Impact of Acute Lymphoblastic Leukemia Treatment on Left Ventricular Function Assessed in 2D and 3D Speckle Tracing Echocardiography—Preliminary Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Echocardiography
2.3. Statistical Analysis
3. Results
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALL | acute lymphoblastic leukemia, |
| 2D | two-dimensional, |
| LV | left ventricle, |
| BSA | body surface area, |
| BMI | body mass index |
| A4Cd | apical four-chamber diastolic, |
| A2Cd | apical two-chamber diastolic, |
| EDV | end diastolic volume, |
| LVEF-2D | two-dimensional left ventricle ejection fraction, |
| LV-GLS-2D | two-dimensional global longitudinal strain of the left ventricle, |
| 3D | three-dimensional, |
| EDVI | end diastolic volume index, |
| ESV | end systolic volume, |
| ESVI | end systolic volume index, |
| LVEF-3D | three-dimensional left ventricle ejection fraction, |
| ED | end diastolic, |
| LV-GLS-3D | three-dimensional left ventricle global longitudinal strain, |
| LV-GCS-3D | three-dimensional left ventricle global circumferential strain, |
| LV-GRS-3D | three-dimensional left ventricle global radial strain. |
References
- Bai, L.; Zhan, Y.; Zhou, Y.; Shi, L.; Gupta, S.; Denburg, A.; Guan, X. Evidence of clinical benefit of WHO essential anticancer medicines for children, 2011–2021. eClinicalMedicine 2023, 59, 101966. [Google Scholar] [CrossRef]
- Childhood Acute Lymphoblastic Leukaemia Collaborative Group (CALLCG). Beneficial and harmful effects of anthracyclines in the treatment of childhood acute lymphoblastic leukaemia: A systematic review and meta-analysis. Br. J. Haematol. 2009, 145, 376–388. [Google Scholar] [CrossRef]
- Feijen, E.A.M.L.; Font-Gonzalez, A.; Van der Pal, H.J.H.; Kok, W.E.M.; Geskus, R.B.; Ronckers, C.M.; Bresters, D.; van Dalen, E.C.; van Dulmen-den Broeder, E.; van den Berg, M.H.; et al. Risk and Temporal Changes of Heart Failure Among 5-Year Childhood Cancer Survivors: A DCOG-LATER Study. J. Am. Heart Assoc. 2019, 8, e009122. [Google Scholar] [CrossRef]
