Factor XI and Cancer: Physiopathological Linkage and Clinical Perspectives
Abstract
1. Introduction
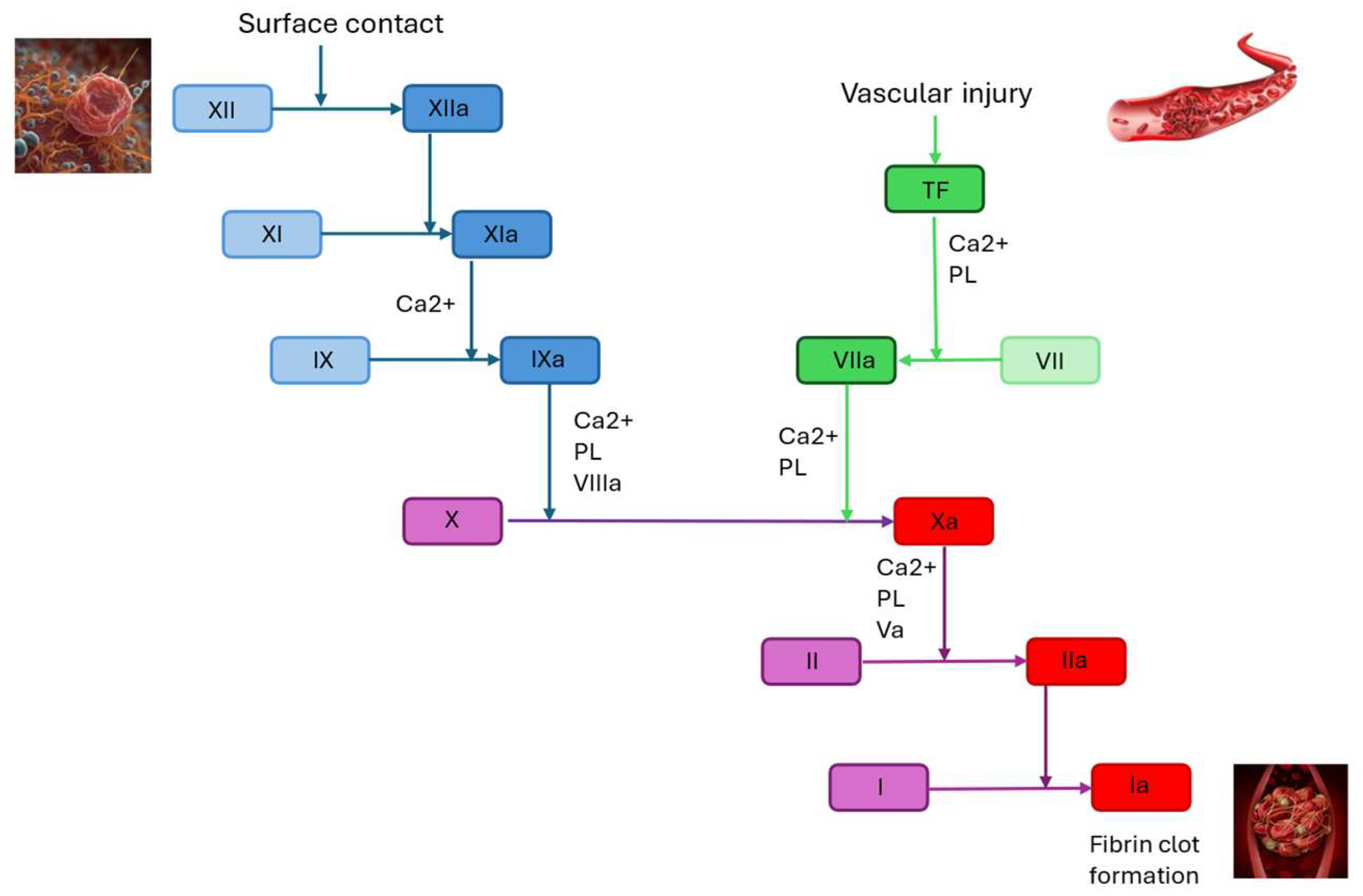
2. Factor XI: Coagulation Cascade and Monitoring
2.1. Role of Cancer in Thrombosis
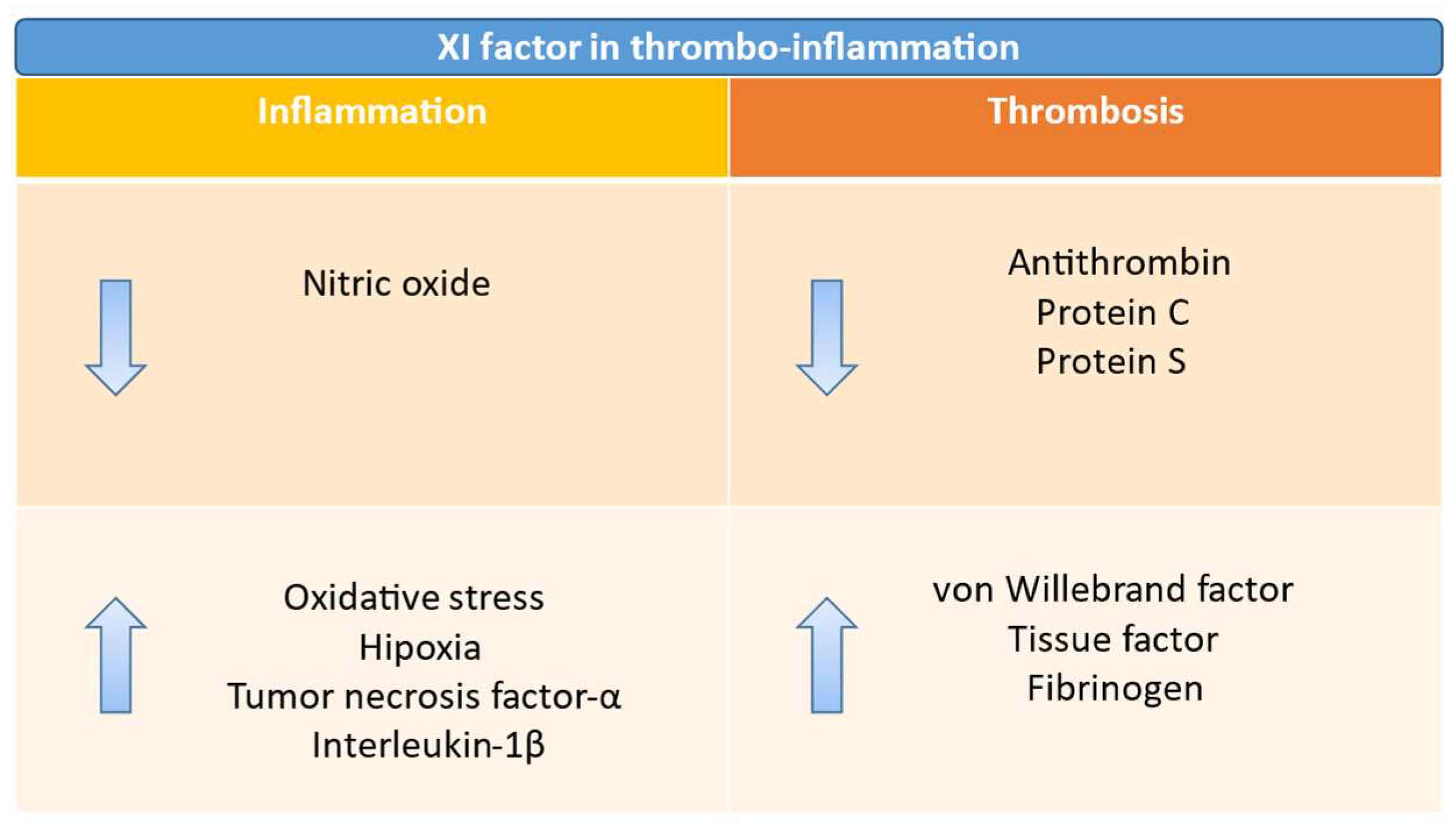
2.2. Role of Factor XI in Hemostasis and Thrombosis
3. Role of Factor XI in Cancer
3.1. Pathophysiology of Venous Thromboembolism
3.2. FactorXI and Cancer Progression
4. Factor XI Inhibitors
4.1. FXI Inhibitors to Prevent Venous Thromboembolic Events
4.2. FXI Inhibitors to Treat Venous Thromboembolic Events
4.3. FXI Inhibitors to Prevent Catheter-Related Thrombosis
4.4. FXI Inhibitors to Prevent Arterial Thromboembolic Events
4.5. Stroke Prevention in Atrial Fibrillation
5. Limitations and Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADAM | a disintegrin and metalloprotease |
| AF | atrial fibrillation |
| AXIOMATIC-SSP | Antithrombotic treatment with Factor XIa inhibition to Optimize Management of Acute Thromboembolic events for Secondary Stroke Prevention |
| AVERT | A Very Early Rehabilitation Trial after stroke |
| BARC | Bleeding Academic Research Consortium |
| CAT | cancer-associated thrombosis |
| CCL 16 | chemokine ligand 16 |
| CD28 | cluster of differentiation 28 |
| CI | confidence interval |
| CTEPH | chronic thromboembolic pulmonary hypertension |
| DVP | deep-venous thrombosis |
| DOACs | direct oral anticoagulants |
| FII | factor II |
| FIII | factor III |
| FIX | factor IX |
| FOX-TROT | Fluoropyrimidine Oxaliplatin and Targeted Receptor Pre-Operative Therapy |
| FV | factor V |
| FVIIa | activated factor VII |
| FVIII | factor VIII |
| FX | factor X |
| FXI-ASO | Active Comparator-Controlled Study to Assess Safety and Efficacy of ISIS-FXIRx in Total Knee Arthroplasty |
| FXI | factor XI |
| FXII | factor XII |
| FXIIa | activated factor XII |
| FXa | activated factor X |
| GSDMD | gasdermin D |
| IL-1β | interleukin-1β |
| IR | incidence rate |
| LMWHs | low molecular weight heparin |
| LRR | leucine-rich repeats |
| MAPK | mitogen-activated protein kinase |
| NACHT | nucleotide-binding domain function |
| NLRP3 | pyrin domain–containing protein 3 |
| PAI-1 | plasminogen activator inhibitor-1 |
| PE | pulmonary embolism |
| PT | prothrombin time |
| PTS | post-thrombotic syndrome |
| PTT | partial thromboplastin time |
| TF | tissue factor |
| TNF-α | tumor necrosis factor-α |
| VE-cadherin | vascular endothelial cadherin |
| VLDLR | very low–density lipoprotein receptor |
| VTE | venous thromboembolism |
| vWF | von Willebrand factor |
References
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.-J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Acute Pulmonary Embolism Developed in Collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- Falanga, A.; Schieppati, F.; Russo, L. Pathophysiology 1. In Mechanisms of Thrombosis in Cancer Patients; Springer: Berlin/Heidelberg, Germany, 2019; pp. 11–36. [Google Scholar]
- Bell, E.J.; Lutsey, P.L.; Basu, S.; Cushman, M.; Heckbert, S.R.; Lloyd-Jones, D.M.; Folsom, A.R. Lifetime Risk of Venous Thromboembolism in Two Cohort Studies. Am. J. Med. 2016, 129, 339.e19–339.e26. [Google Scholar] [CrossRef]
- Abdulla, A.; Davis, W.M.; Ratnaweera, N.; Szefer, E.; Ballantyne Scott, B.; Lee, A.Y.Y. A Meta-Analysis of Case Fatality Rates of Recurrent Venous Thromboembolism and Major Bleeding in Patients with Cancer. Thromb. Haemost. 2020, 120, 702–713. [Google Scholar] [CrossRef]
