Standardized Prospective Intervention in Hospitalized Patients with Bacterial Pneumonia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
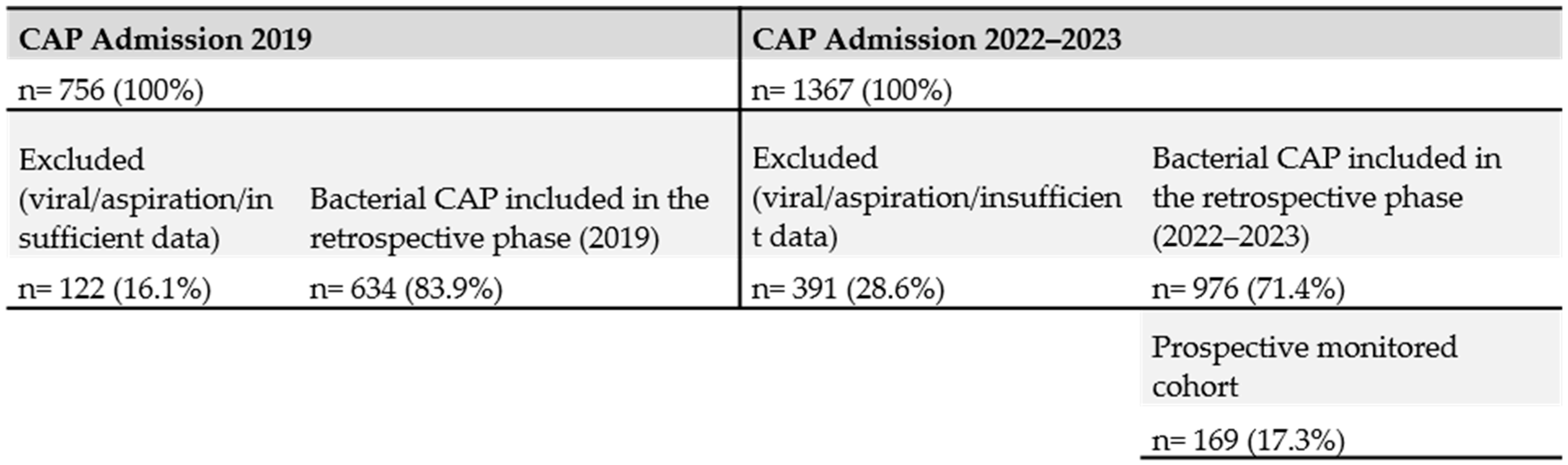
2.2. Study Population
2.3. Intervention
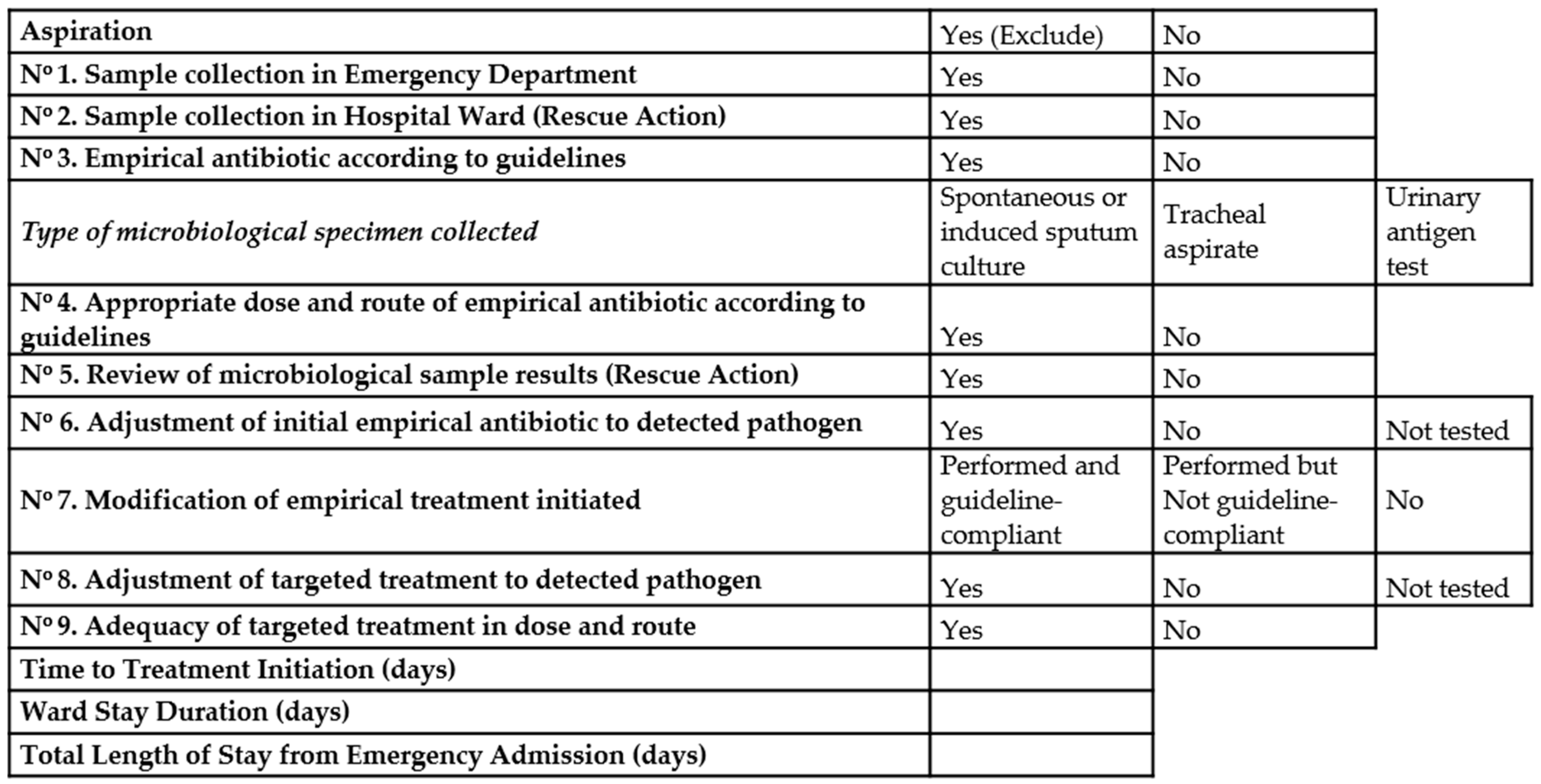
2.4. Protocol of Pneumonia Management Included
- Diagnose of pneumonia at emergency room: Register of comorbidities and risk factors at hospital admission. Register of clinical variables, laboratory tests (biochemical, blood count, gasometry values and inflammation markers) and presence of type pulmonary infiltrates in chest x-ray. Clinical diagnose of pneumonia was based on established criteria. Microbiological specimen’s collection: blood cultures, spontaneous or induced sputum cultures, tracheal aspirate when indicated, and urinary test antigens.
- When pneumonia diagnose was established and appropriate cultures were obtained, antimicrobial therapy should be initiated following the therapeutic guidelines of the hospital.
- When the patients were admitted to hospital wards, the intervention group developed the survey of different items considered as important for the appropriate management and described in Figure 1.
