Biliary Reconstruction in Liver Transplantation with Primary Sclerosing Cholangitis: Roux-en-Y Hepaticojejunostomy or Duct-to-Duct Anastomosis?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Surgery and Immunosuppression
2.3. Study Variables and Outcome Parameters
2.4. Statistics
3. Results
3.1. Study Population
3.2. Postoperative Complications
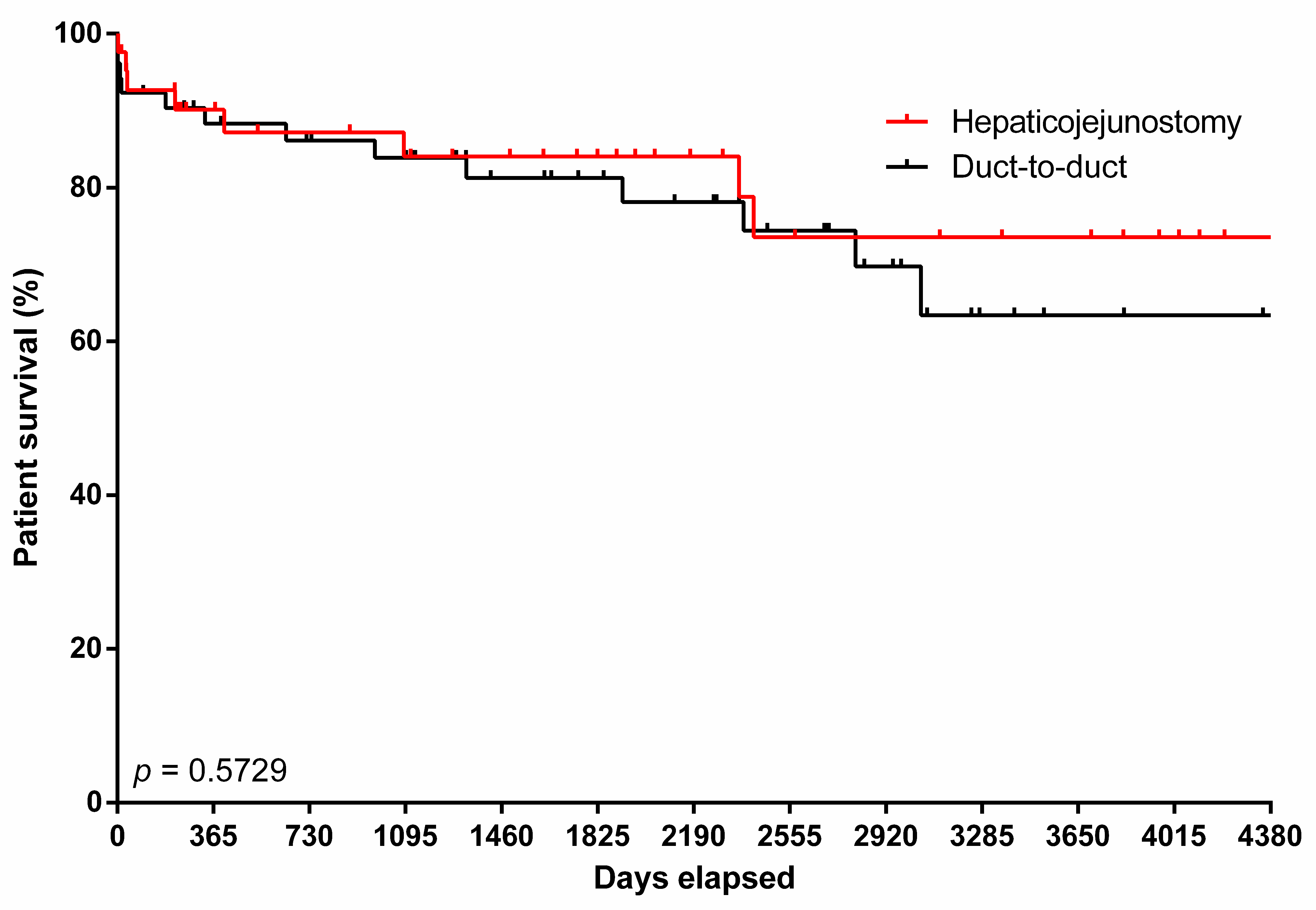
3.3. Predictors of Mortality
3.4. Risk Factors of Postoperative Complications
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCI | Comprehensive Complication Index |
| CI | Confidence Interval |
| DD | Duct-to-Duct Reconstruction |
| ET | Eurotransplant |
| HJ | Roux-en-Y Hepaticojejunostomy |
| HR | Hazards Ratio |
| LT | Liver Transplantation |
| MELD | Model of End-Stage Liver Disease |
| OR | Odds Ratio |
| PSC | Primary Sclerosing Cholangitis |
| WIT | Warm Ischemia Time |
References
- Manns, M.P.; Bergquist, A.; Karlsen, T.H.; Levy, C.; Muir, A.J.; Ponsioen, C.; Trauner, M.; Wong, G.; Younossi, Z.M. Primary sclerosing cholangitis. Nat. Rev. Dis. Primers 2025, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, V.; Neumann, U.P.; Puhl, G.; Tran, Z.V.; Neuhaus, P.; Langrehr, J.M. Surgical complications and long-term outcome of different biliary reconstructions in liver transplantation for primary sclerosing cholangitis-choledochoduodenostomy versus choledochojejunostomy. Am. J. Transplant. 2006, 6, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Welsh, F.K.; Wigmore, S.J. Roux-en-Y Choledochojejunostomy is the method of choice for biliary reconstruction in liver transplantation for primary sclerosing cholangitis. Transplantation 2004, 77, 602–604. [Google Scholar] [CrossRef] [PubMed]
- Esfeh, J.M.; Eghtesad, B.; Hodgkinson, P.; Diago, T.; Fujiki, M.; Hashimoto, K.; Quintini, C.; Aucejo, F.; Kelly, D.; Winans, C.; et al. Duct-to-duct biliary reconstruction in patients with primary sclerosing cholangitis undergoing liver transplantation. HPB 2011, 13, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Al-Judaibi, B.; Hernandez Alejandro, R.; Uhanova, J.; Marotta, P.; Mosli, M.; Chandok, N. Duct-to-Duct Biliary Anastomosis Yields Similar Outcomes to Roux-en-Y Hepaticojejunostomy in Liver Transplantation for Primary Sclerosing Cholangitis. Hepat. Mon. 2015, 15, e18811. [Google Scholar] [CrossRef] [PubMed]
- Alwis, S.M.; Fink, M.A.; Furtado, R.; Lee, E.; Starkey, G.; Jones, R.; Perini, M.V. Untangling biliary reconstruction in liver transplants for primary sclerosing cholangitis. World J. Surg. 2024, 48, 2253–2261. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.E.; Bense, R.D.; Lisman, T.; van der Jagt, E.J.; van den Berg, A.P.; Porte, R.J. Duct-to-duct reconstruction in liver transplantation for primary sclerosing cholangitis is associated with fewer biliary complications in comparison with hepaticojejunostomy. Liver Transpl. 2014, 20, 457–463. [Google Scholar] [CrossRef]
