Quantitative Analysis of the Human Face Skin Thickness—A High-Frequency Ultrasound Study
Abstract
1. Introduction
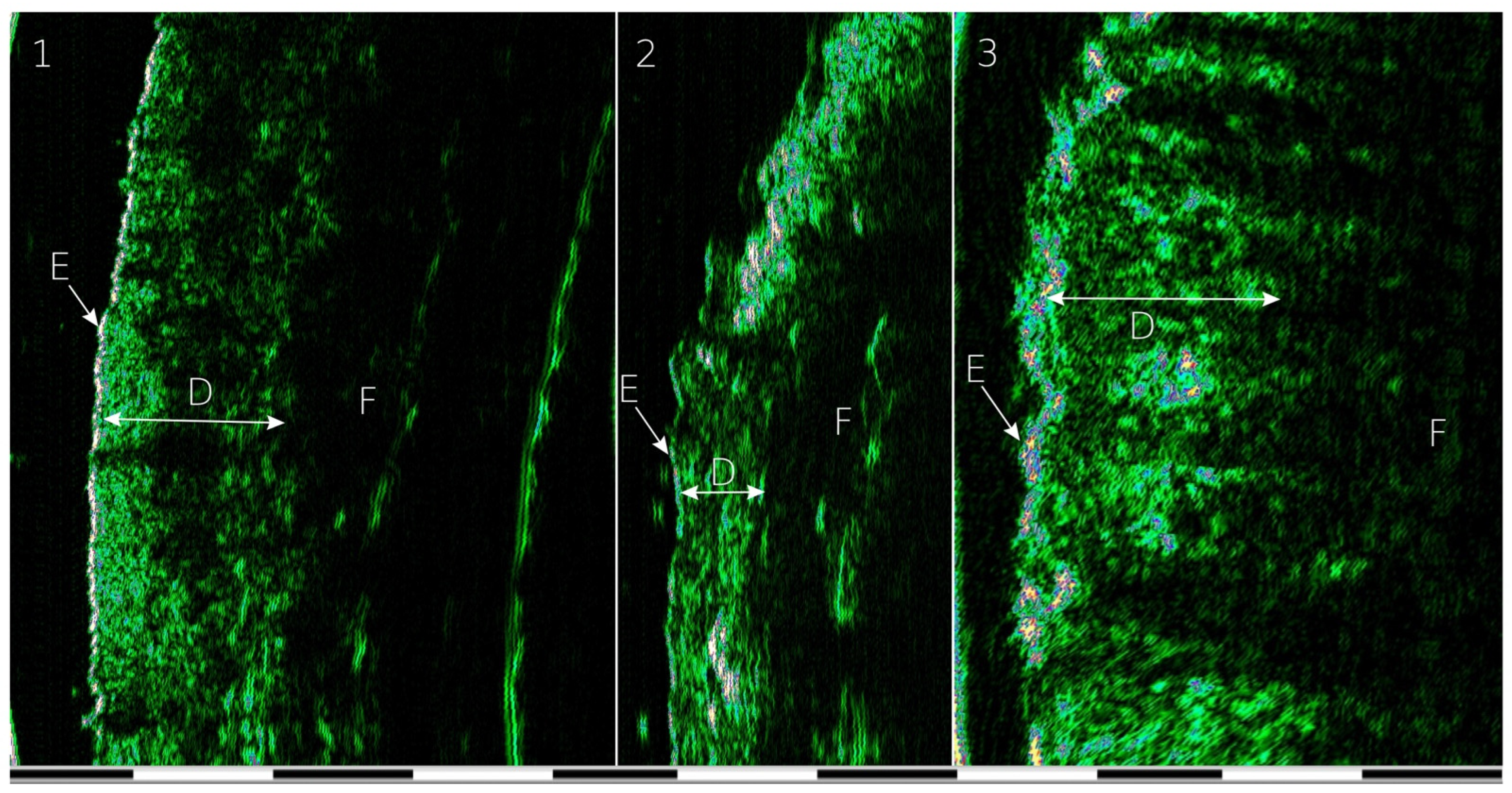
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Differences in Skin Thickness Depending on Anatomical Location

3.2. Differences in Skin Thickness by Anatomical Location
3.3. Variation in Skin Thickness by Location and Age
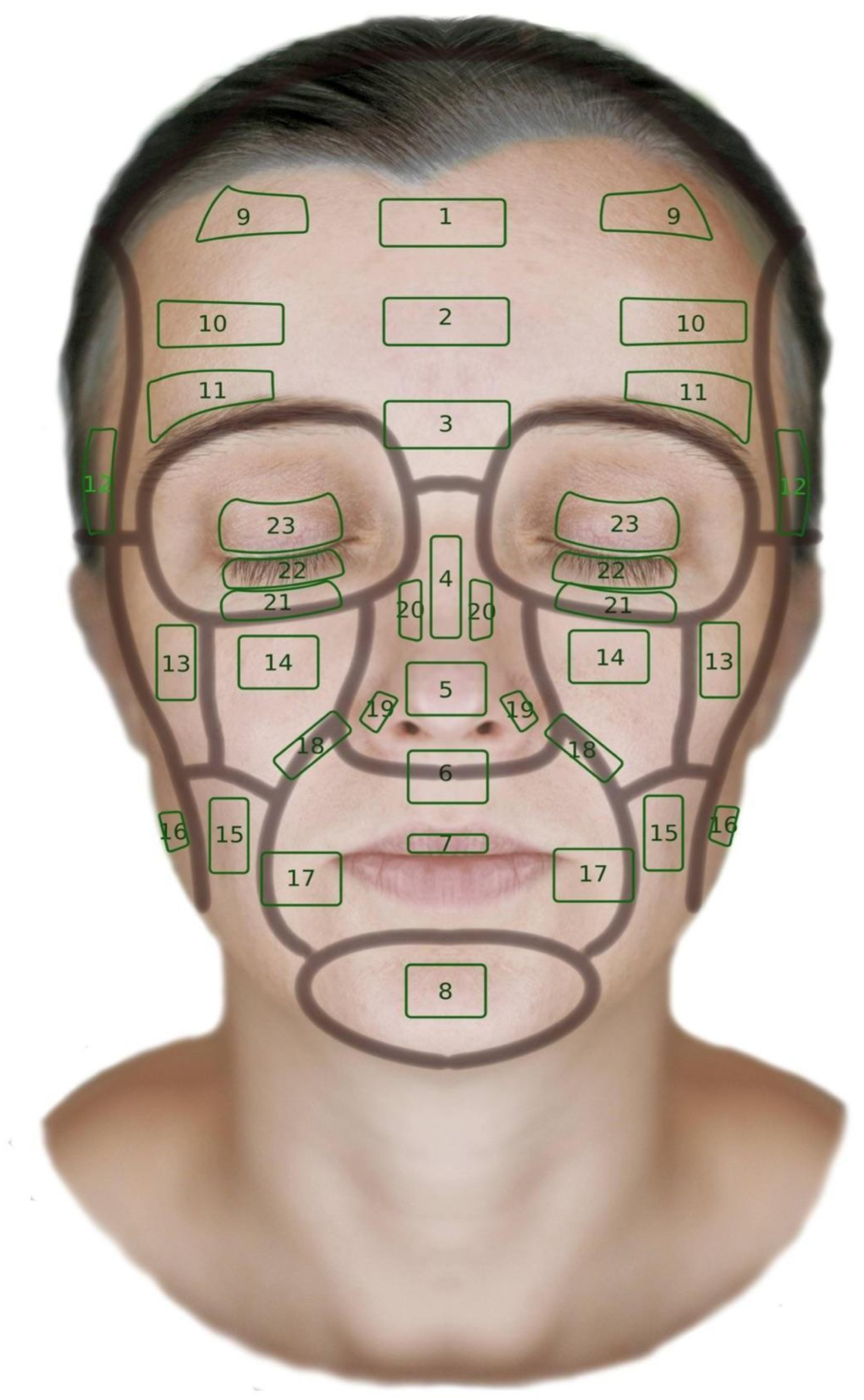
3.4. Facial Skin Thickness Map
3.5. Analysis of Skin Thickness Depending on Facial Location
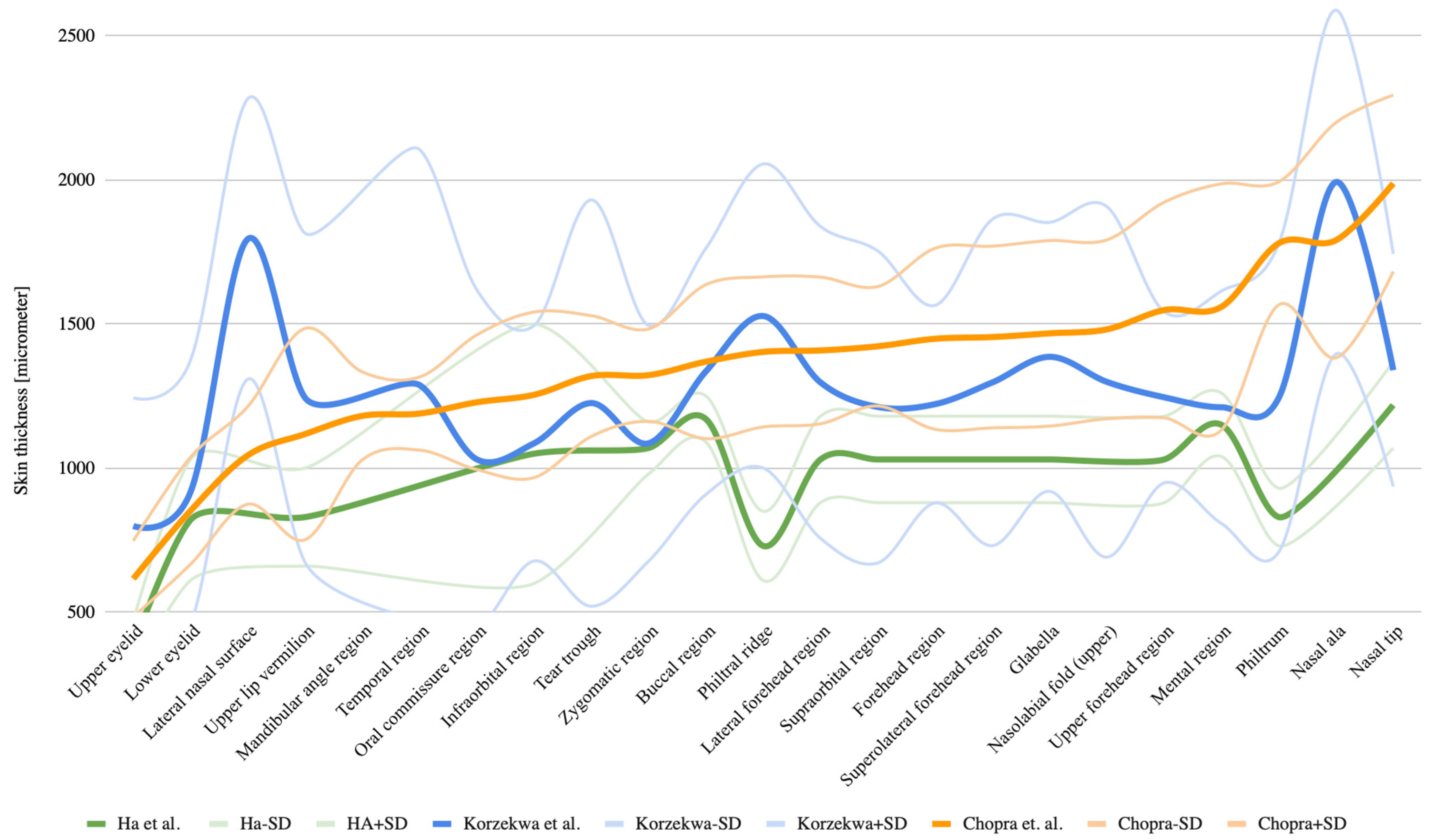
3.6. Analysis of Skin Thickness by Age Group
3.7. Correlation Analysis of Quantitative Variables: Age, Weight, Height, and BMI
3.8. Correlation Between Skin Thickness in Different Anatomical Regions