2.5. Sample Size Calculation
2.6. Data Collection
2.7. Statistical Analysis
2.8. Additional Pre/Post Risk-Adjusted Analysis
2.9. Multivariable Analyses in the Prospective Cohort
3. Results
3.1. Prospective Cohort Analysis (2022–2023)
3.2. Factors Associated with Mortality
3.3. Multivariate Analysis. Prospective Cohort
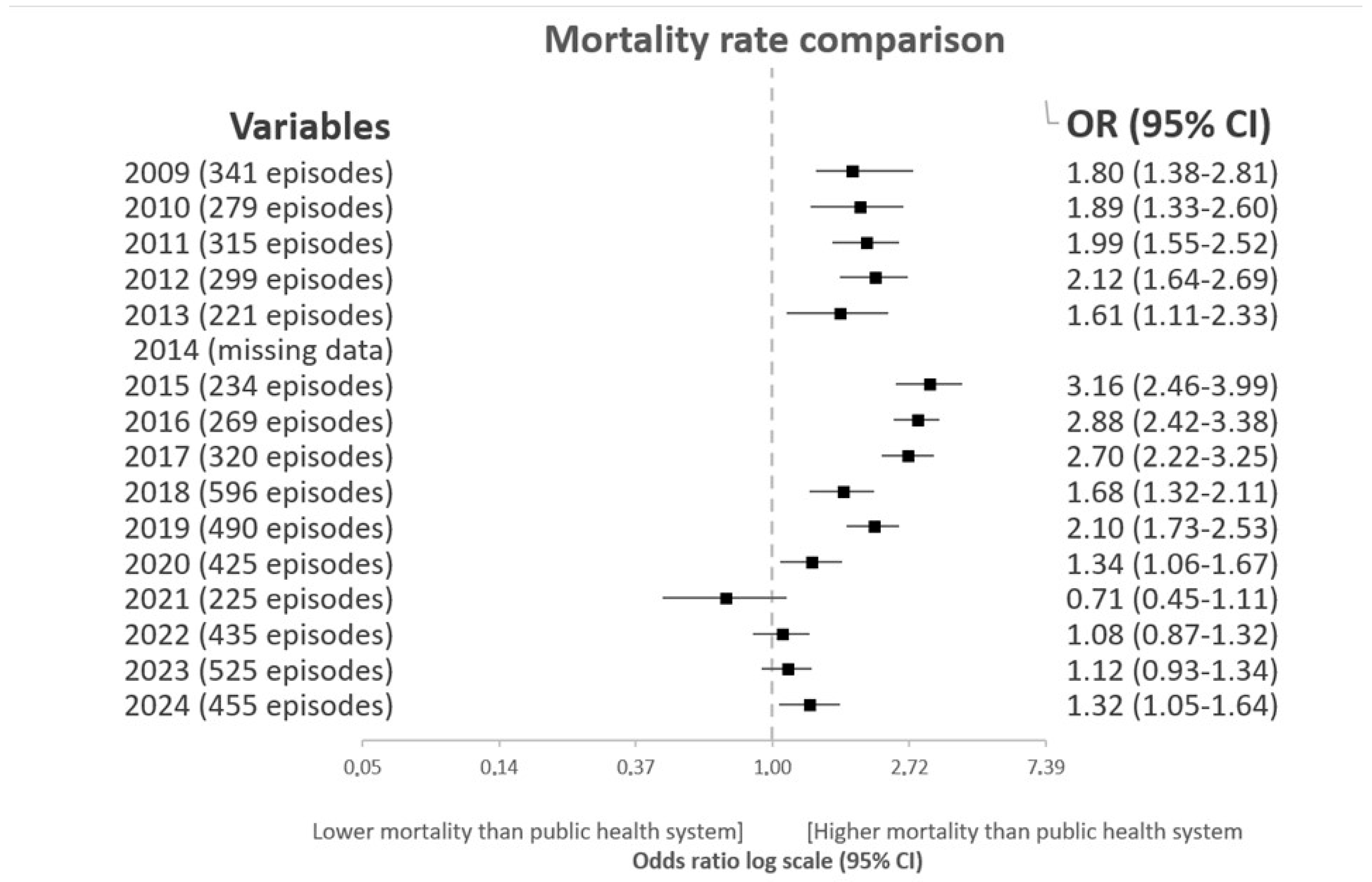
3.4. Trend Analysis of Standardized Mortality Rates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHRQ | Agency for Healthcare Research and Quality |
| ARDS | Acute Respiratory Distress Syndrome |
| CAP | Community-acquired pneumonia |
| HSJDA | Hospital San Juan de Dios del Aljarafe |
| ICU | Intensive care unit |
| INE | National Institute of Statistics |
| PSI | Pneumonia Severity Index |
| SaO2/FiO2 | Arterial Oxygen Saturation to Fraction of Inspired Oxygen Ratio |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
References
- Aliberti, S.; Dela Cruz, C.S.; Amati, F.; Sotgiu, G.; Restrepo, M.I. Community-acquired pneumonia. Lancet 2021, 398, 906–919. [Google Scholar] [CrossRef]
- Vaughn, V.M.; Dickson, R.P.; Horowitz, J.K.; Flanders, S.A. Community-Acquired Pneumonia: A Review. JAMA 2024, 332, 1282–1295. [Google Scholar] [CrossRef]
- File, T.M., Jr.; Ramirez, J.A. Community-Acquired Pneumonia. N. Engl. J. Med. 2023, 389, 632–641. [Google Scholar] [CrossRef]
- Tsoumani, E.; Carter, J.A.; Salomonsson, S.; Stephens, J.M.; Bencina, G. Clinical, economic, and humanistic burden of community acquired pneumonia in Europe: A systematic literature review. Expert Rev. Vaccines 2023, 22, 876–884. [Google Scholar] [CrossRef]
- Bender, R.G.; Sirota, S.B.; Swetschinski, L.R.; Dominguez, R.M.; Novotney, A.; Wool, E.E.; Ikuta, K.S.; Vongpradith, A.; Rogowski, E.L.; Doxey, M.; et al. Global, regional, and national incidence and mortality burden of non-COVID-19 lower respiratory infections and aetiologies, 1990–2021: A systematic analysis from the Global Burden of Disease Study 2021. Lancet Infect. Dis. 2024, 24, 974–1002. [Google Scholar] [CrossRef]
- Gonçalves-Pereira, J.; Froes, F.; Pereira, F.G.; Diniz, A.; Oliveira, H.; Mergulhão, P. Community-acquired pneumonia mortality trends according to age and gender: 2009 to 2019. BMC Pulm. Med. 2025, 25, 391. [Google Scholar] [CrossRef] [PubMed]
- Tasas Estandarizadas de Mortalidad por Causa de Muerte (Causas Más Frecuentes), Sexo y Nivel de Estudio. 25 y Más Años. Available online: https://www.ine.es/jaxi/Datos.htm?tpx=61494#_tabs-tabla (accessed on 20 November 2025).