- Shamsaeefar, A.; Shafiee, M.; Nikeghbalian, S.; Kazemi, K.; Mansorian, M.; Motazedian, N.; Afshinnia, F.; Geramizadeh, B.; Malekhosseini, S.A. Biliary reconstruction in liver transplant patients with primary sclerosing cholangitis, duct-to-duct or Roux-en-Y? Clin. Transplant. 2017, 31, e12964. [Google Scholar] [CrossRef] [PubMed]
- Pandanaboyana, S.; Bell, R.; Bartlett, A.J.; McCall, J.; Hidalgo, E. Meta-analysis of Duct-to-duct versus Roux-en-Y biliary reconstruction following liver transplantation for primary sclerosing cholangitis. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2015, 28, 485–491. [Google Scholar] [CrossRef]
- Saner, F.H.; Frey, A.; Stüben, B.O.; Hoyer, D.P.; Willuweit, K.; Daniel, M.; Rashidi-Alavieh, J.; Treckmann, J.W.; Schmidt, H.H. Transplantation for Primary Sclerosing Cholangitis: Outcomes and Recurrence. J. Clin. Med. 2023, 12, 3405. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, H.; Brockmann, J.G.; Voigt, R.; Rauchfuss, F.; Pascher, A.; Brose, S.; Binner, C.; Bittner, H.; Klar, E. DTG procurement guidelines in heart beating donors. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2011, 24, 733–757. [Google Scholar] [CrossRef]
- Slankamenac, K.; Graf, R.; Barkun, J.; Puhan, M.A.; Clavien, P.A. The comprehensive complication index: A novel continuous scale to measure surgical morbidity. Ann. Surg. 2013, 258, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.M.; Croome, K.P.; Boyce, E.; Chandok, N. Roux-en-Y choledochojejunostomy versus duct-to-duct biliary anastomosis in liver transplantation for primary sclerosing cholangitis: A meta-analysis. Transplant. Proc. 2013, 45, 2263–2271. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Mueller, M.; Muller, X.; Eden, J.; Panconesi, R.; von Felten, S.; Steigmiller, K.; Sousa Da Silva, R.X.; de Rougemont, O.; Mabrut, J.Y.; et al. A multicenter randomized-controlled trial of hypothermic oxygenated perfusion (HOPE) for human liver grafts before transplantation. J. Hepatol. 2023, 78, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Castanedo, S.; Toledo, E.; Fernández-Santiago, R.; Castillo, F.; Echeverri, J.; Rodríguez-Sanjuán, J.C. Influence of postoperative complications on long-term survival in liver transplant patients. World J. Gastrointest. Surg. 2020, 12, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Melandro, F.; Nowak, G.; Nicolini, D.; Iesari, S.; Fasolo, E.; Mennini, G.; Romano, A.; Mocchegiani, F.; Ackenine, K.; et al. The role of the comprehensive complication index for the prediction of survival after liver transplantation. Updates Surg. 2021, 73, 209–221. [Google Scholar] [CrossRef] [PubMed]
