The Levonorgestrel Intrauterine System Attenuates the Expression of Angiopoietin-1, Angiopoietin-2, and Vascular Endothelial Growth Factor in Adenomyosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Immunohistochemistry (IHC)
2.3. Assessment of Staining (H-Score)
2.4. Quantitative Reverse-Transcription PCR (qRT-PCR)
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
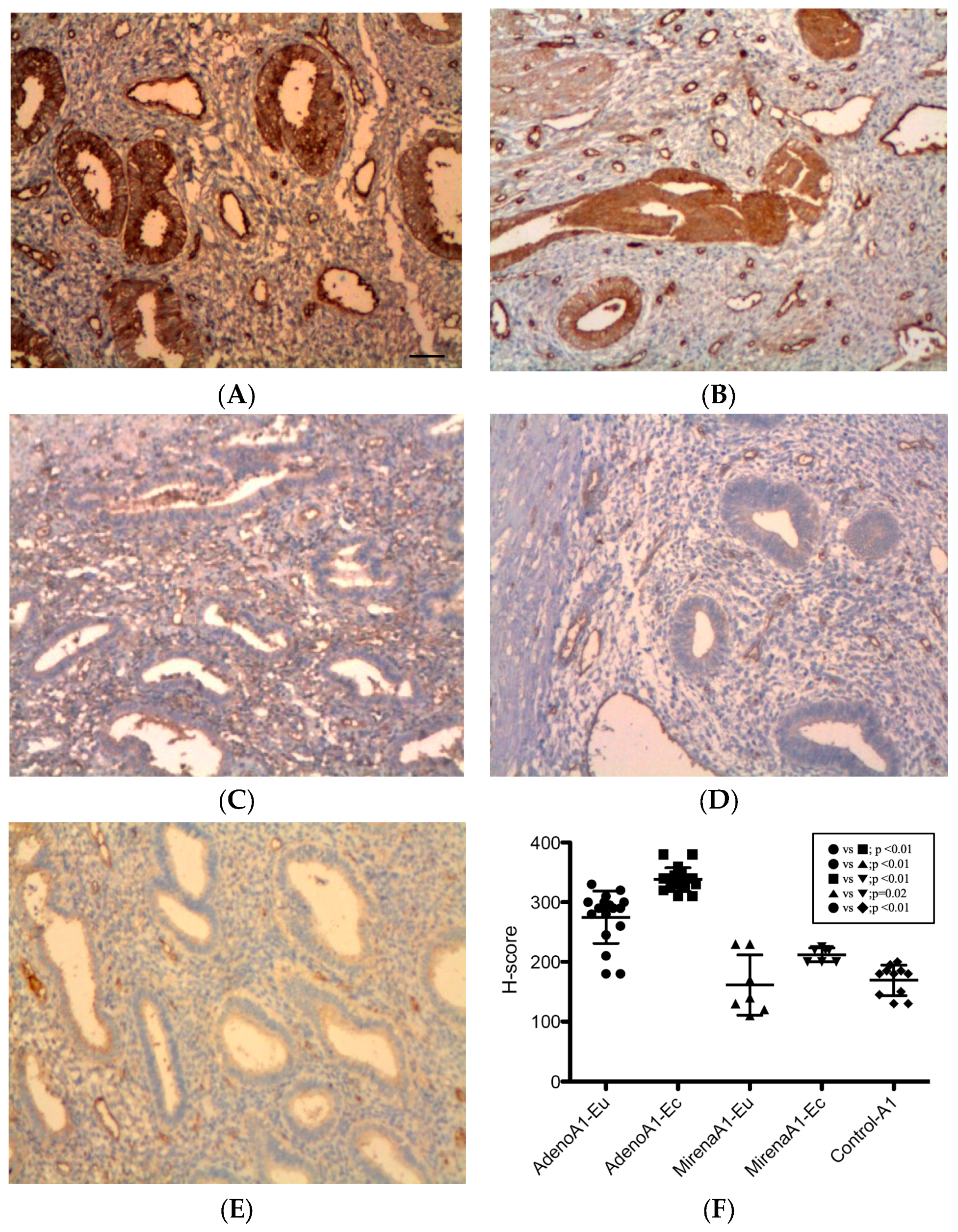
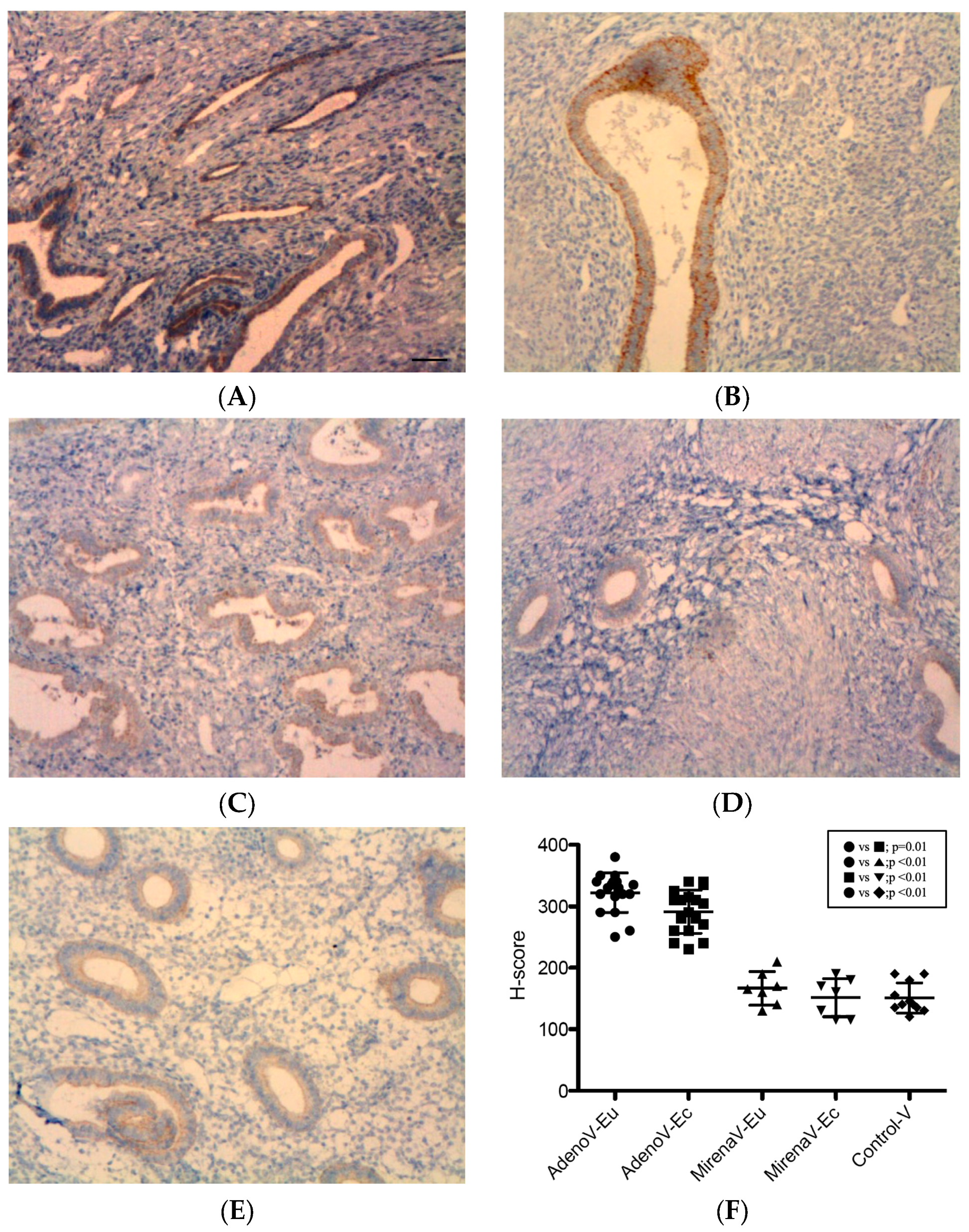
3.2. Immunohistochemical Expression of Angiogenic Factors
3.2.1. ANGPT-1
3.2.2. ANGPT-2
3.2.3. VEGF
3.3. mRNA Expression of ANGPT-1, ANGPT-2, and VEGFA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANGPT | Angiopoietin |
| ANGPT-1 | Angiopoietin-1 |
| ANGPT-2 | Angiopoietin-2 |
| BMI | Body mass index |
| CA-125 | Cancer antigen 125 |
| Ec | Ectopic endometrium |
| Eu | Eutopic endometrium |
| H-score | Histochemical immunoreactivity score |
| LNG-IUS | Levonorgestrel intrauterine system |
| VEGF | Vascular endothelial growth factor |
| TIE-2 | Tyrosine kinase with immunoglobulin-like and EGF-like domains 2 receptor |
References
- Upson, K.; Missmer, S.A. Epidemiology of Adenomyosis. Semin. Reprod. Med. 2020, 38, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Yu, O.; Schulze-Rath, R.; Grafton, J.; Hansen, K.; Scholes, D.; Reed, S.D. Adenomyosis incidence, prevalence and treatment: United States population-based study 2006–2015. Am. J. Obstet. Gynecol. 2020, 223, 94.e1–94.e10. [Google Scholar] [CrossRef] [PubMed]
- Leyendecker, G.; Herbertz, M.; Kunz, G.; Mall, G. Endometriosis results from the dislocation of basal endometrium. Hum. Reprod. 2002, 17, 2725–2736. [Google Scholar] [CrossRef] [PubMed]
- Kelekci, S.; Kelekci, K.H.; Yilmaz, B. Effects of levonorgestrel-releasing intrauterine system and T380A intrauterine copper device on dysmenorrhea and days of bleeding in women with and without adenomyosis. Contraception 2012, 86, 458–463. [Google Scholar] [CrossRef]