- Armstrong, G.T.; Joshi, V.M.; Ness, K.K.; Marwick, T.H.; Zhang, N.; Srivastava, D.; Griffin, B.P.; Grimm, R.A.; Thomas, J.; Phelan, D.; et al. Comprehensive Echocardiographic Detection of Treatment-Related Cardiac Dysfunction in Adult Survivors of Childhood Cancer: Results From the St. Jude Lifetime Cohort Study. J. Am. Coll. Cardiol. 2015, 65, 2511–2522. [Google Scholar] [CrossRef]
- González-Manzanares, R.; Ojeda, S.; Carrasco-Chinchilla, F.; Benito-González, T.; Pascual, I.; Nombela-Franco, L.; Serrador Frutos, A.M.; Estévez-Loureiro, R.; Del Trigo, M.; Freixa, X.; et al. Transcatheter mitral edge-to-edge repair in patients with a prior cancer diagnosis: Insights from the Spanish M-TEER registry. Rev. Espanola De Cardiol. 2025. [Google Scholar] [CrossRef]
- Heredia, G.; Gonzalez-Manzanares, R.; Ojeda, S.; Molina, J.R.; Fernandez-Aviles, C.; Hidalgo, F.; Lopez-Aguilera, J.; Crespin, M.; Mesa, D.; Anguita, M.; et al. Right Ventricular Function in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia: From the CTOXALL Study. Cancers 2023, 15, 5158. [Google Scholar] [CrossRef]
- Azzam, M.; Wasef, M.; Khalaf, H.; Al-Habbaa, A. 3D-based strain analysis and cardiotoxicity detection in cancer patients received chemotherapy. BMC Cancer 2023, 23, 760. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef] [PubMed]
- Leszek, P.; Klotzka, A.; Bartuś, S.; Burchardt, P.; Czarnecka, A.M.; Długosz-Danecka, M.; Gierlotka, M.; Koseła-Paterczyk, H.; Krawczyk-Ożóg, A.; Kubiatowski, T.; et al. A practical approach to the 2022 ESC cardio-oncology guidelines: Comments by a team of experts-cardiologists and oncologists. Kardiol. Pol. 2023, 81, 1047–1063. [Google Scholar] [CrossRef] [PubMed]