- Girardi, L.; Wang, T.-F.; Ageno, W.; Carrier, M. Updates in the Incidence, Pathogenesis, and Management of Cancer and Venous Thromboembolism. Arter. Thromb. Vasc. Biol. 2023, 43, 824–831. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Mahé, I.; Felip, E.; Agnelli, G.; Awada, A.; Cohen, A.; Falanga, A.; Mandala, M.; Peeters, M.; Tsoukalas, N.; et al. Treating Cancer-Associated Venous Thromboembolism: A Practical Approach. Eur. J. Cancer 2024, 209, 114263. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on Cardio-Oncology Developed in Collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef] [PubMed]
- Mahé, I.; Chidiac, J.; Bertoletti, L.; Font, C.; Trujillo-Santos, J.; Peris, M.; Pérez Ductor, C.; Nieto, S.; Grandone, E.; Monreal, M.; et al. The Clinical Course of Venous Thromboembolism May Differ According to Cancer Site. Am. J. Med. 2017, 130, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Tsantes, A.G.; Petrou, E.; Tsante, K.A.; Sokou, R.; Frantzeskaki, F.; Domouchtsidou, A.; Chaldoupis, A.E.; Fortis, S.P.; Piovani, D.; Nikolopoulos, G.K.; et al. Cancer-Associated Thrombosis: Pathophysiology, Laboratory Assessment, and Current Guidelines. Cancers 2024, 16, 2082. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.G.; Monteiro, R.Q. Activation of Blood Coagulation in Cancer: Implications for Tumour Progression. Biosci. Rep. 2013, 33, e00064. [Google Scholar] [CrossRef]
- Hsu, C.; Hutt, E.; Bloomfield, D.M.; Gailani, D.; Weitz, J.I. Factor XI Inhibition to Uncouple Thrombosis From Hemostasis. J. Am. Coll. Cardiol. 2021, 78, 625–631. [Google Scholar] [CrossRef]
- Puy, C.; Moellmer, S.A.; Pang, J.; Vu, H.H.; Melrose, A.R.; Lorentz, C.U.; Tucker, E.I.; Shatzel, J.J.; Keshari, R.S.; Lupu, F.; et al. Coagulation Factor XI Regulates Endothelial Cell Permeability and Barrier Function in Vitro and in Vivo. Blood 2024, 144, 1821–1833. [Google Scholar] [CrossRef]
- Pallares Robles, A.; ten Cate, V.; Schulz, A.; Prochaska, J.H.; Rapp, S.; Koeck, T.; Panova-Noeva, M.; Heitmeier, S.; Schwers, S.; Leineweber, K.; et al. Association of FXI Activity with Thrombo-Inflammation, Extracellular Matrix, Lipid Metabolism and Apoptosis in Venous Thrombosis. Sci. Rep. 2022, 12, 9761. [Google Scholar] [CrossRef]
- Palta, S.; Saroa, R.; Palta, A. Overview of the Coagulation System. Indian J. Anaesth. 2014, 58, 515. [Google Scholar] [CrossRef]
- Bijak, M.; Rzeźnicka, P.; Saluk, J.; Nowak, P. [Cellular Model of Blood Coagulation Process]. Pol. Merkur. Lek. 2015, 39, 5–8. [Google Scholar]
- Fernandes, C.J.; Morinaga, L.T.K.; Alves, J.L.; Castro, M.A.; Calderaro, D.; Jardim, C.V.P.; Souza, R. Cancer-Associated Thrombosis: The When, How and Why. Eur. Respir. Rev. 2019, 28, 180119. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A. Malignancy, Thrombosis and Trousseau: The Case for an Eponym. J. Thromb. Haemost. 2003, 1, 2463–2465. [Google Scholar] [CrossRef] [PubMed]
- Mulder, F.I.; Candeloro, M.; Kamphuisen, P.W.; Di Nisio, M.; Bossuyt, P.M.; Guman, N.; Smit, K.; Büller, H.R.; van Es, N. The Khorana Score for Prediction of Venous Thromboembolism in Cancer Patients: A Systematic Review and Meta-Analysis. Haematologica 2019, 104, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, R.S.; Sullenger, B.; Becker, R.C. The Many Faces of the Contact Pathway and Their Role in Thrombosis. J. Thromb. Thrombolysis 2011, 32, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Laudani, C.; Spagnolo, M.; Agnello, F.; Faro, D.C.; Finocchiaro, S.; Legnazzi, M.; Mauro, M.S.; Mazzone, P.M.; Occhipinti, G.; et al. Pharmacology and Clinical Development of Factor XI Inhibitors. Circulation 2023, 147, 897–913. [Google Scholar] [CrossRef]
- Yau, J.W.; Liao, P.; Fredenburgh, J.C.; Stafford, A.R.; Revenko, A.S.; Monia, B.P.; Weitz, J.I. Selective Depletion of Factor XI or Factor XII with Antisense Oligonucleotides Attenuates Catheter Thrombosis in Rabbits. Blood 2014, 123, 2102–2107. [Google Scholar] [CrossRef]