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barker, D. Skin Thickness In The Human. Plast. Reconstr. Surg. 1951, 7, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.R. Things I Wish I Had Been Taught About Rhinoplasty. Can. J. Plast. Surg. 2010, 18, 129. [Google Scholar] [CrossRef]
- Aldosari, B. Is Nasal Skin Thickness a Prognostic Indicator to Postoperative Edema and Ecchymosis? Ear Nose Throat J. 2019, 100, NP206–NP209. [Google Scholar] [CrossRef]
- Lee, Y.; Hwang, K. Skin thickness of Korean adults. Surg. Radiol. Anat. 2002, 24, 183–189. [Google Scholar] [CrossRef]
- Cobo, R. Managing the Thick Skin in Facial Plastic Surgery. Facial Plast. Surg. 2018, 34, 001–002. [Google Scholar] [CrossRef]
- Hu, A.C.; Lee, S.A.; Clark, E.G.; Yamamoto, M.; Jakowatz, J.G.; Evans, G.R.D. Impact of Immediate Surgical Reconstruction Following Wide Local Excision of Malignant Head and Neck Melanoma. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2661. [Google Scholar] [CrossRef]
- Hom, D.B.; Ingraffea, A. Facial Skin: Contemporary Topics for the Surgeon. Facial Plast. Surg. Clin. N. Am. 2013, 21, xv. [Google Scholar] [CrossRef] [PubMed]
- Anand, C. Facial Contouring With Fillers, Neuromodulators, and Lipolysis to Achieve a Natural Look in Patients With Facial Fullness. J. Drugs Dermatol. 2016, 15, 1536–1542. [Google Scholar]
- Alam, M.; Tung, R. Injection technique in neurotoxins and fillers: Planning and basic technique. J. Am. Acad. Dermatol. 2018, 79, 407–419. [Google Scholar] [CrossRef]
- Ou-Jin, C.; Kang-Woo, L.; Young-Chun, G.; Kyung-Seok, H.; Hee-Jin, K. Ultrasonographic Analyses Of The Forehead Region For Injectable Treatments. Ultrasound Med. Biol. 2019, 45, 18. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, F.; Voorhees, J.J.; Fisher, G.J. Rejuvenation of Aged Human Skin by Injection of Cross-linked Hyaluronic Acid. Plast. Reconstr. Surg. 2020, 147, 43–49. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Shah, V.; Dhinakar, A.; Yildirimer, L.; Cui, W.; Zhao, X. Intradermal fillers for minimally invasive treatment of facial aging. Plast. Aesthet. Res. 2016, 3, 72–82. [Google Scholar] [CrossRef]
- Available online: https://www.plasticsurgery.org/cosmetic-procedures/dermal-fillers/fat-injections (accessed on 22 November 2021).