- Levy, P.T.; Machefsky, A.; Sanchez, A.A.; Patel, M.D.; Rogal, S.; Fowler, S.; Yaeger, L.; Hardi, A.; Holland, M.R.; Hamvas, A.; et al. Reference Ranges of Left Ventricular Strain Measures by Two-Dimensional Speckle-Tracking Echocardiography in Children: A Systematic Review and Meta-Analysis. J. Am. Soc. Echocardiogr. 2016, 29, 209–225.e6. [Google Scholar] [CrossRef] [PubMed]
- Lopez, L.; Saurers, D.L.; Barker, P.C.; Cohen, M.S.; Colan, S.D.; Dwyer, J.; Altman, C.A. Guidelines for Performing a Comprehensive Pediatric Transthoracic Echocardiogram: Recommendations From the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2024, 37, 119–170. [Google Scholar] [CrossRef]
- Kuebler, J.D.; Ghelani, S.; Williams, D.M.; Nathan, M.; Marx, G.; Colan, S.D.; Harrild, D.M. Normal Values and Growth-Related Changes of Left Ventricular Volumes, Stress, and Strain in Healthy Children Measured by 3-Dimensional Echocardiography. Am. J. Cardiol. 2018, 122, 331–339. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Giantris, A.L.; Lipsitz, S.R.; Dalton, V.K.; Asselin, B.L.; Barr, R.D.; Colan, S.D. Doxorubicin administration by continuous infusion is not cardioprotective: The Dana-Farber 91-01 Acute Lymphoblastic Leukemia protocol. J. Clin. Oncol. 2002, 20, 1677–1682. [Google Scholar] [CrossRef]
- Gonzalez-Manzanares, R.; Castillo, J.C.; Molina, J.R.; Ruiz-Ortiz, M.; Mesa, D.; Ojeda, S.; Anguita, M.; Pan, M. Automated Global Longitudinal Strain Assessment in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. Cancers 2022, 14, 1513. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Kokkinidis, D.G.; Kampaktsis, P.N.; Amir, E.A.; Marwick, T.H.; Gupta, D.; Thavendiranathan, P. Assessment of Prognostic Value of Left Ventricular Global Longitudinal Strain for Early Prediction of Chemotherapy-Induced Cardiotoxicity: A Systematic Review and Meta-analysis. JAMA Cardiol. 2019, 4, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Bilir, Ö.A.; Turkey, A.; Çetin, I.I.; Kaçar, D.; Aker, C.B.; Özbek, N.Y.; Yaralı, N. Evaluation of early-onset cardiotoxic effects of anthracyclines used during the treatment of childhood acute lymphoblastic leukemia by speckle-tracking echocardiography. Anatol. J. Cardiol. 2022, 26, 57–62. [Google Scholar] [CrossRef]