- Barg, A.A.; Livnat, T.; Kenet, G. Factor XI Deficiency: Phenotypic Age-Related Considerations and Clinical Approach towards Bleeding Risk Assessment. Blood 2024, 143, 1455–1464. [Google Scholar] [CrossRef]
- Salomon, O.; Steinberg, D.; Zucker, M.; Varon, D.; Zivelin, A.; Seligsohn, U. Patients with Severe Factor XI Deficiency Have a Reduced Incidence of Deep-Vein Thrombosis. Thromb. Haemost. 2011, 105, 269–273. [Google Scholar] [CrossRef]
- Gill, D.; Georgakis, M.K.; Laffan, M.; Sabater-Lleal, M.; Malik, R.; Tzoulaki, I.; Veltkamp, R.; Dehghan, A. Genetically Determined FXI (Factor XI) Levels and Risk of Stroke. Stroke 2018, 49, 2761–2763. [Google Scholar] [CrossRef] [PubMed]
- Rohmann, J.L.; Huo, S.; Sperber, P.S.; Piper, S.K.; Rosendaal, F.R.; Heuschmann, P.U.; Endres, M.; Liman, T.G.; Siegerink, B. Coagulation Factor XII, XI, and VIII Activity Levels and Secondary Events after First Ischemic Stroke. J. Thromb. Haemost. 2020, 18, 3316–3324. [Google Scholar] [CrossRef] [PubMed]
- Preis, M.; Hirsch, J.; Kotler, A.; Zoabi, A.; Stein, N.; Rennert, G.; Saliba, W. Factor XI Deficiency Is Associated with Lower Risk for Cardiovascular and Venous Thromboembolism Events. Blood 2017, 129, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Falco, L.; Tessitore, V.; Mauriello, A.; Catapano, D.; Napolitano, N.; Tariq, M.; Caturano, A.; Ciccarelli, G.; D’Andrea, A.; et al. Anti-Inflammatory and Anticancer Effects of Anticoagulant Therapy in Patients with Malignancy. Life 2023, 13, 1888. [Google Scholar] [CrossRef]
- Cohen, A.; Agnelli, G.; Anderson, F.; Arcelus, J.; Bergqvist, D.; Brecht, J.; Greer, I.; Heit, J.; Hutchinson, J.; Kakkar, A.; et al. Venous Thromboembolism (VTE) in Europe. Thromb. Haemost. 2007, 98, 756–764. [Google Scholar] [CrossRef]
- Lee, L.; Gallus, A.; Jindal, R.; Wang, C.; Wu, C.-C. Incidence of Venous Thromboembolism in Asian Populations: A Systematic Review. Thromb. Haemost. 2017, 117, 2243–2260. [Google Scholar] [CrossRef]
- Cohen, A.T.; Katholing, A.; Rietbrock, S.; Bamber, L.; Martinez, C. Epidemiology of First and Recurrent Venous Thromboembolism in Patients with Active Cancer. Thromb. Haemost. 2017, 117, 57–65. [Google Scholar] [CrossRef]
- Winter, M.-P.; Schernthaner, G.H.; Lang, I.M. Chronic Complications of Venous Thromboembolism. J. Thromb. Haemost. 2017, 15, 1531–1540. [Google Scholar] [CrossRef]
- Lip, G.Y.; Chin, B.S.; Blann, A.D. Cancer and the Prothrombotic State. Lancet Oncol. 2002, 3, 27–34. [Google Scholar] [CrossRef]
- Grover, S.P.; Mackman, N. Tissue Factor. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 709–725. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, Q.; Xu, L.; Feuerstein, G.Z.; Hsu, M.; Smith, P.L.; Seiffert, D.A.; Schumacher, W.A.; Ogletree, M.L.; Gailani, D. Effects of Factor IX or Factor XI Deficiency on Ferric Chloride-induced Carotid Artery Occlusion in Mice. J. Thromb. Haemost. 2005, 3, 695–702. [Google Scholar] [CrossRef]
- Meijers, J.C.M.; Tekelenburg, W.L.H.; Bouma, B.N.; Bertina, R.M.; Rosendaal, F.R. High Levels of Coagulation Factor XI as a Risk Factor for Venous Thrombosis. N. Engl. J. Med. 2000, 342, 696–701. [Google Scholar] [CrossRef]
- Mulder, F.I.; Horváth-Puhó, E.; van Es, N.; van Laarhoven, H.W.M.; Pedersen, L.; Moik, F.; Ay, C.; Büller, H.R.; Sørensen, H.T. Venous Thromboembolism in Cancer Patients: A Population-Based Cohort Study. Blood 2021, 137, 1959–1969. [Google Scholar] [CrossRef]
- Foley, J.H.; Conway, E.M. Cross Talk Pathways Between Coagulation and Inflammation. Circ. Res. 2016, 118, 1392–1408. [Google Scholar] [CrossRef]