- Sheng, J.; Zhang, W.Y.; Zhang, J.P.; Lu, D. The LNG-IUS study on adenomyosis: A 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception 2009, 79, 189–193. [Google Scholar] [CrossRef]
- Bragheto, A.M.; Caserta, N.; Bahamondes, L.; Petta, C.A. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception 2007, 76, 195–199. [Google Scholar] [CrossRef]
- Cho, S.; Nam, A.; Kim, H.; Chay, D.; Park, K.; Cho, D.J.; Park, Y.; Lee, B. Clinical effects of the levonorgestrel-releasing intrauterine device in patients with adenomyosis. Am. J. Obstet. Gynecol. 2008, 198, 373.e1–373.e7. [Google Scholar] [CrossRef]
- Fraser, I.S. Non-contraceptive health benefits of intrauterine hormonal systems. Contraception 2010, 82, 396–403. [Google Scholar] [CrossRef]
- Heikinheimo, O.; Gemzell-Danielsson, K. Emerging indications for the levonorgestrel-releasing intrauterine system (LNG-IUS). Acta Obstet. Gynecol. Scand. 2012, 91, 3–9. [Google Scholar] [CrossRef]
- Choi, Y.S.; Cho, S.; Lim, K.J.; Jeon, Y.E.; Yang, H.I.; Lee, K.E.; Heena, K.; Seo, S.K.; Kim, H.Y.; Lee, B.S. Effects of LNG-IUS on nerve growth factor and its receptors expression in patients with adenomyosis. Growth Factors 2010, 28, 452–460. [Google Scholar] [CrossRef]
- Harmsen, M.J.; Wong, C.F.C.; Mijatovic, V.; Griffioen, A.W.; Groenman, F.; Hehenkamp, W.J.K.; Huirne, J.A.F. Role of angiogenesis in adenomyosis-associated abnormal uterine bleeding and subfertility: A systematic review. Hum. Reprod. Update. 2019, 25, 647–671. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.D.; Lebovic, D.I.; Garrett, E.; Taylor, R.N. Neutrophils infiltrating the endometrium express vascular endothelial growth factor: Potential role in endometrial angiogenesis. Fertil. Steril. 2000, 74, 107–112. [Google Scholar] [CrossRef]
- Ota, H.; Igarashi, S.; Tanaka, T. Morphometric evaluation of stromal vascularization in the endometrium in adenomyosis. Hum. Reprod. 1998, 13, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Plank, M.J.; Sleeman, B.D.; Jones, P.F. The role of the angiopoietins in tumour angiogenesis. Growth Factors 2004, 22, 1–11. [Google Scholar] [CrossRef]