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and treatment of adults with community-acquired pneumonia. Am. J. Respir. Crit. Care Med. 2019, 200, E45–E67. [Google Scholar] [CrossRef]
- Musher, D.M.; Thorner, A.R. Community-Acquired Pneumonia. N. Engl. J. Med. 2014, 371, 1619–1628. [Google Scholar] [CrossRef]
- Jones, B.E.; Ramirez, J.A.; Oren, E.; Soni, N.J.; Sullivan, L.R.; Restrepo, M.I.; Musher, D.M.; Erstad, B.L.; Pickens, C.; Vaughn, V.M.; et al. Diagnosis and Management of Community-acquired Pneumonia. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2025; Epub ahead of print. Available online: https://www.atsjournals.org/doi/pdf/10.1164/rccm.202507-1692ST?download=true (accessed on 20 November 2025).
- Ryu, J.; Kim, N.-H.; Ohn, J.H.; Lim, Y.; Lee, J.; Kim, H.W.; Kim, S.-W.; Park, H.-S.; Kim, E.S.; Yoon, S.; et al. Impact of antibiotic changes on hospital stay and treatment duration in community-acquired pneumonia. Sci. Rep. 2024, 14, 22669. [Google Scholar] [CrossRef] [PubMed]
- Menéndez, R.; Cilloniz, C.; España, P.P.; Almirall, J.; Uranga, A.; Méndez, R.; Rigau, D.; Torres, A. Neumonía adquirida en la comunidad. Normativa de la Sociedad Española de Neumología y Cirugía Torácica (SEPAR). Actualización 2020. Arch. Bronconeumol. 2020, 56, 1–10. [Google Scholar] [CrossRef]
- Rice, T.W.; Wheeler, A.P.; Bernard, G.R.; Hayden, D.L.; Schoenfeld, D.A.; Ware, L.B. Comparison of the SpO2/FIO2 ratio and the PaO 2/FIO2 ratio in patients with acute lung injury or ARDS. Chest 2007, 132, 410–417. [Google Scholar] [CrossRef]
- Lee, J.S.; Giesler, D.L.; Gellad, W.F.; Fine, M.J. Antibiotic Therapy for Adults Hospitalized With Community-Acquired Pneumonia: A Systematic Review. JAMA 2016, 315, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, A.; Garcia-Vidal, C.; Viasus, D.; García-Somoza, D.; Dorca, J.; Gudiol, F.; Carratalà, J. Declining mortality among hospitalized patients with community-acquired pneumonia. Clin. Microbiol. Infect. 2016, 22, 567.e1–567.e7. [Google Scholar] [CrossRef]
- Darbà, J.; Marsà, A. Hospital incidence, in-hospital mortality and medical costs of pneumococcal disease in Spain (2008–2017): A retrospective multicentre study. Curr. Med. Res. Opin. 2021, 37, 523–530. [Google Scholar] [CrossRef]
- Lee, J.S.; Nsa, W.; Hausmann, L.R.M.; Trivedi, A.N.; Bratzler, D.W.; Auden, D.; Mor, M.K.; Baus, K.; Larbi, F.M.; Fine, M.J. Quality of Care for Elderly Patients Hospitalized for Pneumonia in the United States, 2006 to 2010. JAMA Intern. Med. 2014, 174, 1806–1814. [Google Scholar] [CrossRef]
- Daniel, P.; Woodhead, M.; Welham, S.; McKeever, T.M.; Lim, W.S. Mortality reduction in adult community-acquired pneumonia in the UK (2009-2014): Results from the British Thoracic Society audit programme. Thorax 2016, 71, 1061–1063. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Guan, S.; Kong, X.; Ji, W.; Du, C.; Jia, M.; Wang, H. Predictors of mortality in severe pneumonia patients: A systematic review and meta-analysis. Syst. Rev. 2024, 13, 210. [Google Scholar] [CrossRef] [PubMed]
- Sellarès-Nadal, J.; Burgos, J.; Martín-Gómez, M.T.; Antón, A.; Sordé, R.; Romero-Herrero, D.; Bosch-Nicolau, P.; Falcó-Roget, A.; Kirkegaard, C.; Rodríguez-Pardo, D.; et al. Community-acquired pneumonia in hospitalised patients: Changes in aetiology, clinical presentation, and severity outcomes in a 10-year period. Ann. Med. 2022, 54, 3052–3059. [Google Scholar] [CrossRef]
- Theilacker, C.; Sprenger, R.; Leverkus, F.; Walker, J.; Häckl, D.; von Eiff, C.; Schiffner-Rohe, J. Population-based incidence and mortality of community-acquired pneumonia in Germany. PLoS ONE 2021, 16, e0253118. [Google Scholar] [CrossRef]
- Amodio, E.; Vitale, F.; D’angela, D.; Carrieri, C.; Polistena, B.; Spandonaro, F.; Pagliaro, A.; Montuori, E.A. Increased Risk of Hospitalization for Pneumonia in Italian Adults from 2010 to 2019: Scientific Evidence for a Call to Action. Vaccines 2023, 11, 187. [Google Scholar] [CrossRef]
- Lüthi-Corridori, G.; Boesing, M.; Roth, A.; Giezendanner, S.; Leuppi-Taegtmeyer, A.B.; Schuetz, P.; Leuppi, J.D. Predictors of Length of Stay, Rehospitalization and Mortality in Community-Acquired Pneumonia Patients: A Retrospective Cohort Study. J. Clin. Med. 2023, 12, 5601. [Google Scholar] [CrossRef]
- Idigo, A.J.; Wells, J.M.; Brown, M.L.; Wiener, H.W.; Griffin, R.L.; Cutter, G.; Shrestha, S.; Lee, R.A. Socio-demographic and comorbid risk factors for poor prognosis in patients hospitalized with community-acquired bacterial pneumonia in southeastern US. Heart Lung 2024, 65, 31–39. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Q.; Yu, Z. Prognostic Factors of Severe Pneumonia in Adult Patients: A Systematic Review. Altern. Ther. Health Med. 2024, 30, 80–89. [Google Scholar] [PubMed]
- Ferrer, M.; Travierso, C.; Cilloniz, C.; Gabarrus, A.; Ranzani, O.T.; Polverino, E.; Liapikou, A.; Blasi, F.; Torres, A. Severe community-acquired pneumonia: Characteristics and prognostic factors in ventilated and non-ventilated patients. PLoS ONE 2018, 13, e0191721. [Google Scholar] [CrossRef]
- Chen, S.; Hou, C.; Kang, Y.; Li, D.; Rong, J.; Li, Z. Factors affecting hospital discharge outcomes in patients with community-acquired pneumonia: A retrospective epidemiological study (2014–2021). Am. J. Med. Sci. 2023, 366, 143–149. [Google Scholar] [CrossRef]