- de Goeij, F.H.C.; Wehrle, C.J.; Abbassi, F.; Satish, S.; Zhang, M.; Panconesi, R.; Hashimoto, K.; Miller, C.M.; Polak, W.G.; Clavien, P.A.; et al. Mastering the narrative: Precision reporting of risk and outcomes in liver transplantation. J. Hepatol. 2025, 82, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Madadi-Sanjani, O.; Kuebler, J.F.; Brendel, J.; Wiesner, S.; Mutanen, A.; Eaton, S.; Domenghino, A.; Clavien, P.A.; Ure, B.M. Implementation and validation of a novel instrument for the grading of unexpected events in paediatric surgery: Clavien-Madadi classification. Br. J. Surg. 2023, 110, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Boeva, I.; Karagyozov, P.I.; Tishkov, I. Post-liver transplant biliary complications: Current knowledge and therapeutic advances. World J. Hepatol. 2021, 13, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Visseren, T.; Erler, N.S.; Polak, W.G.; Adam, R.; Karam, V.; Vondran, F.W.R.; Ericzon, B.G.; Thorburn, D.; JNM, I.J.; Paul, A.; et al. Recurrence of primary sclerosing cholangitis after liver transplantation—Analysing the European Liver Transplant Registry and beyond. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2021, 34, 1455–1467. [Google Scholar] [CrossRef] [PubMed]
| Entire Cohort | Hepaticojejunostomy | Duct-to-Duct Anastomosis | ||
|---|---|---|---|---|
| Characteristic | n = 94 | n = 42 | n = 52 | p Value |
| Age, y, mean (SD) | 47.8 (11.9) | 49.5 (12.0) | 46.4 (11.7) | 0.208 |
| Sex, n (%) | ||||
| Female | 18 (19.0) | 10 (23.8) | 8 (15.4) | 0.302 |
| Male | 76 (80.9) | 32 (76.2) | 44 (84.6) | |
| MELD score, mean (SD) | 14.9 (7.9) | 13.5 (7.5) | 16.0 (8.1) | 0.135 |
| MELD score, n (%) | ||||
| <15 | 54 (57.4) | 26 (61.9) | 28 (53.8) | |
| 15–20 | 22 (23.4) | 10 (23.8) | 12 (23.1) | |
| 21–25 | 9 (9.6) | 3 (7.1) | 6 (11.5) | |
| 26–30 | 2 (2.1) | 0 (0.0) | 2 (3.8) | |
| >30 | 7 (7.4) | 3 (7.1) | 4 (7.7) | |
| BMI, kg/m2, mean (SD) | 24.2 (4.2) | 24.1 (4.1) | 24.3 (4.2) | 0.657 |
| BMI, n (%) | ||||
| <18.5 | 3 (3.2) | 1 (2.4) | 2 (3.8) | |
| 18.5–24.9 | 59 (62.8) | 27 (64.3) | 32 (61.5) | |
| 25–29.9 | 25 (26.6) | 11 (26.2) | 14 (26.9) | |
| 30–34.9 | 6 (6.4) | 3 (7.1) | 3 (5.8) | |
| ≥35 | 1 (1.1) | 0 (0.0) | 1 (1.9) | |
| Transplant center, n (%) | ||||
| Aachen | 21 (22.3) | 21 (50.0) | 0 (0.0) | <0.001 |
| Essen | 73 (77.7) | 21 (50.0) | 52 (100.0) | |
| Donor Age, y, mean (SD) | 52.6 (17.6) | 51.2 (16.9) | 53.7 (18.1) | 0.500 |
| Donor Sex, n (%) | ||||
| Female | 36 (38.3) | 18 (42.9) | 18 (34.6) | 0.414 |
| Male | 58 (61.7) | 24 (57.1) | 34 (65.4) | |
| Donor BMI, kg/m2, mean (SD) | 27.2 (5.5) | 27.5 (6.3) | 26.9 (4.9) | 0.676 |
| Warm ischemia time, min (SD) | 34 (7) | 37 (7) | 32 (7) | 0.001 |
| Cold ischemia time, h (SD) | 7.7 (1.8) | 7.8 (2.0) | 7.6 (1.6) | 0.483 |
| Entire Cohort | Hepaticojejunostomy | Duct-to-Duct Anastomosis | ||
|---|---|---|---|---|
| Characteristic | n = 94 | n = 42 | n = 52 | p Value |
| CCI, mean (SD) | 52 (34) | 48 (34) | 54 (34) | 0.389 |