- Hyunchul, P.; Eunjin, K.; Jeongeun, K.; Youngsuck, R.; Jooyeon, K. High-Intensity Focused Ultrasound for the Treatment of Wrinkles and Skin Laxity in Seven Different Facial Areas. Ann. Dermatol. 2015, 27, 688. [Google Scholar] [CrossRef] [PubMed]
- Azizzadeh, B. Reshaping Rhytidectomy. In Master Techniques in Facial Rejuvenation; Azizzadeh, B., Johnson, C.M., Jr., Fitzgerald, R., Murphy, M.R., Massry, G.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 219–246. [Google Scholar]
- Walden, J.L.; Aston, S.J. Rhytidectomy. In Plastic Surgery Secrets Plus; Weinzweig, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 504–515. [Google Scholar]
- Ha, R.Y.; Nojima, K.; Adams, W.P.; Brown, S.A. Analysis of Facial Skin Thickness: Defining the Relative Thickness Index. Plast. Reconstr. Surg. 2005, 115, 1769–1773. [Google Scholar] [CrossRef] [PubMed]
- Derraik, J.G.; Rademaker, M.; Cutfield, W.S.; Pinto, T.E.; Tregurtha, S.; Faherty, A.; Peart, J.M.; Drury, P.L.; Hofman, P.L. Effects of Age, Gender, BMI, and Anatomical Site on Skin Thickness in Children and Adults with Diabetes. PLoS ONE 2014, 9, e86637. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hou, Y.; Zhu, Q.L.; Xu, D.; Wang, L.; Li, J.C.; Jiang, Y.X.; Wang, Q.; Li, M.T.; Zhang, F.C.; et al. A preliminary study of skin ultrasound in diffuse cutaneous systemic sclerosis: Does skin echogenicity matter? PLoS ONE 2017, 12, e0174481. [Google Scholar] [CrossRef]
- Chen, L.; Dyson, M.; Rymer, J.; Bolton, P.A.; Young, S.R. The use of high-frequency diagnostic ultrasound toinvestigate the effect of hormone replacement therapy onskin thickness. Ski. Res. Technol. 2001, 7, 95–97. [Google Scholar] [CrossRef]
- Lopez, H.; Beer, J.Z.; Miller, S.A.; Zmudzka, B.Z. Ultrasound measurements of skin thickness after UV exposure: A feasibility study. J. Photochem. Photobiol. B Biol. 2004, 73, 123–132. [Google Scholar] [CrossRef]
- El-Mehallawi, I.H.; Soliman, E.M. Ultrasonic assessment of facial soft tissue thicknesses in adult Egyptians. Forensic Sci. Int. 2001, 117, 99–107. [Google Scholar] [CrossRef]
- Rhine, J.S.; Campbell, H.R. Thickness of facial tissues in American blacks. J. Forensic Sci. 1980, 25, 847–858. [Google Scholar] [CrossRef]
- Saxena, T.; Panat, S.R.; Sangamesh, N.C.; Choudhary, A.; Aggarwal, A.; Yadav, N. Facial Soft Tissue Thickness in North Indian Adult Population. Indian Acad. Oral Med. Radiol. 2012, 24, 121–125. [Google Scholar] [CrossRef]
- Aulsebrook, W.A.; Becker, P.J.; İşcan, M.Y. Facial soft-tissue thicknesses in the adult male Zulu. Forensic Sci. Int. 1996, 79, 83–102. [Google Scholar] [CrossRef]
- Toneva, D.; Nikolova, S.; Georgiev, I.; Harizanov, S.; Zlatareva, D.; Hadjidekov, V.; Lazarov, N. Facial soft tissue thicknesses in Bulgarian adults: Relation to sex, body mass index and bilateral asymmetry. Folia Morphol. 2018, 77, 570–582. [Google Scholar] [CrossRef]
- De Greef, S.; Claes, P.; Vandermeulen, D.; Mollemans, W.; Suetens, P.; Willems, G. Large-scale in-vivo Caucasian facial soft tissue thickness database for craniofacial reconstruction. Forensic Sci. Int. 2006, 159, S126–S146. [Google Scholar] [CrossRef]