- Furman, C.D.; Leinenbach, A.; Usher, R.; Elikkottil, J.; Arnold, F.W. Pneumonia in older adults. Curr. Opin. Infect. Dis. 2021, 34, 135–141. [Google Scholar] [CrossRef]
- Putot, A.; Garin, N.; Rello, J.; Prendki, V. Comprehensive management of pneumonia in older patients. Eur. J. Intern. Med. 2025, 135, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Clotet-Vidal, S.; Saez Prieto, M.E.; Duch Llorach, P.; Gutiérrez, Á.S.; Casademont Pou, J.; Torres Bonafonte, O.H. Malnutrition, Functional Decline, and Institutionalization in Older Adults after Hospital Discharge Following Community-Acquired Pneumonia. Nutrients 2024, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Fimognari, F.L.; Corsonello, A.; Rizzo, M.; Bambara, V.; Fabbietti, P.; Arone, A.; Cuccurullo, O.; Pilotto, A.; Ferrari, A.; Cristiano, G.; et al. Contribution of clinical severity and geriatric risk factors in predicting short-term mortality of older hospitalized pneumonia patients: The Pneumonia in Italian Acute Care for Elderly units (PIACE) study. Aging Clin. Exp. Res. 2022, 34, 1419–1427. [Google Scholar] [CrossRef]
- Schweitzer, V.A.; van Heijl, I.; Boersma, W.G.; Rozemeijer, W.; Verduin, K.; Grootenboers, M.J.; Sankatsing, S.U.C.; van der Bij, A.K.; de Bruijn, W.; Ammerlaan, H.S.M.; et al. Narrow-spectrum antibiotics for community-acquired pneumonia in Dutch adults (CAP-PACT): A cross-sectional, stepped-wedge, cluster-randomised, non-inferiority, antimicrobial stewardship intervention trial. Lancet Infect. Dis. 2022, 22, 274–283. [Google Scholar] [CrossRef]
- Ablakimova, N.; Rachina, S.; de Silva, H.R.; Vlasenko, A.; Smagulova, G.; Mussina, A.; Sakhanova, S.; Zhylkybekova, A.; Tleumagambetova, B.; Karimoldayeva, D.; et al. Antimicrobial stewardship interventions in hospitalized adults with community-acquired pneumonia: A systematic review and meta-analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2025, 44, 1533–1550. [Google Scholar] [CrossRef]
- Ghadimi, M.; Siemieniuk, R.A.; Loeb, M.; Lima, J.P.; Aminaei, D.; Gomaa, H.; Wang, Y.; Hazzan, A.A.; Basmaji, J.; Yao, L.; et al. Empiric antibiotic therapy for moderate-to-severe community-acquired pneumonia: A systematic review and network meta-analysis. Clin. Microbiol. Infect. 2025, 31, 1807–1815. [Google Scholar] [CrossRef]
- Bai, A.D.; Srivastava, S.; Wong, B.K.C.; Digby, G.C.; Razak, F.; Verma, A.A. Comparative Effectiveness of First-Line and Alternative Antibiotic Regimens in Hospitalized Patients With Nonsevere Community-Acquired Pneumonia: A Multicenter Retrospective Cohort Study. Chest 2024, 165, 68–78. [Google Scholar] [CrossRef]
- Fally, M.; Von Plessen, C.; Anhøj, J.; Benfield, T.; Tarp, B.; Clausen, L.N.; Kolte, L.; Diernaes, E.; Molzen, L.; Seerup, R.; et al. Improved treatment of community-acquired pneumonia through tailored interventions: Results from a controlled, multicentre quality improvement project. PLoS ONE 2020, 15, e0234308. [Google Scholar] [CrossRef]
- Ablakimova, N.; Rachina, S.; Smagulova, G.; Vlasenko, A.; Mussina, A.; Zhylkybekova, A.; Yessenzhulova, A.; Koshmaganbetova, G.K.; Iztleuov, Y. Impact of complex interventions on antibacterial therapy and etiological diagnostics in community-acquired pneumonia: A 12-month pre- and post-intervention study. Front. Pharmacol. 2025, 16, 1627858. [Google Scholar] [CrossRef] [PubMed]
- Marrie, T.J.; Lau, C.Y.; Wheeler, S.L.; Wong, C.J.; Vandervoort, M.K.; Feagan, B.G. A Controlled Trial of a Critical Pathway for Treatment of Community-Acquired Pneumonia. JAMA 2000, 283, 749–755. [Google Scholar] [CrossRef]
- Kanjee, Z.; Metlay, J.P.; Moskowitz, A.; Reynolds, E.E. How Would You Treat This Patient Hospitalized With Community-Acquired Pneumonia?: Grand Rounds Discussion From Beth Israel Deaconess Medical Center. Ann. Intern. Med. 2021, 174, 1719–1726. [Google Scholar] [CrossRef]
- Cilloniz, C.; Pericas, J.M.; Curioso, W.H. Interventions to improve outcomes in community-acquired pneumonia. Expert. Rev. Anti Infect. Ther. 2023, 21, 1071–1086. [Google Scholar] [CrossRef] [PubMed]
- Waterer, G.; Bennett, L. Improving outcomes from community-acquired pneumonia. Curr. Opin. Pulm. Med. 2015, 21, 219–225. [Google Scholar] [CrossRef] [PubMed]
| Before (2019) N (%) | After (2022–2023) N (%) | Significance | |
|---|---|---|---|
| Patients with community-acquired bacterial pneumonia | 634 (39.4) | 976 (60.6) | |
| Sex (male) | 384 (60.6) | 534 (54.7) | 0.020 * |
| Severity level | <0.001 * | ||
| Mild | 141 (22.2) | 142 (14.5) | |
| Moderate | 301 (47.5) | 430 (44.1) | |
| High | 160 (25.2) | 322 (33.0) | |
| Extreme | 32 (5.0) | 82 (8.4) | |
| Mortality risk | <0.001 * | ||
| Mild | 112 (17.7) | 120 (12.3) | |
| Moderate | 321 (50.6) | 314 (32.2) | |
| High | 160 (25.2) | 388 (39.8) | |
| Extreme | 41 (6.5) | 154 (15.8) | |
| ICU 1 admission | 13 (2.1) | 37 (3.8) | 0.049 * |
| Discharge due to death | 105 (16.6) (95% CI 13.7–19.5) | 110 (11.3) (95% CI 9.3–13.2) | 0.002 * |
| Median (Q1–Q3) 2 | Median (Q1–Q3) 2 | Significance | |
| Age (years) | 79.1 (67.8–86.5) | 78.5 (66.5–86.2) | 0.455 |