- Al-Biltagi, M.; Tolba, O.A.R.E.; El-Shanshory, M.R.; El-Shitany, N.A.E.-A.; El-Hawary, E.E.-S. Strain echocardiography in early detection of Doxorubicin-induced left ventricular dysfunction in children with acute lymphoblastic leukemia. ISRN Pediatr. 2012, 2012, 870549. [Google Scholar] [CrossRef] [PubMed]
- Toro, C.; Felmingham, B.; Jessop, S.; Celermajer, D.S.; Kotecha, R.S.; Govender, D.; Conyers, R. Cardio-Oncology Recommendations for Pediatric Oncology Patients: An Australian and New Zealand Delphi Consensus. JACC Adv. 2022, 1, 100155. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, H.; Werner, B. Three-dimensional echocardiography in the assessment of ventricular function in children: Pros, cons, and hopes. Kardiol. Pol. 2019, 77, 12–17. [Google Scholar] [CrossRef]
- Lazar, D.R.; Maniu, D.; Lazar, F.-L.; Blag, C.; Bota, M.; Zdrenghea, M.; Cainap, S. Exploring the baseline cardiac function and its correlation with risk stratification in children diagnosed with acute lymphoblastic leukemia. Ann. Hematol. 2025, 104, 4157–4164. [Google Scholar] [CrossRef]
- Jackowska, T.; Wasilewski, R.; Golabek, M.; Syczewska, M. Natriuretic Peptides in Children with Acute Lymphoblastic Leukaemia. Blood 2004, 104, 4437. [Google Scholar] [CrossRef]

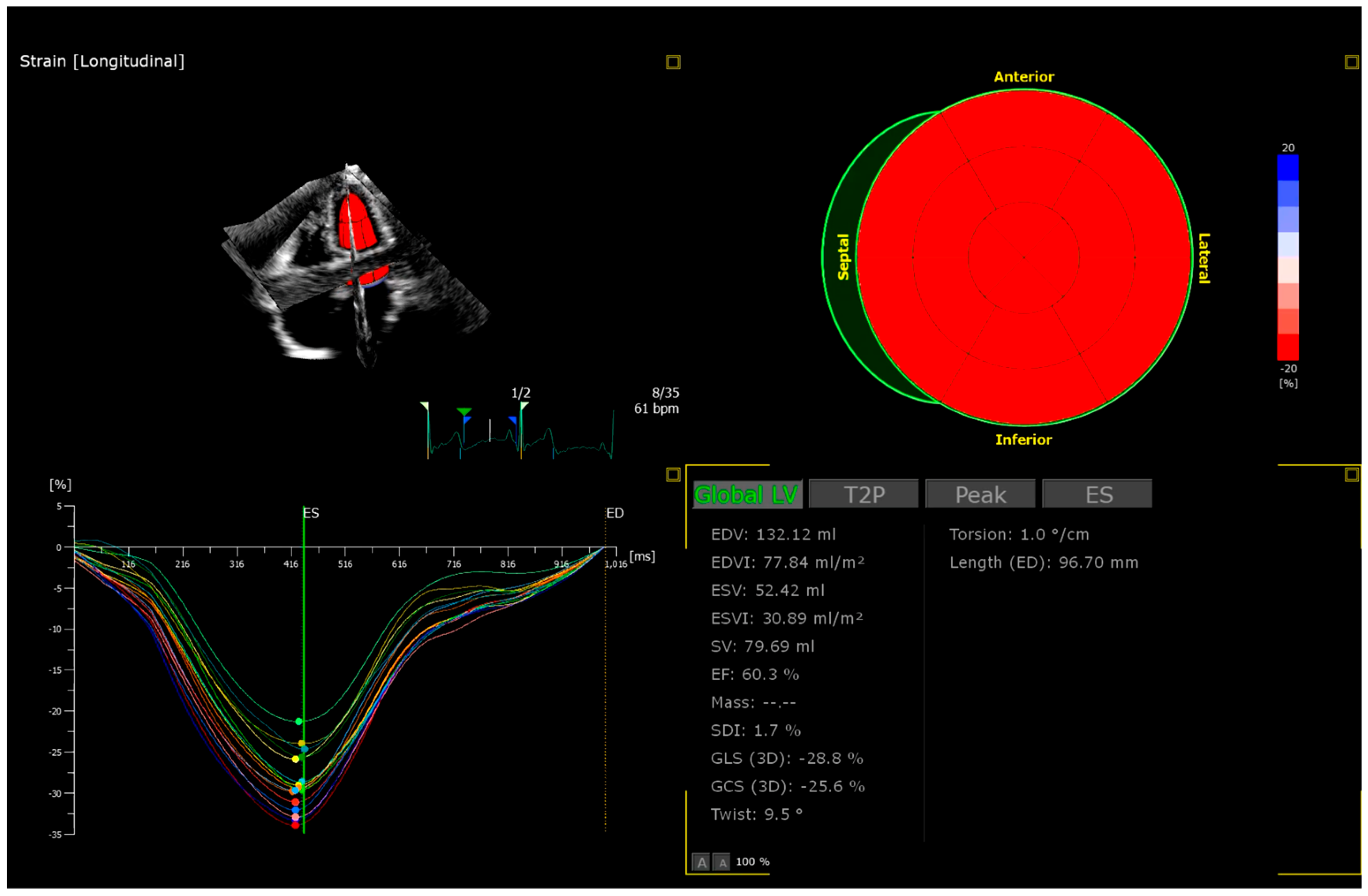
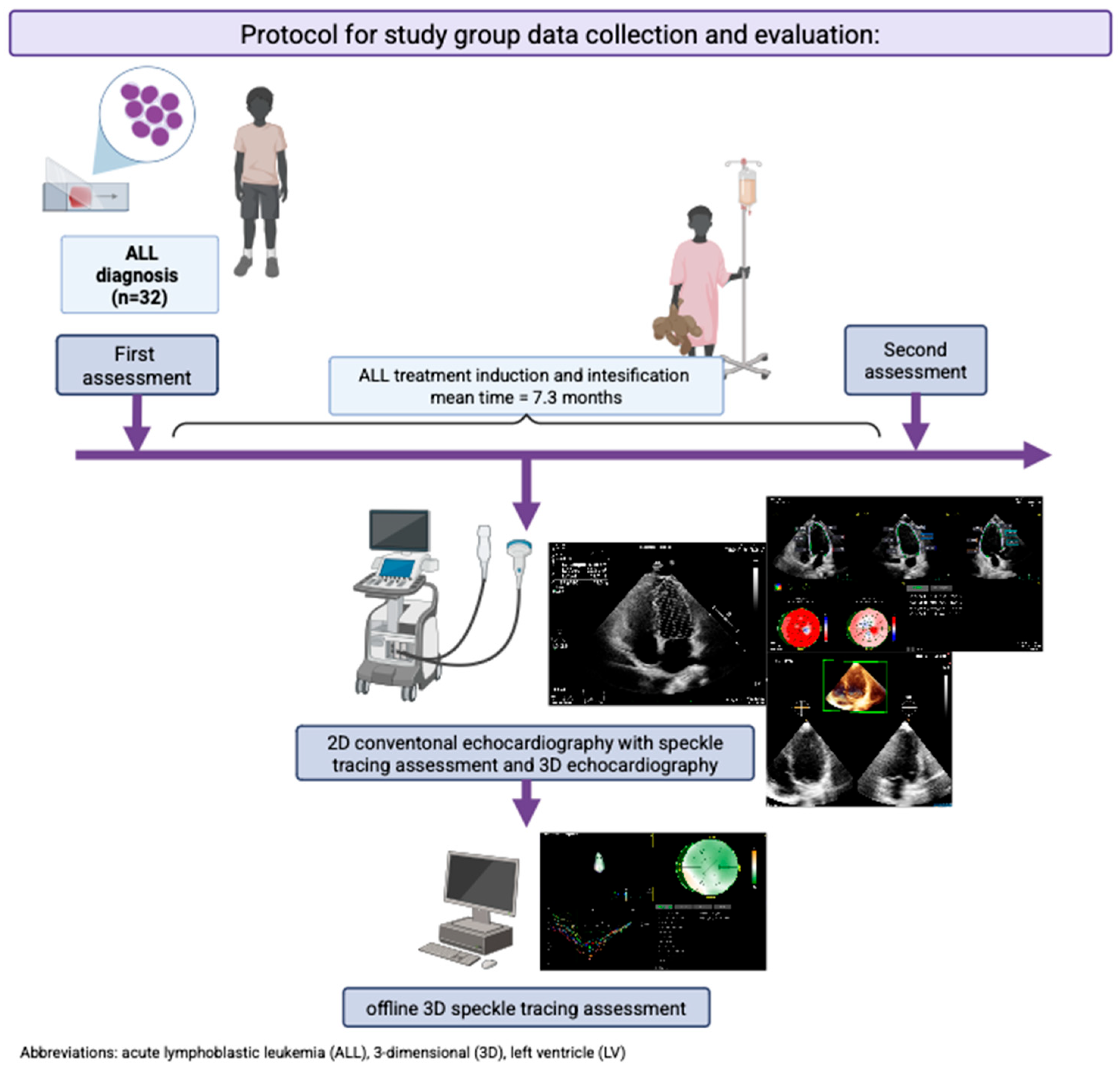
| Inclusion criteria |
| - age 0–18 years, - newly diagnosed acute lymphoblastic leukemia (ALL) based on bone marrow cytology (taken by fine-needle aspiration biopsy from one of the spines of the hip plate or tibia, according to the FAB classification of bone marrow smears stained by the May–Grünwald and Giemsa method), - qualification for treatment of acute lymphoblastic leukemia/lymphoblastic lymphoma with anthracycline chemotherapy, - written informed consent signed by parents and patient ≥ 12 years of age, |