- Hur, S.E.; Lee, J.Y.; Moon, H.S.; Chung, H.W. Angiopoietin-1, angiopoietin-2 and Tie-2 expression in eutopic endometrium in advanced endometriosis. Mol. Hum. Reprod. 2006, 12, 421–426. [Google Scholar] [CrossRef]
- Hwang, S.M.; Lee, C.W.; Lee, B.S.; Park, J.H. Clinical features of thoracic endometriosis: A single center analysis. Obstet. Gynecol. Sci. 2015, 58, 223–231. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Z.; Liu, H.; Niu, W.; Wang, X.; Liang, N.; Wang, X.; Wang, Y.; Shi, Y.; Xu, L.; et al. Single-cell transcriptomic analysis of eutopic endometrium and ectopic lesions of adenomyosis. Cell Biosci. 2021, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, C.; Li, W.; Xin, W.; Qin, T. Research Advances in Adenomyosis-Related Signaling Pathways and Promising Targets. Biomolecules 2024, 14, 1402. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, C.; Bonifacio, M.; Tommaselli, G.A.; Bifulco, G.; Guerra, G.; Nappi, C. Metalloproteinases, vascular endothelial growth factor, and angiopoietin 1 and 2 in eutopic and ectopic endometrium. Fertil. Steril. 2009, 91, 2315–2323. [Google Scholar] [CrossRef] [PubMed]
- Shifren, J.L.; Tseng, J.F.; Zaloudek, C.J.; Ryan, I.P.; Meng, Y.G.; Ferrara, N.; Jaffe, R.B.; Taylor, R.N. Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: Implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J. Clin. Endocrinol. Metab. 1996, 81, 3112–3118. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.D.; Shifren, J.L.; Foulk, R.A.; Taylor, R.N.; Jaffe, R.B. Angiogenesis in the human female reproductive tract. Obstet. Gynecol. Surv. 1995, 50, 688–697. [Google Scholar] [CrossRef]
- Harfouche, R.; Hassessian, H.M.; Guo, Y.; Faivre, V.; Srikant, C.B.; Yancopoulos, G.D.; Hussain, S.N. Mechanisms which mediate the antiapoptotic effects of angiopoietin-1 on endothelial cells. Microvasc. Res. 2002, 64, 135–147. [Google Scholar] [CrossRef]