| Resource intensity weight | 0.605 (0.605–0.956) | 0.711 (0.583–0.850) | 0.061 |
| Average length of stay (days) | 8.13 (8.01–9.27) | 7.89 (7.47–8.85) | <0.001 * |
| Multivariate Coefficient (B) | Multivariate OR 3 (95% CI) 4 | Significance | |
| Period (2022–2023) | 0.48 | 0.62 (0.45–0.85) | 0.003 * |
| Age (years) | 0.07 | 1.07 (1.05–1.09) | <0.001 * |
| Sex (female) | 0.21 | 0.81 (0.60–1.10) | 0.172 |
| Mortality risk (moderate) | 0.36 | 1.44 (0.87–2.37) | 0.153 |
| Mortality risk (high) | 0.48 | 1.62 (0.97–2.71) | 0.066 |
| Mortality risk (extreme) | 0.36 | 1.43 (0.77–2.69) | 0.261 |
| Sample Characteristics | N (%) |
|---|---|
| Gender | |
| Male | 90 (53.3) |
| Female | 79 (46.7) |
| Comorbidities | |
| Active smoking | 37 (21.9) |
| Active alcohol use | 30 (17.8) |
| Use of other drugs | 8 (4.7) |
| Immunosuppressed | 29 (17.2) |
| Active neoplastic disease | 18 (10.7) |
| Chronic heart failure | 70 (41.4) |
| Chronic liver failure | 10 (5.9) |
| Chronic kidney failure | 33 (19.5) |
| Pre-existing neurological deficit | 36 (21.3) |
| Chronic obstructive pulmonary disease | 33 (19.5) |
| Diabetes mellitus | 46 (27.2) |
| Pre-existing dysphagia | 8 (4.7) |
| Other Possible Risk Factors at Admission | |
| Pre-existing dependency | 64 (37.9) |
| Institutionalized | 14 (8.3) |
| Taking immunosuppressants | 12 (7.1) |
| Taking oral corticosteroids | 14 (8.3) |
| Hospitalization in the previous 12 months | 45 (26.6) |
| Median (Q1–Q3) 1 | |
| Age | 77.1 (62.7–84.6) |
| Lab Tests at Admission | |
| Hematocrit (%) | 35 (30–40) |
| Leukocytes (×109/L) | 10.6 (7.6–14.5) |
| Creatinine (mg/dL) | 0.90 (0.65–1.88) |
| Urea (mg/dL) | 56. 5 (38.0–84.5) |
| Albumin (g/dL) | 2.6 (2.2–2.9) |
| Glucose (mg/dL) | 117.5 (101.0–148.8) |
| Sodium (mmol/L) | 139.0 (136.0–141.8) |
| Potassium (mmol/L) | 4.1 (3.8–4.5) |
| Arterial pH | 7.37 (7.34–7.41) |
| Arterial pCO2 (mmHg) | 45.1 (40.2–52.3) |
| C-reactive protein (mg/L) | 140.5 (62.0–210.3) |
| Procalcitonin (ng/mL) | 0.49 (0.11–2.98) |
| SaO2/FiO2 2 | 313.0 (243.0–360.0) |
| Clinical Examination Variables at Admission | |
| Temperature (°C) | 37.0 (36.5–38.0) |
| Heart rate (bpm) | 98.0 (81.5–116.8) |
| Respiratory rate (rpm) | 20.0 (15.0–25.0) |
| Systolic blood pressure (mmHg) | 125.0 (110.0–141.8) |
| Diastolic blood pressure (mmHg) | 68.0 (60.0–78.0) |
| SatO2 (%) 3 | 92.0 (88.0–97.0) |
| Barthel Index | 100 (60.0–100) |
| CURB 65 4 | 2.0 (2.0–2.0) |
| Number of comorbidities | 2.0 (1.0–3.0) |
| Clinical Diagnosis | N (%) | Laboratory Diagnosis | N (%) |
|---|---|---|---|
| Pulmonary Auscultation | Patients with Microbiological Analysis | ||
| Good vesicular breath sounds without pathological noises | 34 (20) | Yes | 160 (95.2) |
| Good vesicular breath sounds with pathological noises | 72 (42.9) | Sample Collection Site | |
| Decreased vesicular breath sounds without pathological noises | 15 (8.9) | Emergency Department | 132 (78.1) |
| Decreased vesicular breath sounds with pathological noises | 47 (28.0) | Hospital Ward | 118 (69.8) |
| Infiltrate | Type of Sample Collected 2 * | ||
| Yes | 116 (98.2) | Spontaneous or induced sputum culture | 126 (30.0) |
| Alveolar | 140 (82.8) | Tracheal aspirate | 6 (1.4) |
| Interstitial | 13 (7.7) | Urinary antigen test | 160 (38.1) |
| Mixed | 13 (7.7) | Blood culture | 128 (30.5) |
| Respiratory Support | Sample Where Pathogen Was Identified 3 * | ||
| Nasal cannula | 98 (58.7) | Spontaneous or induced sputum culture | 167 (85.2) |
| Oxygen mask | 22 (13.2) | Tracheal aspirate | 0 (0) |
| HFNO 1 | 3 (1.8) | Urinary antigen test | 19 (9.7) |
| Non-Invasive Mechanical Ventilation (NIV) | 9 (5.4) | Blood culture | 10 (5.1) |
| Invasive Mechanical Ventilation (IMV) | 6 (3.6) | ||
| No respiratory support | 29 (17.4) | ||
| Treatments | N (%) | Complications | N (%) |
| Use of vasopressors | 7 (4.1) | Acute Respiratory Distress Syndrome (ARDS) | 62 (36.7) |
| Nebulizers | 30 (17.8) | Stroke (Cerebrovascular Accident) | 1 (0.6) |
| Inhalers | 125 (74.0) | Acute Kidney Injury (AKI) | 39 (23.1) |
| Systemic corticosteroids | 116 (68.6) | Septic Shock | 18 (10.7) |
| Mortality | N (%) | Extrapulmonary Infection | 25 (14.8) |
| Intra-episode death | 13 (7.7) | Arrhythmias | 15 (8.9) |
| Intra-episode and/or within 30 days post-discharge death | 28 (16.6) | Congestive Heart Failure | 71 (42.0) |
| Myocardial Ischemia | 7 (4.1) | ||
| Altered Mental Status | 32 (18.9) | ||
| Admission to Intensive Care Unit (ICU) | 9 (5.3) | ||
| Electronic Health Record Documentation | N (%) | ||
| Suspected aspiration: dysphagia test performed | 3 (1.8) | ||
| Discharge report: secondary diagnoses included | 162 (95.9) | ||
| Therapeutic effort limitation indicated | 77 (45.6) | ||
| Prior Pneumococcal Vaccination | 94 (55.6) | ||
| Proper Protocol Implementation | N (%) |
|---|---|
| N°1. Sample collection in Emergency Department | 132 (78.6) |
| N°2. Sample collection in Hospital Ward | 118 (70.2) |
| N°3. Empirical antibiotic according to guidelines | 107 (63.3) |
| N°4. Appropriate dose and route of empirical antibiotic according to guidelines | 104 (61.5) |
| N°5. Review of microbiological sample results | 161 (95.3) |
| N°6. Adjustment of initial empirical antibiotic to detected pathogen 1 | 49 (77.8) |