| Exclusion criteria |
| - a history of cancer/ALL and treatment of cancer/ALL with chemotherapy, especially with anthracyclines, - acute infection, - platelet count < 20 G/L, - severe conditions: protein loss syndrome, peritoneal, pericardial, or pleural effusion, arrhythmia, metabolic disorders, - after initial chemotherapy that is common to all patients treated for ALL on the basis of response to treatment, qualification for a very high-risk group, and planned inclusion of radiotherapy or hematopoietic cell transplantation, - previously diagnosed chronic cardiovascular disease, i.e., cardiomyopathy, heart failure, congenital heart defects, arrhythmias. - significant chronic comorbidities involving the endocrine, neurological, and digestive systems, as well as the respiratory tract, and kidney diseases, - lack of consent from parent and/or patient > 12 years of age, |
| Interobserver | Intraobserver | |||
|---|---|---|---|---|
| Parameter | p Value | R, 95% Confidence Interval | p Value | R, 95% Confidence Interval |
| LV-GLS-2D average | 0.91 | 0.965, 0.835–0.978 | 0.95 | 0.996, 0.992–0.998 |
| LVEF-3D | 0.92 | 0.932, 0.732–0.984 | 0.94 | 0.986, 0.920–0.997 |
| LV-GLS-3D | 0.92 | 0.934, 0.738–0.985 | 0.97 | 0.986, 0.923–0.998 |
| LV-GCS-3D | 0.92 | 0.982, 0.925–0.996 | 0.95 | 0.981, 0.895–0.997 |
| LV-GRS-3D | 0.93 | 0.978, 0.906–0.995 | 0.93 | 0.976, 0.864–0.996 |
| Preliminary Study Group Characteristics (n = 32) | |
|---|---|
| age at diagnosis (years old) | 5.98 ± 3.7 (min. 1.6, max. 16.0) |
| sex n (%) | 14 (43%) males, 18 (56%) females |
| Ethnicity n (%) | Caucasian n = 32 (100%) |
| diagnosis n (%) | 31 (96%) ALL type C, 1 (4%) ALL type T |