- Gupta, N.; Sahu, A.; Prabhakar, A.; Chatterjee, T.; Tyagi, T.; Kumari, B.; Khan, N.; Nair, V.; Bajaj, N.; Sharma, M.; et al. Activation of NLRP3 Inflammasome Complex Potentiates Venous Thrombosis in Response to Hypoxia. Proc. Natl. Acad. Sci. USA 2017, 114, 4763–4768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, J.; Zhang, G.; Wu, C.; Abdel-Latif, A.; Smyth, S.S.; Shiroishi, T.; Mackman, N.; Wei, Y.; Tao, M.; et al. Inflammasome Activation Promotes Venous Thrombosis through Pyroptosis. Blood Adv. 2021, 5, 2619–2623. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.; Ponomaryov, T.; De Prendergast, A.; Whitworth, K.; Smith, C.W.; Khan, A.O.; Kavanagh, D.; Brill, A. Neutrophil Extracellular Traps and Inflammasomes Cooperatively Promote Venous Thrombosis in Mice. Blood Adv. 2021, 5, 2319–2324. [Google Scholar] [CrossRef]
- Potere, N.; Abbate, A.; Kanthi, Y.; Carrier, M.; Toldo, S.; Porreca, E.; Di Nisio, M. Inflammasome Signaling, Thromboinflammation, and Venous Thromboembolism. JACC Basic. Transl. Sci. 2023, 8, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Im, J.H.; Fu, W.; Wang, H.; Bhatia, S.K.; Hammer, D.A.; Kowalska, M.A.; Muschel, R.J. Coagulation Facilitates Tumor Cell Spreading in the Pulmonary Vasculature during Early Metastatic Colony Formation. Cancer Res. 2004, 64, 8613–8619. [Google Scholar] [CrossRef]
- Donati, M.B.; Lorenzet, R. Coagulation Factors and Tumor Cell Biology: The Role of Tissue Factor. Pathophysiol. Haemost. Thromb. 2003, 33, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Wu, X.; Xiang, M.; Wang, C.; Novakovic, V.A.; Shi, J. Microparticle Phosphatidylserine Mediates Coagulation: Involvement in Tumor Progression and Metastasis. Cancers 2023, 15, 1957. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Rosas, P.; Nagy, M.; Spronk, H.M.H.; Russo, L.; Gamba, S.; Tartari, C.J.; Bolognini, S.; Ticozzi, C.; Schieppati, F.; Sarmiento, R.; et al. Activated Factor XI-Antithrombin and Thrombin-Antithrombin Complexes in the Prediction of Venous Thromboembolism and Mortality in Patients with Non-Small-Cell Lung Cancer. J. Thromb. Haemost. 2025, 23, 1908–1919. [Google Scholar] [CrossRef] [PubMed]
- Poenou, G.; Heestermans, M.; Lafaie, L.; Accassat, S.; Moulin, N.; Rodière, A.; Petit, B.; Duvillard, C.; Mismetti, P.; Bertoletti, L. Inhibition of Factor XI: A New Era in the Treatment of Venous Thromboembolism in Cancer Patients? Int. J. Mol. Sci. 2023, 24, 14433. [Google Scholar] [CrossRef]
- Piccini, J.P.; Patel, M.R.; Steffel, J.; Ferdinand, K.; Van Gelder, I.C.; Russo, A.M.; Ma, C.S.; Goodman, S.G.; Oldgren, J.; Hammett, C.; et al. Asundexian versus Apixaban in Patients with Atrial Fibrillation. N. Engl. J. Med. 2024, 392, 23–32. [Google Scholar] [CrossRef]
- Jain, S.S.; Mahaffey, K.W.; Pieper, K.S.; Shimizu, W.; Potpara, T.; Ruff, C.T.; Kamel, H.; Lewis, B.S.; Cornel, J.H.; Kowey, P.R.; et al. Milvexian vs Apixaban for Stroke Prevention in Atrial Fibrillation: The LIBREXIA Atrial Fibrillation Trial Rationale and Design. Am. Heart J. 2024, 277, 145–158. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Kohs, T.C.L.; Vu, H.H.; Jordan, K.R.; Wang, J.S.H.; Lorentz, C.U.; Tucker, E.I.; Puy, C.; Olson, S.R.; DeLoughery, T.G.; et al. Factor XI Inhibition for the Prevention of Catheter-Associated Thrombosis in Patients With Cancer Undergoing Central Line Placement: A Phase 2 Clinical Trial. Arter. Thromb. Vasc. Biol. 2024, 44, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Ruff, C.T.; Patel, S.M.; Giugliano, R.P.; Morrow, D.A.; Hug, B.; Kuder, J.F.; Goodrich, E.L.; Chen, S.-A.; Goodman, S.G.; Joung, B.; et al. Abelacimab versus Rivaroxaban in Patients with Atrial Fibrillation. N. Engl. J. 2025, 392, 361–371. [Google Scholar] [CrossRef]
- A Study Comparing Abelacimab to Apixaban in the Treatment of Cancer-Associated VTE (ASTER). Available online: https://clinicaltrials.gov/study/NCT05171049 (accessed on 4 September 2025).