| N°7. Modification of empirical treatment initiated 2 | 65 (38.5) |
| Performed and guideline-compliant | 48 (28.4) |
| Performed but not guideline-compliant | 17 (10.1) |
| N°8. Adjustment of targeted treatment to detected pathogen 3 | 52 (81.3) |
| N°9. Adequacy of targeted treatment in dose and route 4 | 55 (84.6) |
| Rescue Actions (N°2 y N°5) | |
| None | 36 (21.3) |
| One rescue action | 83 (49.1) |
| Two rescue actions | 50 (29.6) |
| Median (Q1–Q3) 5 1.0 (1.0–2.0) 4.0 (2.5–5.0) | |
| Rescue Actions (N°2 y N°5) | |
| Primary Actions (N° 1. 3. 4 6–9) | |
| Total Actions | 5.0 (4.0–7.0) |
| Time to Treatment Initiation (days) | 0 (0–1) |
| Ward Stay Duration (days) | 5.9 (3.7–8.4) |
| Total Length of Stay from Emergency Admission (days) | 7.5 (4.8–9.8) |
| No Mortality N (%) | Intra-Episode Mortality N (%) | Significance | |
|---|---|---|---|
| Total | 156 (93.3%) | 13 (7.7%) | |
| Gender Male (N = 169) | 84 (53.8) | 6 (46.2) | 0.593 a |
| Comorbidities | |||
| Active smoking (N = 167) | 35 (22.7) | 2 (15.4) | 0.735 b |
| Active alcoholism (N = 167) | 28 (18.2) | 2 (15.4) | 1 b |
| Substance abuse (N = 169) | 8 (5.2) | 0 (0) | 1 b |
| Immunocompromised (N = 167) | 27 (17.3) | 2 (15.4) | 1 b |
| Active neoplastic disease (N = 169) | 16 (10.3) | 2 (15.4) | 0.633 b |
| Chronic heart failure (N = 167) | 61 (39.0) | 6 (69.2) | 0.038 * a |
| Chronic liver failure (N = 169) | 10 (6.4) | 0 (0) | 1 b |
| Chronic kidney failure (N = 169) | 31 (19.9) | 2 (15.4) | 1 b |
| Previous neurological deficit (N = 169) | 32 (20.5) | 4 (30.8) | 0.478 b |
| Chronic obstructive pulmonary disease (COPD) (N = 169) | 29 (18.6) | 4 (30.8) | 0.258 b |
| Diabetes mellitus (N = 169) | 45 (28.8) | 1 (7.7) | 0.118 b |
| Previous dysphagia (N = 169) | 7 (4.5) | 1 (7.7) | 0.482 b |
| Other potential risk factors | |||
| Previous dependency (N = 169) | 57 (36.5) | 7 (53.8) | 0.243 b |
| Institutionalized (N = 168) | 11 (7.1) | 3 (23.1) | 0.080 b |
| Use of immunosuppressants (N = 169) | 11 (7.1) | 1 (7.7) | 1 b |
| Use of oral corticosteroids (N = 169) | 13 (8.3) | 1 (7.7) | 1 b |
| Hospitalization in the last 12 months (N = 169) | 43 (27.6) | 2 (15.4) | 0.517 b |
| Infiltrate (N = 169) | 156 (98.1) | 13 (100) | 1 b |
| Respiratory Support (N = 167) | |||
| None | 29 (18.8) | 0 (0) | |
| Conventional oxygen therapy (Nasal cannula or mask) | 113 (73.4) | 7 (53.8) | |
| HFNO 1 | 3 (1.9) | 0 (0) | |
| Non-invasive mechanical ventilation | 6 (3.9) | 3 (23.1) | |
| Invasive mechanical ventilation | 3 (1.9) | 3 (23.1) | <0.001 * a |
| Complications During Hospitalization | |||
| Acute Respiratory Distress Syndrome (ARDS) (N = 169) | 51 (32.7) | 11 (84.6) | <0.001 * b |
| Stroke (Cerebrovascular Accident) (N = 169) | 0 (0) | 1 (7.7) | 0.077 b |
| Acute Kidney Failure (N = 169) | 33 (21.2) | 6 (46.2) | 0.078 b |
| Septic Shock (N = 169) | 15 (9.6) | 3 (23.1) | 0.147 b |
| Extrapulmonary Infection (N = 169) | 22 (14.1) | 3 (23.1) | 0.412 b |
| Arrhythmias (N = 169) | 12 (7.7) | 3 (23.1) | 0.094 b |
| Congestive Heart Failure (N = 169) | 62 (39.7) | 9 (69.2) | 0.038 * a |
| Myocardial Ischemia (N = 168) | 5 (3.2) | 2 (15.4) | 0.093 b |
| Altered Mental Status (N = 169) | 26 (16.7) | 6 (46.2) | 0.019 * b |
| Admission to ICU 2 (N = 169) | 6 (3.8) | 3 (23.1) | 0.023 * b |
| Treatment | |||
| Use of vasopressors (N = 169) | 5 (3.2) | 2 (15.4) | 0.092 b |
| Nebulizers (N = 169) | 26 (16.7) | 4 (30.8) | 0.250 b |
| Inhalers (N = 169) | 114 (73.1) | 11 (84.6) | 0.518 b |
| Systemic corticosteroids (N = 169) | 104 (66.7) | 12 (92.3) | 0.065 b |
| Proper Protocol Implementation | |||
| N°1 Sample collection in Emergency Department (N = 168) | 124 (80.0) | 8 (61.5) | 0.155 b |
| N°2 Sample collection in Hospital Ward (N = 168) | 107 (69.0) | 11 (84.6) | 0.348 b |
| N°3 Empirical antibiotic according to guidelines (N = 168) | 101 (65.2) | 6 (46.2) | 0.230 b |
| N°4 Appropriate dose and route of empirical antibiotic according to guidelines (N = 168) | 98 (63.2) | 6 (46.2) | 0.246 b |
| N°5 Review of microbiological sample results (N = 168) | 149 (96.1) | 12 (92.3) | 0.437 b |
| N°6 Adjustment of initial empirical antibiotic to detected pathogen (N = 63) | 46 (78.0) | 3 (75.0) | 1 b |
| N°7 Modification of empirical treatment initiated (N = 164) | 59 (39.1) | 6 (46.2) | 0.616 a |
| N°8 Adjustment of targeted treatment to detected pathogen (N = 64) | 49 (81.7) | 3 (75.0) | 0.574 b |
| N°9 Adequacy of targeted treatment in dose and route (N = 65) | 52 (85.2) | 3 (75.0) | 0.496 b |
| Rescue Actions (N = 169) | |||
| None | 34 (21.8) | 2 (15.4) | |
| One rescue action | 78 (50.0) | 5 (38.5) | |
| Two rescue actions | 44 (28.2) | 6 (46.2) | 0.394 a |
| Electronic Health Record Documentation | |||
| Suspected aspiration: dysphagia test performed (N = 168) | 3 (1.9) | 0 (0) | 1 b |
| Discharge report: secondary diagnoses included (N = 168) | 149 (96.1) | 13 (100) | 1 b |
| Therapeutic effort limitation indicated (N = 168) | 65 (41.9) | 12 (92.3) | <0.001 * a |
| Prior pneumococcal vaccination (N = 166) | 86 (56.2) | 8 (61.5) | 0.710 a |
| No mortality (Median (Q1–Q3) 3) | Intra-episode mortality (Median (Q1–Q3) 3) | Significance | |