- Suri, C.; Jones, P.F.; Patan, S.; Bartunkova, S.; Maisonpierre, P.C.; Davis, S.; Sato, T.N.; Yancopoulos, G.D. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 1996, 87, 1171–1180. [Google Scholar] [CrossRef]
- Zagzag, D.; Hooper, A.; Friedlander, D.R.; Chan, W.; Holash, J.; Wiegand, S.J.; Yancopoulos, G.D.; Grumet, M. In situ expression of angiopoietins in astrocytomas identifies angiopoietin-2 as an early marker of tumor angiogenesis. Exp. Neurol. 1999, 159, 391–400. [Google Scholar] [CrossRef]
- Maisonpierre, P.C.; Suri, C.; Jones, P.F.; Bartunkova, S.; Wiegand, S.J.; Radziejewski, C.; Compton, D.; McClain, J.; Aldrich, T.H.; Papadopoulos, N.; et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997, 277, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, M.J.; Juffermans, L.J.M.; Kroon, M.O.; Griffioen, A.W.; Huirne, J.A.F. Anti-angiogenic therapy as potential treatment for adenomyosis. Angiogenesis 2025, 28, 12. [Google Scholar] [CrossRef] [PubMed]
- Ramey, J.W.; Archer, D.F. Peritoneal fluid: Its relevance to the development of endometriosis. Fertil. Steril. 1993, 60, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Conklin, B.S.; Zhao, W.; Zhong, D.S.; Chen, C. Nicotine and cotinine up-regulate vascular endothelial growth factor expression in endothelial cells. Am. J. Pathol. 2002, 160, 413–418. [Google Scholar] [CrossRef]
- Middelkoop, M.A.; Don, E.E.; Hehenkamp, W.J.K.; Polman, N.J.; Griffioen, A.W.; Huirne, J.A.F. Angiogenesis in abnormal uterine bleeding: A narrative review. Hum. Reprod. Update. 2023, 29, 457–485. [Google Scholar] [CrossRef]
- Graubert, M.D.; Ortega, M.A.; Kessel, B.; Mortola, J.F.; Iruela-Arispe, M.L. Vascular repair after menstruation involves regulation of vascular endothelial growth factor-receptor phosphorylation by sFLT-1. Am. J. Pathol. 2001, 158, 1399–1410. [Google Scholar] [CrossRef]