- A Study Comparing Abelacimab to Dalteparin in the Treatment of Gastrointestinal/Genitourinary Cancer and Associated VTE (MAGNOLIA). 2024. Available online: https://clinicaltrials.gov/study/NCT05171075?intr=Abelacimab&rank=3 (accessed on 4 September 2025).
- Weitz, J.I.; Tankó, L.B.; Floege, J.; Fox, K.A.A.; Bhatt, D.L.; Thadhani, R.; Hung, J.; Pap, Á.F.; Kubitza, D.; Winkelmayer, W.C. Anticoagulation with Osocimab in Patients with Kidney Failure Undergoing Hemodialysis: A Randomized Phase 2 Trial. Nat. Med. 2024, 30, 435–442. [Google Scholar] [CrossRef]
- Winkelmayer, W.C.; Lensing, A.W.A.; Thadhani, R.I.; Mahaffey, K.W.; Walsh, M.; Pap, Á.F.; Willmann, S.; Thelen, K.; Hodge, S.; Solms, A.; et al. A Phase II Randomized Controlled Trial Evaluated Antithrombotic Treatment with Fesomersen in Patients with Kidney Failure on Hemodialysis. Kidney Int. 2024, 106, 145–153. [Google Scholar] [CrossRef]
- Majima, T.; Oshima, Y. Venous Thromboembolism in Major Orthopedic Surgery. J. Nippon. Med. Sch. 2021, 88, 268–272. [Google Scholar] [CrossRef]
- Büller, H.R.; Bethune, C.; Bhanot, S.; Gailani, D.; Monia, B.P.; Raskob, G.E.; Segers, A.; Verhamme, P.; Weitz, J.I. Factor XI Antisense Oligonucleotide for Prevention of Venous Thrombosis. N. Engl. J. Med. 2015, 372, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.I.; Strony, J.; Ageno, W.; Gailani, D.; Hylek, E.M.; Lassen, M.R.; Mahaffey, K.W.; Notani, R.S.; Roberts, R.; Segers, A.; et al. Milvexian for the Prevention of Venous Thromboembolism. N. Engl. J. Med. 2021, 385, 2161–2172. [Google Scholar] [CrossRef]
- Verhamme, P.; Yi, B.A.; Segers, A.; Salter, J.; Bloomfield, D.; Büller, H.R.; Raskob, G.E.; Weitz, J.I. Abelacimab for Prevention of Venous Thromboembolism. N. Engl. J. Med. 2021, 385, 609–617. [Google Scholar] [CrossRef]
- Weitz, J.I.; Bauersachs, R.; Becker, B.; Berkowitz, S.D.; Freitas, M.C.S.; Lassen, M.R.; Metzig, C.; Raskob, G.E. Effect of Osocimab in Preventing Venous Thromboembolism among Patients Undergoing Knee Arthroplasty: The FOXTROT Randomized Clinical Trial. J. Am. Med. Assoc. 2020, 323, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Heit, J.A.; Mohr, D.N.; Silverstein, M.D.; Petterson, T.M.; O’fallon, M.; Melton, W.; Joseph, L. Predictors of Recurrence After Deep Vein Thrombosis and Pulmonary Embolism A Population-Based Cohort Study. Arch. Intern. Med. 2000, 160, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.J.; Dewilde, S.; Noble, S.; Reimer, E.; Lee, A.Y.Y. What Impact Does Venous Thromboembolism and Bleeding Have on Cancer Patients’ Quality of Life? Value Health 2018, 21, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Kuderer, N.M. When to Offer Thromboprophylaxis to Patients with Advanced Pancreatic Cancer: Shedding Light on the Path Forward. J. Clin. Oncol. 2015, 33, 1995–1997. [Google Scholar] [CrossRef]
- Maraveyas, A.; Waters, J.; Roy, R.; Fyfe, D.; Propper, D.; Lofts, F.; Sgouros, J.; Gardiner, E.; Wedgwood, K.; Ettelaie, C.; et al. Gemcitabine versus Gemcitabine plus Dalteparin Thromboprophylaxis in Pancreatic Cancer. Eur. J. Cancer 2012, 48, 1283–1292. [Google Scholar] [CrossRef]
- Haas, S.K.; Freund, M.; Heigener, D.; Heilmann, L.; Kemkes-Matthes, B.; Von Tempelhoff, G.F.; Melzer, N.; Kakkar, A.K. Low-Molecular-Weight Heparin versus Placebo for the Prevention of Venous Thromboembolism in Metastatic Breast Cancer or Stage III/IV Lung Cancer. Clin. Appl. Thromb. Hemost. 2012, 18, 159–165. [Google Scholar] [CrossRef]
- Khorana, A.A.; Francis, C.W.; Kuderer, N.M.; Carrier, M.; Ortel, T.L.; Wun, T.; Rubens, D.; Hobbs, S.; Iyer, R.; Peterson, D.; et al. Dalteparin Thromboprophylaxis in Cancer Patients at High Risk for Venous Thromboembolism: A Randomized Trial. Thromb. Res. 2017, 151, 89–95. [Google Scholar] [CrossRef]