| white blood cells at diagnosis | 18.2 × 103 (5.7 × 103; 33.3 × 103) |
| percentage of blasts detected in manual count (%) | 85.2 ± 12.5 |
| treatment protocol n (%) | AIEOP BMF 2017 n = 32 (100%) |
| initial treatment response n (%) | M1 n = 29 (90%) M2 n = 2 (6%) M3 n = 1 (4%) |
| BSA at diagnosis (m2) | 0.87 ± 0.36 |
| BMI at diagnosis (kg/m2) | 15.9 ± 2.2 |
| BMI percentile at diagnosis | 37.9 ± 24.9 |
| type of anthracycline used n (%) | Doxorubicin n = 32 (100%) Daunorubicin n = 32 (100%) |
| time from the first anthracycline dose (months) | 7.3 ± 1.5 min 5.3–max 11.4 |
| cumulative equivalent anthracycline dose (mg/m2) | 165.6 ± 54.0 min. 97.2–max. 293.7 |
| cumulative equivalent anthracycline dose > 200 mg/m2 n (%) | n = 5 (16%) |
| NT-proBNP (pg/mL) | |
| 1st assessment | 383.5 (116.0; 926.0) |
| Troponin I/T (ng/mL) | |
| 1st assessment | 0.0 * (0.0; 0.0) |
| Study Group—Children with ALL n = 32 | |||||
|---|---|---|---|---|---|
| Variable | First Assessment—at the Time of Diagnosis | Second Assessment—After Induction and Intensification Phase of Therapy Completion | p Value | Δ Difference (95% CI Lower/Upper) | Effect Size |
| 2D Echocardiography | |||||
| LV volume (A4Cd) (mL) | 52.0 ± 20.9 | 58.7 ± 24.1 | 0.13 t | −4.9 (−11.3/1.5) | −0.329 cd |
| LV volume (A2Cd) (mL) | 44.4 ± 17.1 | 50.9 ± 20.2 | 0.21 t | −4.7 (−12.3/2.9) | −0.267 cd |
| EDV (BP) (mL) | 49.0 ± 18.0 | 54.6 ± 21.4 | 0.35 t | −3.2 (−10.0/3.7) | −0.206 cd |
| LVEF-2D (Simpson Biplane) (%) | 56.8 ± 8.4 | 55.4 ± 4.8 | 0.45 t | 3.1 (−0.8/7.0) | −0.329 cd |
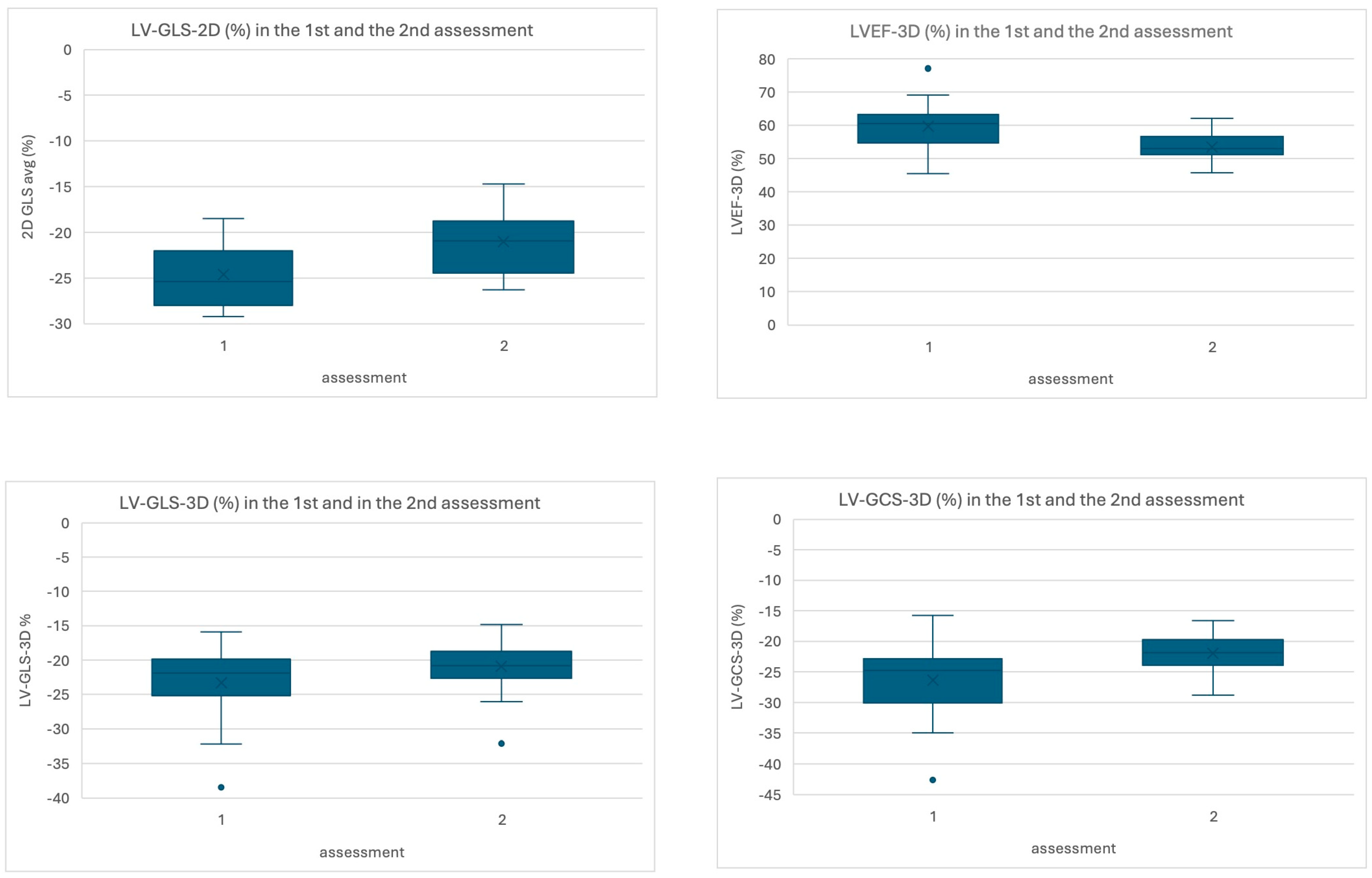
| LV-GLS-2D (%) | −24.6 ± 3.3 | −21.0 ± 3.3 | <0.001 t* | −3.5 (−5.1/−1.8) | −0.865 cd |
| 3D Echocardiography | |||||
| EDV-3D (mL) | 52.0 ± 23.1 | 55.6 ± 22.8 | 0.55 t | −2.2 (−9.5/5.2) | −0.134 cd |
| EDVI-3D (mL/m2) | 58.8 (52.7; 64.9) | 56.5 (47.3; 64.8) | 0.31 w | 4.5 (−4.6/13.6) | 0.233 wr |