- Herve, M.A.; Meduri, G.; Petit, F.G.; Domet, T.S.; Lazennec, G.; Mourah, S.; Perrot-Applanat, M. Regulation of the vascular endothelial growth factor (VEGF) receptor Flk-1/KDR by estradiol through VEGF in uterus. J. Endocrinol. 2006, 188, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Bourlev, V.; Volkov, N.; Pavlovitch, S.; Lets, N.; Larsson, A.; Olovsson, M. The relationship between microvessel density, proliferative activity and expression of vascular endothelial growth factor-A and its receptors in eutopic endometrium and endometriotic lesions. Reproduction 2006, 132, 501–509. [Google Scholar] [CrossRef]
- Garrido, N.; Albert, C.; Krussel, J.S.; O’Connor, J.E.; Remohi, J.; Simon, C.; Pellicer, A. Expression, production, and secretion of vascular endothelial growth factor and interleukin-6 by granulosa cells is comparable in women with and without endometriosis. Fertil. Steril. 2001, 76, 568–575. [Google Scholar] [CrossRef]
- Etoh, T.; Inoue, H.; Tanaka, S.; Barnard, G.F.; Kitano, S.; Mori, M. Angiopoietin-2 is related to tumor angiogenesis in gastric carcinoma: Possible in vivo regulation via induction of proteases. Cancer Res. 2001, 61, 2145–2153. [Google Scholar]
- Hess, A.P.; Hirchenhain, J.; Schanz, A.; Talbi, S.; Hamilton, A.E.; Giudice, L.C.; Krussel, J.S. Angiopoietin-1 and -2 mRNA and protein expression in mouse preimplantation embryos and uteri suggests a role in angiogenesis during implantation. Reprod. Fertil. Dev. 2006, 18, 509–516. [Google Scholar] [CrossRef]
- Fedele, L.; Bianchi, S.; Raffaelli, R.; Portuese, A.; Dorta, M. Treatment of adenomyosis-associated menorrhagia with a levonorgestrel-releasing intrauterine device. Fertil. Steril. 1997, 68, 426–429. [Google Scholar] [CrossRef]
- Charnock-Jones, D.S.; Macpherson, A.M.; Archer, D.F.; Leslie, S.; Makkink, W.K.; Sharkey, A.M.; Smith, S.K. The effect of progestins on vascular endothelial growth factor, oestrogen receptor and progesterone receptor immunoreactivity and endothelial cell density in human endometrium. Hum. Reprod. 2000, 15 (Suppl. S3), 85–95. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Heavy Menstrual Bleeding: Assessment and Management. Available online: https://www.nice.org.uk/guidance/ng88 (accessed on 20 November 2025).