| CCI > 75, n (%) | 24 (25.5) | 9 (22.4) | 15 (28.8) | 0.412 |
| Anastomotic stricture, n (%) | 20 (21.3) | 4 (9.5) | 16 (30.8) | 0.012 |
| Bile leakage, n (%) | 7 (7.4) | 2 (4.8) | 5 (9.6) | 0.373 |
| Episode of cholangitis, n (%) | 6 (6.4) | 2 (4.8) | 4 (7.7) | 0.563 |
| Biliary duct ischemia, n (%) | 3 (3.2) | 1 (2.4) | 2 (3.8) | 0.688 |
| PSC recurrence, n (%) | 5 (5.3) | 2 (4.8) | 3 (5.8) | 0.829 |
| Revision surgery because of bile duct complication, n (%) | 8 (8.5) | 3 (7.1) | 5 (9.6) | 0.669 |
| Variable | HR (95% CI) | p Value |
|---|---|---|
| Biliary Reconstruction Technique | 1.116 (0.436–2.855) | 0.820 |
| Anastomotic Stricture | 1.429 (0.431–4.735) | 0.559 |
| MELD Score | 1.099 (1.045–1.155) | <0.001 |
| Warm Ischemia Time | 1.034 (0.971–1.100) | 0.299 |
| Variable | OR (95% CI) | p Value |
|---|---|---|
| MELD Score | 1.193 (1.094–1.302) | <0.001 |
| Biliary Reconstruction Technique | 1.627 (0.390–6.781) | 0.504 |
| Warm Ischemia Time | 0.966 (0.094–1.044) | 0.384 |
| Variable | OR (95% CI) | p Value |
|---|---|---|
| MELD Score | 0.932 (0.857–1.014) | 0.101 |
| Biliary Reconstruction Technique | 4.565 (1.265–16.477) | 0.020 |
| Warm Ischemia Time | 0.984 (0.920–1.054) | 0.650 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dancs, P.T.; Pohlmann, M.C.; Bednarsch, J.; Rashidi-Alavijeh, J.; Schmitz, S.M.; Kroh, A.; Ulmer, F.; Vondran, F.W.R.; Neumann, U.P.; Hoyer, D.P.; et al. Biliary Reconstruction in Liver Transplantation with Primary Sclerosing Cholangitis: Roux-en-Y Hepaticojejunostomy or Duct-to-Duct Anastomosis? J. Clin. Med. 2025, 14, 8518. https://doi.org/10.3390/jcm14238518
Dancs PT, Pohlmann MC, Bednarsch J, Rashidi-Alavijeh J, Schmitz SM, Kroh A, Ulmer F, Vondran FWR, Neumann UP, Hoyer DP, et al. Biliary Reconstruction in Liver Transplantation with Primary Sclerosing Cholangitis: Roux-en-Y Hepaticojejunostomy or Duct-to-Duct Anastomosis? Journal of Clinical Medicine. 2025; 14(23):8518. https://doi.org/10.3390/jcm14238518
Chicago/Turabian StyleDancs, Peter T., Mira C. Pohlmann, Jan Bednarsch, Jassin Rashidi-Alavijeh, Sophia M. Schmitz, Andreas Kroh, Florian Ulmer, Florian W. R. Vondran, Ulf P. Neumann, Dieter P. Hoyer, and et al. 2025. "Biliary Reconstruction in Liver Transplantation with Primary Sclerosing Cholangitis: Roux-en-Y Hepaticojejunostomy or Duct-to-Duct Anastomosis?" Journal of Clinical Medicine 14, no. 23: 8518. https://doi.org/10.3390/jcm14238518
APA StyleDancs, P. T., Pohlmann, M. C., Bednarsch, J., Rashidi-Alavijeh, J., Schmitz, S. M., Kroh, A., Ulmer, F., Vondran, F. W. R., Neumann, U. P., Hoyer, D. P., & Heise, D. (2025). Biliary Reconstruction in Liver Transplantation with Primary Sclerosing Cholangitis: Roux-en-Y Hepaticojejunostomy or Duct-to-Duct Anastomosis? Journal of Clinical Medicine, 14(23), 8518. https://doi.org/10.3390/jcm14238518