- Phillips, V.M.; Smuts, N.A. Facial reconstruction: Utilization of computerized tomography to measure facial tissue thickness in a mixed racial population. Forensic Sci. Int. 1996, 83, 51–59. [Google Scholar] [CrossRef]
- Dykes, P.J.; Francis, A.J.; Marks, R. Measurement of Dermal Thickness with the Harpenden Skinfold Caliper. Arch. Derm. Res. 1976, 256, 261–263. [Google Scholar] [CrossRef]
- Chopra, K.; Calva, D.; Sosin, M.; Tadisina, K.K.; Banda, A.; De La Cruz, C.; Chaudhry, M.R.; Legesse, T.; Drachenberg, C.B.; Manson, P.N.; et al. A Comprehensive Examination of Topographic Thickness of Skin. in the Human Face. Aesthetic Surg. J. 2015, 35, 1007–1013. [Google Scholar] [CrossRef]
- Vyas, S.; Meyerle, J.; Burlina, P. Non-invasive estimation of skin thickness from hyperspectral imaging and validation using echography. Comput. Biol. Med. 2015, 57, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Thiboutot, D.M. Dermatological Applications of High-Frequency Ultrasound. In Proceedings of the SPIE Conference, San Diego, CA, USA, 24–25 February 1999. [Google Scholar]
- Sedky, M.M.; Fawzy, S.M.; Baki, N.A.E.; Eishi, N.H.E.; Bohy, A.E.M.M.E. Systemic sclerosis: An ultrasonographic study of skin and subcutaneous tissue in relation to clinical findings. Skin. Res. Technol. 2012, 19, e78–e84. [Google Scholar] [CrossRef]
- Alharethy, S.; Alohali, S.; Alquniabut, I.; Ju Jang, Y. Bone and Soft Tissue Nasal Angles Discrepancies and Overlying Skin Thickness: A Computed Tomography Study. Aesthetic Plast. Surg. 2018, 42, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1997, 571, 289–300. [Google Scholar] [CrossRef]
- Haedersdal, M.; Efsen, J.; Gniadecka, M.; Fogh, H.; Keiding, J.; Wulf, H.C. Changes in Skin Redness, Pigmentation, Echostructure, Thickness, and Surface Contour After 1 Pulsed Dye Laser Treatment of Port-wine Stains in Children. Arch. Dermatol. 1998, 134, 175–181. [Google Scholar] [CrossRef]
- Gambichler, T.; Moussa, G.; Bahrenberg, K.; Vogt, M.; Ermert, H.; Weyhe, D.; Altmeyer, P.; Hoffmann, K. Preoperative Ultrasonic Assessment Of Thin Melanocytic Skin Lesions Using A 100-Mhz Ultrasound Transducer: A Comparative Study. Dermatol. Surg. 2007, 33, 818–824. [Google Scholar] [CrossRef]
- Gawriołek, K.; Klatkiewicz, T.; Przystańska, A.; Maciejewska-Szaniec, Z.; Gedrange, T.; Czajka-Jakubowska, A. Standardization of the ultrasound examination of the masseter muscle with size-independent calculation of records. Adv. Clin. Exp. Med. 2021, 30, 441–447. [Google Scholar] [CrossRef]
- Meyer, N.; Lauwers-Cances, V.; Lourari, S.; Laurent, J.; Konstantinou, M.P.; Lagarde, J.M.; Krief, B.; Batatia, H.; Lamant, L.; Paul, C. High-frequency ultrasonography but not 930-nm optical coherence tomography reliably evaluates melanoma thickness in vivo: A prospective validation study. Br. J. Dermatol. 2014, 171, 799–805. [Google Scholar] [CrossRef]
- Hoffmann, K.; Jung, J.; El Gammal, S.; Altmeyer, P. Malignant Melanoma in 20MHz B Scan Sonography. Dermatology 1992, 185, 49–55. [Google Scholar] [CrossRef]