- Carrier, M.; Abou-Nassar, K.; Mallick, R.; Tagalakis, V.; Shivakumar, S.; Schattner, A.; Kuruvilla, P.; Hill, D.; Spadafora, S.; Marquis, K.; et al. Apixaban to Prevent Venous Thromboembolism in Patients with Cancer. N. Engl. J. Med. 2019, 380, 711–719. [Google Scholar] [CrossRef]
- Khorana, A.A.; Soff, G.A.; Kakkar, A.K.; Vadhan-Raj, S.; Riess, H.; Wun, T.; Streiff, M.B.; Garcia, D.A.; Liebman, H.A.; Belani, C.P.; et al. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. N. Engl. J. Med. 2019, 380, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Al-Samkari, H.; Connors, J.M. Managing the Competing Risks of Thrombosis, Bleeding, and Anticoagulation in Patients with Malignancy. Blood Adv. 2019, 3, 3770–3779. [Google Scholar] [CrossRef]
- Frere, C.; Farge, D.; Schrag, D.; Prata, P.H.; Connors, J.M. Direct Oral Anticoagulant versus Low Molecular Weight Heparin for the Treatment of Cancer-Associated Venous Thromboembolism: 2022 Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Hematol. Oncol. 2022, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Raskob, G.E.; van Es, N.; Verhamme, P.; Carrier, M.; Di Nisio, M.; Garcia, D.; Grosso, M.A.; Kakkar, A.K.; Kovacs, M.J.; Mercuri, M.F.; et al. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N. Engl. J. Med. 2018, 378, 615–624. [Google Scholar] [CrossRef]
- McBane, R.D.; Wysokinski, W.E.; Le-Rademacher, J.G.; Zemla, T.; Ashrani, A.; Tafur, A.; Perepu, U.; Anderson, D.; Gundabolu, K.; Kuzma, C.; et al. Apixaban and Dalteparin in Active Malignancy-Associated Venous Thromboembolism: The ADAM VTE Trial. J. Thromb. Haemost. 2020, 18, 411–421. [Google Scholar] [CrossRef]
- Young, A.M.; Marshall, A.; Thirlwall, J.; Chapman, O.; Lokare, A.; Hill, C.; Hale, D.; Dunn, J.A.; Lyman, G.H.; Hutchinson, C.; et al. Comparison of an Oral Factor Xa Inhibitor With Low Molecular Weight Heparin in Patients With Cancer With Venous Thromboembolism: Results of a Randomized Trial (SELECT-D). J. Clin. Oncol. 2018, 36, 2017–2023. [Google Scholar] [CrossRef]
- Planquette, B.; Bertoletti, L.; Charles-Nelson, A.; Laporte, S.; Grange, C.; Mahé, I.; Pernod, G.; Elias, A.; Couturaud, F.; Falvo, N.; et al. Rivaroxaban vs Dalteparin in Cancer-Associated Thromboembolism. Chest J. 2022, 161, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, G.; Becattini, C.; Meyer, G.; Muñoz, A.; Huisman, M.V.; Connors, J.M.; Cohen, A.; Bauersachs, R.; Brenner, B.; Torbicki, A.; et al. Apixaban for the Treatment of Venous Thromboembolism Associated with Cancer. N. Engl. J. Med. 2020, 382, 1599–1607. [Google Scholar] [CrossRef]
- Schrag, D.; Uno, H.; Pam Greenerger Rosovsky, R.; Rutherford, C.; Marie Sanfilippo, K.; Villano, J.L.; Drescher, M.R.; Jayaram, N.H.; Holmes, C.E.; Eric Feldman, L.; et al. 2020 Poster Discussion Session The Comparative Effectiveness of Direct Oral Anti-Coagulants and Low Molecular Weight Heparins for Prevention of Recurrent Venous Thromboembolism in Cancer: The CANVAS Pragmatic Randomized Trial. J. Clin. Oncol. 2021, 39, 12020. [Google Scholar] [CrossRef]
- O’Connell, C.; Escalante, C.P.; Goldhaber, S.Z.; McBane, R.; Connors, J.M.; Raskob, G.E. Treatment of Cancer-Associated Venous Thromboembolism with Low-Molecular-Weight Heparin or Direct Oral Anticoagulants: Patient Selection, Controversies, and Caveats. Oncologist 2021, 26, e8–e16. [Google Scholar] [CrossRef]
- Gibb, S.; Engelhardt, S.; von Dincklage, F.; Kuhn, S.O. Incidence and Onset of Central Venous Catheter-Related Thrombosis in Critically Ill Surgical Patients: A Prospective Observational Single-Center Study. J. Clin. Anesth. 2024, 97, 111556. [Google Scholar] [CrossRef]
- Xisomab 3G3 for the Prevention of Catheter-Associated Thrombosis in Patients With Cancer Receiving Chemotherapy. 2024. Available online: https://clinicaltrials.gov/study/NCT04465760 (accessed on 4 September 2025).