| Variables at Admission | |||
| Age | 77.1 (61.2–84.1) | 83.4 (71.1–89.1) | 0.122 c |
| CURB-65 score 4 | 2.0 (1.0–2.0) | 3.0 (2.0–3.0) | 0.001 * c |
| Barthel index | 100 (60.0–100) | 80.0 (40.0–100) | 0.127 c |
| Number of comorbidities | 2.0 (1.0–3.0) | 2.0 (1.0–3.5) | 0.978 c |
| Laboratory Analysis at Admission | |||
| Hematocrit | 0.35 (0.30–0.40) | 0.39 (0.31–0.42) | 0.257 c |
| Leukocytes | 10.5 (7.4–13.8) | 12.1 (8.0–17.4) | 0.391 c |
| Creatinine | 0.90 (0.66–1.38) | 1.11 (0.54–1.50) | 0.955 c |
| Urea | 56.0 (38.0–82.0) | 70.0 (40.0–134.5) | 0.280 c |
| Albumin | 2.65 (2.20–2.90) | 2.40 (1.70–2.65) | 0.063 c |
| Glucose | 116.0 (100.0–148.0) | 132.0 (100.5–147.0) | 0.245 c |
| Sodium | 139.0 (136.0–141.0) | 140.0 (134.0–143.5) | 0.653 c |
| Potassium | 4.10 (3.80–4.50) | 4.05 (3.23–4.28) | 0.273 c |
| Arterial pH | 7.38 (7.34–7.41) | 7.34 (7.22–7.44) | 0.121 c |
| Arterial PCO2 | 44.8 (40.2–52.0) | 46.9 (37.8–73.7) | 0.360 c |
| C-reactive protein | 140.0 (61.9–203.0) | 168.0 (60.0–264.5) | 0.748 c |
| Procalcitonin | 0.49 (0.11–2.78) | 0.61 (0.29–5.62) | 0.451 c |
| Clinical Examination Variables at Admission | |||
| SaO2/FiO2 ratio 5 (Mean [SD] 6) | 315.8 (90.84) | 151.2 (71.31) | <0.001 * d |
| Temperature (Median (Q1–Q3) 3) | 37.2 (36.5–38.0) | 36.2 (36.0–36.7) | <0.001 * c |
| Heart rate (bpm) (Mean (SD) 6) | 99.8 (22.44) | 99.9 (35.01) | 0.992 d |
| Respiratory rate (rpm) (Median (Q1–Q3) 3) | 20.0 (15.0–20.0) | 15.0 (15.0–27.5) | 0.987 c |
| Systolic blood pressure (mmHg) (Mean (SD) 6) | 127.9 (25.85) | 120.2 (28.72) | 0.309 d |
| Diastolic blood pressure (mmHg) (Median (Q1–Q3) 3) | 70.0 (60.0–79.0) | 63.0 (59.0–66.0) | 0.059 c |
| Oxygen saturation (%) 7 | 92.0 (88.0–94.0) | 92.0 (82.5–96.0) | 0.805 c |
| Proper Protocol Implementation | |||
| Primary Actions | 4.0 (3.0–5.0) | 3.0 (2.0–4.0) | 0.122 c |
| Total actions | 5. 0 (4.0–7.0) | 4.0 (3.0–6.0) | 0.430 c |
| Time to treatment initiation (days) | 0 (0–1) | 0 (0–1) | 0.967 c |
| No Mortality N (%) | Mortality Within 30 Days N (%) | Significance | |
|---|---|---|---|
| Gender Male (N = 156) | 76 (53.9) | 14 (50.0) | 0.706 a |
| Comorbidities | |||
| Active smoking (N = 154) | 34 (24.5) | 3 (10.7) | 0.110 a |
| Active alcoholism (N = 154) | 28 (20.1) | 2 (7.1) | 0.102 a |
| Substance abuse (N = 154) | 8 (5.8) | 0 (0) | 0.354 b |
| Immunocompromised (N = 156) | 23 (16.3) | 6 (21.4) | 0.583 b |
| Active neoplastic disease (N = 156) | 13 (9.2) | 5 (17.9) | 0.185 b |
| Chronic heart failure (N = 154) | 50 (36.0) | 20 (71.4) | 0.001 * a |
| Chronic liver failure (N = 156) | 9 (6.4) | 1 (3.6) | 1 b |
| Chronic kidney failure (N = 156) | 25 (17.7) | 8 (28.6) | 0.186 a |
| Previous neurological deficit (N = 156) | 28 (19.9) | 8 (28.6) | 0.304 a |
| Chronic obstructive pulmonary disease (COPD) (N = 156) | 26 (18.4) | 7 (25.0) | 0.424 a |
| Diabetes mellitus (N = 156) | 36 (25.5) | 10 (35.7) | 0.269 a |
| Previous dysphagia (N = 155) | 7 (5.0) | 1 (3.6) | 1 b |
| Other potential risk factors | |||
| Previous dependency (N = 156) | 45 (31.9) | 19 (67.9) | <0.001 * a |
| Institutionalized (N = 155) | 7 (5.0) | 7 (25.0) | 0.003 * b |
| Use of immunosuppressants (N = 156) | 11 (7.8) | 1 (3.6) | 0.693 b |
| Use of oral corticosteroids (N = 156) | 13 (9.2) | 1 (3.6) | 0.470 b |
| Hospitalization in the last 12 months (N = 156) | 36 (25.5) | 9 (32.1) | 0.470 a |
| Infiltrate (N = 156) | 138 (97.9) | 28 (100) | 1 b |
| Respiratory Support (N = 154) | |||
| None | 29 (20.9) | 0 (0) | |
| Conventional oxygen therapy (Nasal cannula or mask) | 101 (72.7) | 12 (80.0) | |
| HFNO 1 | 3 (2.2) | 0 (0) | |
| Non-invasive mechanical ventilation | 5 (3.6) | 1 (6.7) | |
| Invasive mechanical ventilation | 1 (0.7) | 2 (13.3) | <0.001 * a |
| Complications During Hospitalization | |||
| Acute Respiratory Distress Syndrome (ARDS) (N = 156) | 40 (28.4) | 22 (78.6) | <0.001 * a |
| Stroke (Cerebrovascular Accident) (N = 156) | 0 (0) | 1 (3.6) | 0.166 b |
| Acute Kidney Failure (N = 156) | 25 (17.7) | 14 (50.0) | <0.001 * a |
| Septic Shock (N = 156) | 10 (7.1) | 8 (28.6) | 0.003 * b |
| Extrapulmonary Infection (N = 156) | 17 (12.1) | 8 (28.6) | 0.038 * b |
| Arrhythmias (N = 156) | 10 (7.1) | 5 (17.9) | 0.078 b |
| Congestive Heart Failure (N = 156) | 52 (36.9) | 19 (67.9) | 0.002 * a |
| Myocardial Ischemia (N = 155) | 3 (2.1) | 4 (14.3) | 0.015 b |
| Altered Mental Status (N = 156) | 20 (14.2) | 12 (42.9) | <0.001 * a |
| Admission to ICU 2 (N = 156) | 4 (2.8) | 5 (17.9) | 0.007 b |
| Treatment | |||
| Use of vasopressors (N = 156) | 3 (2.1) | 4 (14.3) | 0.015 b |
| Nebulizers (N = 156) | 25 (17.7) | 5 (17.9) | 1 b |
| Inhalers (N = 156) | 102 (72.3) | 23 (82.1) | 0.280 a |
| Systemic corticosteroids (N = 156) | 94 (66.7) | 22 (78.6) | 0.215 a |
| Proper Protocol Implementation | |||
| N°1 Sample collection in Emergency Department (N = 155) | 114 (81.4) | 18 (64.3) | 0.044 a |
| N°2 Sample collection in Hospital Ward (N = 155) | 95 (67.9) | 23 (82.1) | 0.131 a |
| N°3 Empirical antibiotic according to guidelines (N = 155) | 90 (64.3) | 17 (60.7) | 0.720 a |
| N°4 Appropriate dose and route of empirical antibiotic according to guidelines (N = 155) | 89 (63.6) | 15 (53.6) | 0.320 a |