- Salmasi, S.; Sharifi, M.; Rashidi, B. Ovarian stimulation and exogenous progesterone affect the endometrial miR-16-5p, VEGF protein expression, and angiogenesis. Microvasc. Res. 2021, 133, 104074. [Google Scholar] [CrossRef]
- Arlier, S.; Kayisli, U.A.; Semerci, N.; Ozmen, A.; Larsen, K.; Schatz, F.; Lockwood, C.J.; Guzeloglu-Kayisli, O. Enhanced ZBTB16 Levels by Progestin-Only Contraceptives Induces Decidualization and Inflammation. Int. J. Mol. Sci. 2023, 24, 10532. [Google Scholar] [CrossRef] [PubMed]
- Ozer, H.; Boztosun, A.; Acmaz, G.; Atilgan, R.; Akkar, O.B.; Kosar, M.I. The efficacy of bevacizumab, sorafenib, and retinoic acid on rat endometriosis model. Reprod. Sci. 2013, 20, 26–32. [Google Scholar] [CrossRef] [PubMed]


| Gene | Direction | Primer Sequence (5′-3′) | Accession No. | Amplicon (bp) |
|---|---|---|---|---|
| GAPDH | Forward | GTCTCCTCTGACTTCAACAGCG | NM_002046.7 | 131 |
| Reverse | ACCACCCTGTTGCTGTAGCCAA | |||
| ANGPT1 | Forward | CAACAGTGTCCTTCAGAAGCAGC | NM_001199859.3 | 150 |
| Reverse | CCAGCTTGATATACATCTGCACAG | |||
| ANGPT2 | Forward | ATTCAGCGACGTGAGGATGGCA | NM_001118887.2 | 139 |
| Reverse | GCACATAGCGTTGCTGATTAGTC | |||
| VEGFA | Forward | TTGCCTTGCTGCTCTACCTCCA | NM_001025366.3 | 126 |
| Reverse | GATGGCACTAGCTGCGCTGATA |
| Characteristic | Adenomyosis | Adenomyosis-LNG-IUS | Control Endometrium | p-Value |
|---|---|---|---|---|
| N | 20 | 18 | 12 | NA |
| Age (years) | 45.33 ± 3.35 | 43.40 ± 4.62 | 41.73 ± 7.81 | 0.25 |
| BMI (kg/m2) | 23.11 ± 2.32 | 23.24 ± 2.81 | 21.99 ± 2.89 | 0.47 |
| Parity | 2.07 ± 1.10 | 1.60 ± 0.70 | 1.64 ± 0.81 | 0.36 |
| Duration of LNG-IUS insertion (months) | NA | 5.43 ± 2.5 | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.; Kim, H.K.; Choi, Y.S.; Park, J.H. The Levonorgestrel Intrauterine System Attenuates the Expression of Angiopoietin-1, Angiopoietin-2, and Vascular Endothelial Growth Factor in Adenomyosis. J. Clin. Med. 2025, 14, 8629. https://doi.org/10.3390/jcm14248629
Cho S, Kim HK, Choi YS, Park JH. The Levonorgestrel Intrauterine System Attenuates the Expression of Angiopoietin-1, Angiopoietin-2, and Vascular Endothelial Growth Factor in Adenomyosis. Journal of Clinical Medicine. 2025; 14(24):8629. https://doi.org/10.3390/jcm14248629
Chicago/Turabian StyleCho, SiHyun, Hyun Kyung Kim, Young Sik Choi, and Joo Hyun Park. 2025. "The Levonorgestrel Intrauterine System Attenuates the Expression of Angiopoietin-1, Angiopoietin-2, and Vascular Endothelial Growth Factor in Adenomyosis" Journal of Clinical Medicine 14, no. 24: 8629. https://doi.org/10.3390/jcm14248629
APA StyleCho, S., Kim, H. K., Choi, Y. S., & Park, J. H. (2025). The Levonorgestrel Intrauterine System Attenuates the Expression of Angiopoietin-1, Angiopoietin-2, and Vascular Endothelial Growth Factor in Adenomyosis. Journal of Clinical Medicine, 14(24), 8629. https://doi.org/10.3390/jcm14248629