| ESV-3D (mL) | 20.4 ± 8.5 | 25.8 ± 10.5 | 0.13 t | −4.0 (−9.3/1.3) | −0.337 cd |
| ESVI-3D (mL/m2) | 23.2 ± 5.6 | 27.7 ± 7.0 | 0.02 t* | −4.8 (−7.4/−2.3) | −0.883 cd |
| LVEF-3D (%) | 59.7 ± 7.3 | 55.1 ± 3.1 | 0.01 t* | 5.5 (1.8/9.2) | 0.656 cd |
| Length (ED) 3D (mm) | 64.5 ± 8.9 | 66.2 ± 8.3 | 0.23 t | −1.2 (−3.3/0.8) | −0.684 cd |
| LV-GLS-3D (%) | −23.3 ± 5.3 | −20.4 ± 2.8 | 0.03 t* | −2.1 (−4.9/0.7) | −0.756 cd |
| LV-GCS-3D (%) | −26.3 ± 5.9 | −21.9 ± 3.2 | 0.02 t* | −3.8 (−6.8/−0.8) | −0.555 cd |
| LV-GRS-3D (%) | 35.4 ± 20.0 | 35.9 ± 3.6 | 0.45 t | 2.4 (−4.1/8.9) | −0.165 cd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haponiuk-Skwarlińska, J.; Kamińska, H.; Albrecht, K.; Łaguna, P.; Werner, B. Impact of Acute Lymphoblastic Leukemia Treatment on Left Ventricular Function Assessed in 2D and 3D Speckle Tracing Echocardiography—Preliminary Results. J. Clin. Med. 2025, 14, 8682. https://doi.org/10.3390/jcm14248682
Haponiuk-Skwarlińska J, Kamińska H, Albrecht K, Łaguna P, Werner B. Impact of Acute Lymphoblastic Leukemia Treatment on Left Ventricular Function Assessed in 2D and 3D Speckle Tracing Echocardiography—Preliminary Results. Journal of Clinical Medicine. 2025; 14(24):8682. https://doi.org/10.3390/jcm14248682
Chicago/Turabian StyleHaponiuk-Skwarlińska, Julia, Halszka Kamińska, Katarzyna Albrecht, Paweł Łaguna, and Bożena Werner. 2025. "Impact of Acute Lymphoblastic Leukemia Treatment on Left Ventricular Function Assessed in 2D and 3D Speckle Tracing Echocardiography—Preliminary Results" Journal of Clinical Medicine 14, no. 24: 8682. https://doi.org/10.3390/jcm14248682
APA StyleHaponiuk-Skwarlińska, J., Kamińska, H., Albrecht, K., Łaguna, P., & Werner, B. (2025). Impact of Acute Lymphoblastic Leukemia Treatment on Left Ventricular Function Assessed in 2D and 3D Speckle Tracing Echocardiography—Preliminary Results. Journal of Clinical Medicine, 14(24), 8682. https://doi.org/10.3390/jcm14248682