- Donadini, M.P.; Calcaterra, F.; Romualdi, E.; Ciceri, R.; Cancellara, A.; Lodigiani, C.; Bacci, M.; Della Bella, S.; Ageno, W.; Mavilio, D. The Link Between Venous and Arterial Thrombosis: Is There a Role for Endothelial Dysfunction? Cells 2025, 14, 144. [Google Scholar] [CrossRef] [PubMed]
- Blann, A.D.; Dunmore, S. Arterial and Venous Thrombosis in Cancer Patients. Cardiol. Res. Pract. 2011, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mauriello, A.; Correra, A.; Molinari, R.; Del Vecchio, G.E.; Tessitore, V.; D’Andrea, A.; Russo, V. Mitochondrial Dysfunction in Atrial Fibrillation: The Need for a Strong Pharmacological Approach. Biomedicines 2024, 12, 2720. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330. [Google Scholar] [CrossRef]
- Al Said, S.; Patel, S.; Giugliano, R.; Morrow, D.; Goodrich, E.; Murphy, S.; Hug, B.; Parker, S.; Chen, S.A.; Goodman, S.; et al. Bleeding with the FXI Inhibitor Abelacimab Compared with Rivaroxaban in Patients on Antiplatelet Therapy: A Prespecified Analysis of the AZALEA-TIMI 71 Trial. Circulation 2024, 150, 1. [Google Scholar] [CrossRef]
- Mauriello, A.; Ascrizzi, A.; Roma, A.S.; Molinari, R.; Caturano, A.; Imbalzano, E.; D’Andrea, A.; Russo, V. Effects of Heart Failure Therapies on Atrial Fibrillation: Biological and Clinical Perspectives. Antioxidants 2024, 13, 806. [Google Scholar] [CrossRef] [PubMed]
- Kavousi, M. Differences in Epidemiology and Risk Factors for Atrial Fibrillation Between Women and Men. Front. Cardiovasc. Med. 2020, 7, 3. [Google Scholar] [CrossRef]
- Mauriello, A.; Correra, A.; Maratea, A.C.; Caturano, A.; Liccardo, B.; Perrone, M.A.; Giordano, A.; Nigro, G.; D’Andrea, A.; Russo, V. Serum Lipids, Inflammation, and the Risk of Atrial Fibrillation: Pathophysiological Links and Clinical Evidence. J. Clin. Med. 2025, 14, 1652. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.H.; Lew, L.Z.W.; Franke, K.B.; Elliott, A.D.; Lau, D.H.; Thiyagarajah, A.; Linz, D.; Arstall, M.; Tully, P.J.; Baune, B.T.; et al. Predictive Role of Atrial Fibrillation in Cognitive Decline: A Systematic Review and Meta-Analysis of 2.8 Million Individuals. Europace 2022, 24, 1229–1239. [Google Scholar] [CrossRef]
- Gunasekaran, K.; Rajasurya, V.; Devasahayam, J.; Rahi, M.S.; Chandran, A.; Elango, K.; Talari, G. A Review of the Incidence Diagnosis and Treatment of Spontaneous Hemorrhage in Patients Treated with Direct Oral Anticoagulants. J. Clin. Med. 2020, 9, 2984. [Google Scholar] [CrossRef]
- Mauriello, A.; Correra, A.; Ascrizzi, A.; Del Vecchio, G.E.; Benfari, G.; Ilardi, F.; Lisi, M.; Malagoli, A.; Mandoli, G.E.; Pastore, M.C.; et al. Relationship Between Left Atrial Strain and Atrial Fibrillation: The Role of Stress Echocardiography. Diagnostics 2024, 15, 7. [Google Scholar] [CrossRef]
- Shoamanesh, A.; Mundl, H.; Smith, P.E.E.; Masjuan, P.J.; Milanov, P.I.; Hirano, T.; Agafina, P.A.; Campbell, P.B.; Caso, P.V.; Mas, P.J.-L.; et al. Factor XIa Inhibition with Asundexian after Acute Non-Cardioembolic Ischaemic Stroke (PACIFIC-Stroke): An International, Randomised, Double-Blind, Placebo-Controlled, Phase 2b Trial. Lancet 2022, 400, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Study to EvaLuate the EffIcacy and Safety of AbeLacimab in High-Risk Patients With Atrial Fibrillation Who Have Been Deemed Unsuitable for Oral AntiCoagulation (LILAC-TIMI 76) (LILAC-TIMI 76). Available online: https://clinicaltrials.gov/study/NCT05712200 (accessed on 4 September 2025).
