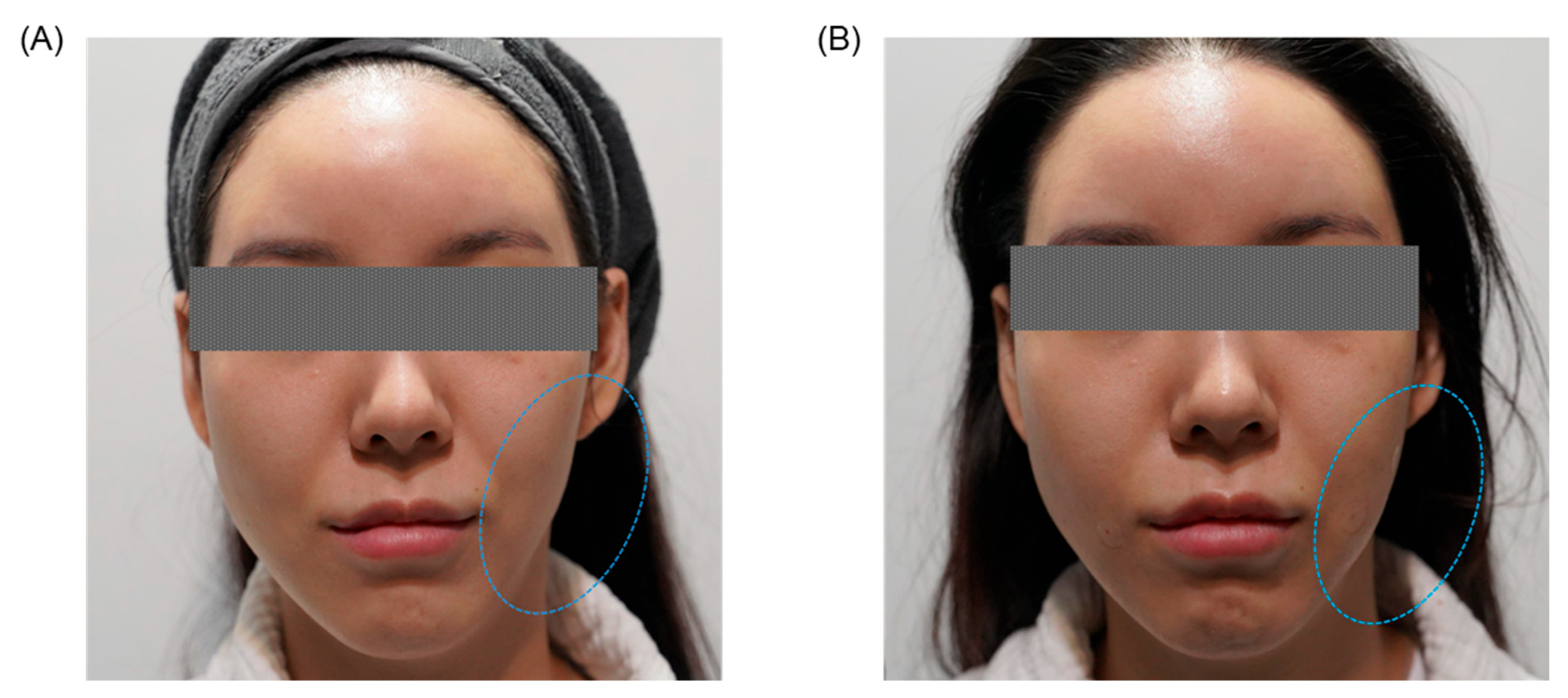
| Clinical Category | Key Clinical Findings |
|---|---|
| Volume-related Asymmetry | Uneven cheek contours, lip asymmetry, infraorbital hollowing (tear trough deformity) |
| Soft Tissue Sagging and Skin Laxity | Drooping eyebrows, accentuated nasolabial folds, irregular jawlines (jowls formation) |
| Dynamic (Muscular) Asymmetry | Asymmetric facial expressions, uneven smiles, involuntary muscle contractions (synkinesis) |
| Superficial Skin Texture Irregularities | Fine lines, wrinkles, acne scars, uneven skin texture and tone |
| Composite (Mixed) Asymmetry | A combination of volume depletion, skin laxity, muscular imbalance, and superficial skin irregularities |
| Aesthetic Modality | Key Mechanisms and Clinical Effects |
|---|---|
| Dermal Fillers | Immediate volume restoration; stimulates collagen production, enhances facial symmetry and contours |
| Collagen Stimulators (Polydioxanone Powder) | Gradual biostimulation; significant collagen synthesis, enhances skin elasticity, volume, and structural support |
| Polydioxanone Thread Lifting | Immediate mechanical lifting; stimulates collagen synthesis, provides structural support, enhances skin elasticity and facial contours |
| Energy-based Non-invasive Devices | Stimulates dermal collagen remodeling through controlled thermal/mechanical injury; improves skin firmness, elasticity, and contours |
| Extracorporeal Shockwave Therapy | Mechanical stimulation inducing biological tissue regeneration; promotes neocollagenesis, angiogenesis, improves tissue elasticity and symmetry |
| Modality | Mechanism of Action | Onset of Effect | Duration of Effect | Major Advantages | Limitations/Complications |
|---|---|---|---|---|---|
| Dermal Fillers (HA, CaHA, PLLA, PMMA) | Immediate volume restoration and collagen stimulation | Immediate to 1 week | 6–24 months (depends on filler type) | Instant results, reversible (HA), customizable | Edema, bruising, nodule or migration (<5%) |
| Collagen Stimulator (PDO Powder) | Induces fibroblast activation and neocollagenesis | 4–6 weeks | 12–24 months | Gradual natural correction, safe biodegradation, minimal downtime | Mild swelling, erythema, rare granuloma (<1%) |
| PDO Thread Lifting | Mechanical lifting + collagen induction | Immediate | 12–18 months | Dual effect (instant lift + collagen), minimal invasiveness | Temporary dimpling, mild asymmetry, thread migration (<5%) |
| Energy-based Devices (RF, HIFU, Laser) | Controlled dermal heating → collagen remodeling | 2–4 weeks | 6–24 months | Non-invasive, improves texture and firmness | Multiple sessions required, transient erythema |