| N°5 Review of microbiological sample results (N = 155) | 135 (96.4) | 26 (92.9) | 0.330 b |
| N°6 Adjustment of initial empirical antibiotic to detected pathogen (N = 60) | 43 (79.6) | 6 (66.7) | 0.403 b |
| N°7 Modification of empirical treatment initiated (N = 151) | 51 (37.5) | 14 (50.0) | 0.218 a |
| N°8 Adjustment of targeted treatment to detected pathogen (N = 59) | 45 (81.8) | 7 (77.8) | 0.672 b |
| N°9 Adequacy of targeted treatment in dose and route (N = 61) | 48 (85.7) | 7 (77.8) | 0.619 b |
| Rescue Actions (N = 155) | |||
| None | 31 (22.0) | 3 (20.0) | |
| One rescue action | 74 (52.5) | 4 (26.7) | |
| Two rescue actions | 36 (25.5) | 8 (53.3) | 0.062 a |
| Electronic Health Record Documentation | |||
| Suspected aspiration: dysphagia test performed (N = 155) | 2 (1.4) | 1 (3.6) | 0.423 b |
| Discharge report: secondary diagnoses included (N = 155) | 136 (97.1) | 26 (92.9) | 0.262 b |
| Therapeutic effort limitation indicated (N = 155) | 53 (37.9) | 24 (85.7) | <0.001 * a |
| Prior pneumococcal vaccination (N = 153) | 77 (55.4) | 17 (63.0) | 0.468 a |
| No Mortality (Median (Q1–Q3) 3) | Mortality within 30 days (Median (Q1–Q3) 3) | Significance | |
| Variables at Admission | |||
| Age | 74.9 (58.7–83.7) | 84.0 (74.9–87.7) | 0.002 * c |
| CURB-65 score 4 | 2.0 (1.0–2.0) | 2.0 (2.0–3.0) | 0.003 c |
| Barthel index | 100 (70.0–100) | 50.0 (40.0–95.0) | <0.001 * c |
| Number of comorbidities | 2.0 (1.0–3.0) | 2.0 (1.3–3.0) | 0.131 c |
| Laboratory Analysis at Admission | |||
| Hematocrit | 0.35 (0.30–0.40) | 0.33 (0.30–0.40) | 0.630 c |
| Leukocytes | 10.21 (7.35–13.69) | 11.39 (7.90–17.88) | 0.289 c |
| Creatinine | 0.89 (0.65–1.29) | 1.21 (0.67–1.80) | 0.141 c |
| Urea | 54.0 (38.0–77.3) | 86.5 (52.5–153.8) | 0.001 * c |
| Albumin | 2.70 (2.28–2.93) | 2.40 (2.05–2.70) | 0.046 c |
| Glucose | 114.0 (99.0–142.75) | 136.0 (112.8–218.3) | 0.004 * c |
| Sodium | 139.0 (136.0–141.0) | 140.0 (136.0–143.0) | 0.137 c |
| Potassium | 4.10 (3.80–4.50) | 4.10 (3.70–4.50) | 0.690 c |
| Arterial pH | 7.38 (7.35–7.41) | 7.34 (7.28–7.39) | 0.005 * c |
| Arterial PCO2 | 44.5 (40.1–50.8) | 49.4 (40.3–56.4) | 0.107 c |
| C–reactive protein | 143.5 (60.6–200.0) | 116.5 (67.5–230.8) | 0.666 c |
| Procalcitonin | 0.46 (0.11–3.12) | 0.59 (0.19–2.10) | 0.693 c |
| Clinical Examination Variables at Admission | |||
| SaO2/FiO2 ratio 5 (Mean [SD] 6) | 323.2 (87.81) | 201.9 (94.70) | <0.001 * d |
| Temperature (Median (Q1–Q3) 3) | 37.4 (36.5–38.2) | 36.5 (36.0–37.0) | <0.001 * d |
| Heart rate (bpm) (Mean (SD) 6) | 100.1 (22.45) | 98.6 (28.67) | 0.757 d |
| Respiratory rate (rpm) (Median (Q1–Q3) 3) | 20.0 (15.0–25.0) | 20.0 (15.0–25.0) | 0.689 c |
| Systolic blood pressure (mmHg) (Mean (SD) 6) | 128.1 (26.45) | 123.5 (24.20) | 0.398 d |
| Diastolic blood pressure (mmHg) (Median (Q1–Q3) 3) | 70.0 (60.0–80.0) | 65.0 (59.0–74.3) | 0.209 c |
| Oxygen saturation (%) 7 | 92.0 (88.0–94.8) | 90.5 (85.0–95.8) | 0.420 c |
| Proper Protocol Implementation | |||
| Primary Actions | 4.0 (3.0–5.0) | 4.0 (2.0–4.0) | 0.146 c |
| Total actions | 5.0 (4.0–7.0) | 5.0 (3.3–6.0) | 0.698 c |
| Time to treatment initiation (days) | 0 (0–1) | 0 (0–0) | 0.712 c |
| Intra-Episode Death | ||
| Variables in the Equation | Adjusted OR a (95% CI) b | p-Value |
| Sex (Female) | 2.69 (0.56–16.09) | 0.221 |
| Therapeutic Effort Limitation Indicated (Yes) | 9.10 (1.36–121.57) | 0.021 * |
| SaO2/FiO2 c (per unit increase) | 0.98 (0.97–0.99) | <0.001 * |
| Empirical Antibiotic According to Guidelines (Yes) | 0.33 (0.06–1.44) | 0.140 |
| Age (per year) | 1.01 (0.95–1.07) | 0.743 |
| Death within 30 Days after Discharge | ||
| Adjusted OR a (95% CI) b | p-value | |
| ARDS (Yes) d | 4.29 (1.05–19.93) | 0.043 * |
| SaO2/FiO2 c (per unit increase) | 0.99 (0.98–1.00) | 0.005 * |
| Age (per year) | 1.06 (1.02–1.12) | 0.005 * |
| Barthel Index (per point) | 0.97 (0.94–0.99) | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Ojeda, M.R.; Galán-Azcona, M.D.; Garrido-Alfaro, R.A.; Ruiz-Romero, M.V.; Fernández-Moyano, A.; García-Garmendia, J.L. Standardized Prospective Intervention in Hospitalized Patients with Bacterial Pneumonia. J. Clin. Med. 2025, 14, 8704. https://doi.org/10.3390/jcm14248704
Fernández-Ojeda MR, Galán-Azcona MD, Garrido-Alfaro RA, Ruiz-Romero MV, Fernández-Moyano A, García-Garmendia JL. Standardized Prospective Intervention in Hospitalized Patients with Bacterial Pneumonia. Journal of Clinical Medicine. 2025; 14(24):8704. https://doi.org/10.3390/jcm14248704
Chicago/Turabian StyleFernández-Ojeda, María Rocío, María Dolores Galán-Azcona, Rosa Anastasia Garrido-Alfaro, María Victoria Ruiz-Romero, Antonio Fernández-Moyano, and José Luis García-Garmendia. 2025. "Standardized Prospective Intervention in Hospitalized Patients with Bacterial Pneumonia" Journal of Clinical Medicine 14, no. 24: 8704. https://doi.org/10.3390/jcm14248704
APA StyleFernández-Ojeda, M. R., Galán-Azcona, M. D., Garrido-Alfaro, R. A., Ruiz-Romero, M. V., Fernández-Moyano, A., & García-Garmendia, J. L. (2025). Standardized Prospective Intervention in Hospitalized Patients with Bacterial Pneumonia. Journal of Clinical Medicine, 14(24), 8704. https://doi.org/10.3390/jcm14248704