- Guitera, P.; Li, L.X.; Crotty, K.; Fitzgerald, P.; Mellenbergh, R.; Pellacani, G.; Menzies, S.W. Melanoma histological Breslow thickness predicted by 75-MHz ultrasonography. Br. J. Dermatol. 2008, 159, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.K.; Konda, S.; Perez, O.A.; Amini, S.; Elgart, G.; Berman, B. Newer Technologies/Techniquesand Tools in the Diagnosis Of Melanoma. Eur. J. Dermatol. 2008, 18, 617–631. [Google Scholar]
- Di Battista, M.; Barsotti, S.; Vitali, S.; Palma, M.; Granieri, G.; Oranges, T.; Aringhieri, G.; Dini, V.; Della Rossa, A.; Neri, E.; et al. Multiparametric Skin Assessment in a Monocentric Cohort of Systemic Sclerosis Patients: Is There a Role for Ultra-High Frequency Ultrasound? Diagnostics 2023, 13, 1495. [Google Scholar] [CrossRef]
- Huang, D.-M.; Wang, S.-H. In Situ Monitoring and Assessment of Ischemic Skin Flap by High-Frequency Ultrasound and Quantitative Parameters. Sensors 2024, 24, 363. [Google Scholar] [CrossRef] [PubMed]
- Mlosek, R.K.; Malinowska, S.P. High-Frequency Ultrasound in the Assessment of Cellulite—Correlation between Ultrasound-Derived Measurements, Clinical Assessment, and Nürnberger–Müller Scale Scores. Diagnostics 2024, 14, 1878. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L. Skin ageing and its treatment. J. Pathol. 2007, 211, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Varga, N.N.; Boostani, M.; Farkas, K.; Bánvölgyi, A.; Lőrincz, K.; Posta, M.; Lihacova, I.; Lihachev, A.; Medvecz, M.; Holló, P.; et al. Optically Guided High-Frequency Ultrasound Shows Superior Efficacy for Preoperative Estimation of Breslow Thickness in Comparison with Multispectral Imaging: A Single-Center Prospective Validation Study. Cancers 2024, 16, 157. [Google Scholar] [CrossRef] [PubMed]

| Side | Area | Sample Size | Median | Interquartile Range |
|---|---|---|---|---|
| bilaterally symmetrical | tear trough | 390 | 1241.5 | 343 |
| upper nasolabial fold | 390 | 1636.5 | 579 | |
| nasal ala | 160 | 1744 | 223 | |
| lateral forehead | 350 | 1492 | 528.75 | |
| superolateral forehead region | 360 | 1523 | 505.75 | |
| zygomatic region | 418 | 1303.5 | 199.75 | |
| oral commissure | 390 | 1234 | 240.75 | |
| mandibular angle | 126 | 1195.5 | 220.25 | |
| infraorbital region | 350 | 1445 | 509 | |
| supraorbital region | 440 | 1362.5 | 298.5 | |
| buccal region | 390 | 1367.5 | 373.5 | |
| temporal region | 390 | 1126.5 | 251.75 | |
| lower eyelid | 430 | 807.5 | 260.75 | |
| upper eyelid | 430 | 573.5 | 128.75 | |
| lateral nasal surface | 50 | 1163 | 371 | |
| central | philtrum | 388 | 1624 | 403.25 |
| nasal tip | 430 | 1907 | 455.75 | |
| upper vermilion lip | 390 | 1061 | 270.25 | |
| glabella | 360 | 1503.5 | 522.75 | |
| nasal dorsum | 118 | 1282 | 259.75 | |
| mental region | 430 | 1606 | 560.25 | |
| upper forehead region | 450 | 1480 | 432.25 | |
| forehead region | 360 | 1456.5 | 440.25 |
| Group 1 | Group 2 | Sample Size Group 1 | Sample Size Group 2 | Dunn’s Test Statistic | p Value |
|---|---|---|---|---|---|
| tear trough (L) | Nasolabial fold (L) | 390 | 390 | 14.38 | <0.001 |
| Upper lip vermilion (C) | 390 | 390 | −5.89 | <0.001 | |
| Glabella (C) | 390 | 360 | 8.93 | <0.001 | |
| Mental region (C) | 390 | 430 | 13.03 | <0.001 | |
| Lateral forehead (L) | 390 | 350 | 9.09 | <0.001 | |