| Extracorporeal Shockwave Therapy (ESWT) | Mechanical acoustic stimulation → angiogenesis and collagen synthesis | Gradual (2–4 weeks) | 6–12 months | Enhances elasticity, no downtime, safe | Mild erythema or tenderness |
| Modality | Representative Complication | Estimated Incidence | Underlying Cause/Mechanism | Recommended Management or Prevention |
|---|---|---|---|---|
| Dermal Fillers (HA, CaHA, PLLA, PMMA) | Edema, bruising, nodule formation, vascular occlusion (rare) | <5% | Improper injection depth, intravascular injection, overcorrection | Gentle massage for minor irregularities; hyaluronidase for HA fillers; avoid high-pressure injection; use aspiration technique |
| Collagen Stimulator (PDO Powder) | Mild swelling, erythema, transient tenderness | <3% | Local inflammatory reaction to polymer degradation | Cold compress, short-course NSAIDs; adhere to aseptic injection technique |
| PDO Thread Lifting | Thread migration, surface irregularity, dimpling, asymmetry | 3–5% | Inaccurate vector placement, superficial insertion, excessive tension | Early manual correction or thread removal; meticulous vector planning; proper depth and tension control |
| Energy-based Devices (RF, HIFU, Laser) | Transient erythema, edema, rare burns or dyschromia | 1–3% | Excessive energy or overlap of treatment passes | Cooling, topical steroid or emollient; proper energy calibration and operator training |
| Extracorporeal Shockwave Therapy (ESWT) | Temporary erythema, tenderness, petechiae | <2% | Local mechanical stress or vascular dilation | Usually self-limited; avoid high energy density and maintain optimal probe contact |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.; Chae, S.; Kwon, H.-J.; Jeong, W.; Lee, K.K.; Chae, M. Non-Surgical Correction of Facial Asymmetry: A Narrative Review of Non-Surgical Modalities and Clinical Case Examples. J. Clin. Med. 2025, 14, 8828. https://doi.org/10.3390/jcm14248828
Lee C, Chae S, Kwon H-J, Jeong W, Lee KK, Chae M. Non-Surgical Correction of Facial Asymmetry: A Narrative Review of Non-Surgical Modalities and Clinical Case Examples. Journal of Clinical Medicine. 2025; 14(24):8828. https://doi.org/10.3390/jcm14248828
Chicago/Turabian StyleLee, Clara, Sumin Chae, Han-Jin Kwon, Wonwoo Jeong, Kyung Kwan Lee, and Minsuk Chae. 2025. "Non-Surgical Correction of Facial Asymmetry: A Narrative Review of Non-Surgical Modalities and Clinical Case Examples" Journal of Clinical Medicine 14, no. 24: 8828. https://doi.org/10.3390/jcm14248828
APA StyleLee, C., Chae, S., Kwon, H.-J., Jeong, W., Lee, K. K., & Chae, M. (2025). Non-Surgical Correction of Facial Asymmetry: A Narrative Review of Non-Surgical Modalities and Clinical Case Examples. Journal of Clinical Medicine, 14(24), 8828. https://doi.org/10.3390/jcm14248828