- Patel, S.M.; Giugliano, R.P.; Morrow, D.A.; Parkar, S.; Shapiro, H.; Hug, B.; Kuder, J.F.; Goodrich, E.L.; Chen, S.A.; Goodman, S.G. Long-Acting Factor XI Inhibition and Periprocedural Bleeding: A Secondary Analysis from AZALEA-TIMI 71. J. Am. Coll. Cardiol. 2024, 85, 2288–2298. [Google Scholar] [CrossRef] [PubMed]

| Trial | Population | Coagulation Factor | Inhibitor | Endpoints | Results |
|---|---|---|---|---|---|
| Preclinical [42] | Mouse model | Thrombin | Hirudin | Interaction of tumor cells with platelets and fibrinogen in isolated lung preparations | Tumor cell spreading and subsequent retention of the tumor cells in the lung were markedly inhibited in the anticoagulated mice |
| Preclinical [43] | Human cell lines | Tissue Factor | Anti-Tissue Factor monoclonal antibodies | The formation of platelet/fibrin/tumor cell aggregates may be causally related to endothelial adhesion and metastatic potential | Abolished prolonged adherence of metastatic cells in the vasculature and inhibited metastasis |
| Preclinical [44] | Human cell lines | Thrombin | R-hirudin and thrombin inhibitor peptides | The formation of platelet/fibrin/tumor cell aggregates may be causally related to endothelial adhesion and metastatic potential | To inhibit tumor progression, spread, and spontaneous metastasis |
| Clinical [45,46] | Factor XI | NA | In a prospective cohort of patients with NSCLC starting chemotherapy, contact system activation and thrombin generation biomarkers were assessed in relation to 6-month VTE occurrence and mortality | The 6-month VTE and mortality cumulative incidences were 11% and 27%, respectively. Basal levels of Factor XI activated: Antithrombin complexes were higher in patients who developed VTE than those in VTE-free patients |
| Molecule | Mechanism of Action | Phase of Clinical Trial |
|---|---|---|
| Asundexian | bind to the active site of FXIa | OCEANIC-AF (NCT05643573) phase 3 [47] |
| Milvexian | bind to the active site of FXIa | LIBREXIA-AF trial (NCT05757869) phase 3 [48] |
| Xisomab | antibodies directed against FXI and FXII | (NCT04465760) Phase 2 [49] |
| Abelacimab | antibodies directed against FXI | AZALEA-TIMI 71 (NCT04755283) phase 2b [50] ASTER (NCT05171049) phase 3 [51] MAGNOLIA (NCT05171075) phase 3 [52] |
| Osocimab | antibodies directed against FXI | (NCT04523220) Phase 2b [53] |
| Fesomersen | antisense oligonucleotides | Phase 2b RE-THINC ESRD (NCT04534114) [54] |
| Trial | Agent | Phase of Clinical Study | Population (N) | Endpoints | Results |
|---|---|---|---|---|---|
| Active Comparator-Controlled Study to Assess Safety and Efficacy of ISIS-FXIRx in Total Knee Arthroplasty (FXI-ASO TKA) (NCT01713361) [56] | IONIS-FXIRx | Phase II | 300 patients | The primary efficacy outcome was the incidence of venous thromboembolism (assessed by mandatory bilateral venography or report of symptomatic events). | 300 mg of IONIS-FXIRx significantly reduced VTE incidence (4%) compared to enoxaparin (30%) (p < 0.001), with similar bleeding rates (3% vs. 8%) (p < 0.001) |
| ANT-005 Total Knee Arthroplasty (TKA) trial [58] | Abelacimab | Phase II | 412 patients | The primary efficacy outcome was venous thromboembolism, detected by mandatory venography of the leg involved in the operation or objective confirmation of symptomatic events. | Both 75 mg (p < 0.001) and 150 mg (p < 0.001) of abelacimab were more effective than enoxaparin in preventing VTE, without increasing bleeding risk; risk difference (95% CI) 1.9 (−0.7 to 4.5) and 0 for abelacimab 75 mg group and abelacimab 150 mg group, respectively |
| Antithrombotic treatment with Factor XIa inhibition to Optimize Management of Acute Thromboembolic events for Secondary Stroke Prevention (AXIOMATIC-TKA) trial (NCT03891524) [57] | Milvexian | Phase II | 1242 patients | The primary efficacy outcome was venous thromboembolism (which was a composite of asymptomatic deep-vein thrombosis, confirmed symptomatic venous thromboembolism, or death from any cause) | Milvexian at a dose of at least 100 mg (administered once or twice daily) outperformed enoxaparin in VTE prevention (relative risk vs. enoxaparin 0.52; 0.42, 0.37, and 0.30), without a notable rise in bleeding risk (relative risks were 1.15, 1.14, 0.81, and 1.51) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauriello, A.; Maratea, A.C.; Fonderico, C.; Quagliariello, V.; Maurea, F.; Maurea, N. Factor XI and Cancer: Physiopathological Linkage and Clinical Perspectives. J. Clin. Med. 2025, 14, 6341. https://doi.org/10.3390/jcm14176341
Mauriello A, Maratea AC, Fonderico C, Quagliariello V, Maurea F, Maurea N. Factor XI and Cancer: Physiopathological Linkage and Clinical Perspectives. Journal of Clinical Medicine. 2025; 14(17):6341. https://doi.org/10.3390/jcm14176341
Chicago/Turabian StyleMauriello, Alfredo, Anna Chiara Maratea, Celeste Fonderico, Vincenzo Quagliariello, Fabrizio Maurea, and Nicola Maurea. 2025. "Factor XI and Cancer: Physiopathological Linkage and Clinical Perspectives" Journal of Clinical Medicine 14, no. 17: 6341. https://doi.org/10.3390/jcm14176341
APA StyleMauriello, A., Maratea, A. C., Fonderico, C., Quagliariello, V., Maurea, F., & Maurea, N. (2025). Factor XI and Cancer: Physiopathological Linkage and Clinical Perspectives. Journal of Clinical Medicine, 14(17), 6341. https://doi.org/10.3390/jcm14176341