| Upper forehead region (C) | 390 | 450 | 9.6 | <0.001 | |
| Superolateral forehead region (L) | 390 | 360 | 9.39 | <0.001 | |
| Frontal region (C) | 390 | 360 | 8.81 | <0.001 | |
| Zygomatic region (L) | 390 | 418 | 3.87 | 0.007 | |
| Supraorbital region (L) | 390 | 350 | 8.01 | <0.001 | |
| Infraorbital region (L) | 390 | 440 | 6.03 | <0.001 | |
| Buccal region (L) | 390 | 390 | 6.5 | <0.001 | |
| Temporal region (L) | 390 | 390 | −3.73 | 0.012 | |
| Lower eyelid (L) | 390 | 430 | −13.9 | <0.001 | |
| Upper eyelid (L) | 390 | 430 | −17.61 | <0.001 | |
| Philtrum (C) | 390 | 388 | 15.71 | <0.001 | |
| Nasal ala (L) | 390 | 160 | 15.5 | <0.001 | |
| Nasal tip (C) | 390 | 430 | 22.85 | <0.001 | |
| Philtrum (L) | Upper lip vermilion (C) | 390 | 390 | −20.27 | <0.001 |
| Glabella (C) | 390 | 360 | −5.16 | <0.001 | |
| Nasal dorsum (C) | 390 | 118 | −7.94 | <0.001 | |
| Lateral forehead (L) | 390 | 350 | −4.89 | <0.001 | |
| Upper forehead region (C) | 390 | 450 | −5.28 | <0.001 | |
| Superolateral forehead region (L) | 390 | 360 | −4.7 | <0.001 | |
| Frontal region (C) | 390 | 360 | −5.28 | <0.001 | |
| Zygomatic region (L) | 390 | 418 | −10.76 | <0.001 | |
| Oral commissure region (L) | 390 | 390 | −13.42 | <0.001 | |
| Mandibular angle region (L) | 390 | 126 | −11.23 | <0.001 | |
| Supraorbital region (L) | 390 | 350 | −5.97 | <0.001 | |
| Infraorbital region (L) | 390 | 440 | −8.78 | <0.001 | |
| Buccal region (L) | 390 | 390 | −7.88 | <0.001 | |
| Temporal region (L) | 390 | 390 | −18.11 | <0.001 | |
| Lower eyelid (L) | 390 | 430 | −28.63 | <0.001 | |
| Upper eyelid (L) | 390 | 430 | −32.34 | <0.001 | |
| Lateral nasal surface (L) | 390 | 50 | −7.17 | <0.001 | |
| Nasal ala (L) | 390 | 160 | 4.53 | <0.001 | |
| Nasal tip (C) | 390 | 430 | 8.13 | <0.001 | |
| Upper lip vermilion (C) | Glabella (C) | 390 | 360 | 14.7 | <0.001 |
| Nasal dorsum (C) | 390 | 118 | 5.87 | <0.001 | |
| Mental region (C) | 390 | 430 | 19.07 | <0.001 | |
| Lateral forehead (L) | 390 | 350 | 14.82 | <0.001 | |
| Upper forehead region (C) | 390 | 450 | 15.7 | <0.001 | |
| Superolateral forehead region (L) | 390 | 360 | 15.16 | <0.001 | |
| Frontal region (C) | 390 | 360 | 14.58 | <0.001 | |
| Zygomatic region (L) | 390 | 418 | 9.86 | <0.001 | |
| Oral commissure region (L) | 390 | 390 | 6.85 | <0.001 | |
| Supraorbital region (L) | 390 | 350 | 13.74 | <0.001 | |
| Infraorbital region (L) | 390 | 440 | 12.1 | <0.001 | |
| Buccal region (L) | 390 | 390 | 12.39 | <0.001 | |
| Lower eyelid (L) | 390 | 430 | −7.87 | <0.001 | |
| Upper eyelid (L) | 390 | 430 | −11.58 | <0.001 | |
| Philtrum (C) | 390 | 388 | 21.59 | <0.001 | |
| Nasal ala (L) | 390 | 160 | 19.99 | <0.001 | |
| Nasal tip (C) | 390 | 430 | 28.88 | <0.001 | |
| Glabella (C) | Nasal dorsum (C) | 360 | 118 | −4.31 | 0.001 |
| Mental region (C) | 360 | 430 | 3.62 | 0.017 | |
| Zygomatic region (L) | 360 | 418 | −5.29 | <0.001 | |
| Oral commissure region (L) | 360 | 390 | −7.99 | <0.001 | |
| Mandibular angle region (L) | 360 | 126 | −7.48 | <0.001 | |
| Temporal region (L) | 360 | 390 | −12.59 | <0.001 | |
| Lower eyelid (L) | 360 | 430 | −22.74 | <0.001 | |
| Upper eyelid (L) | 360 | 430 | −26.38 | <0.001 | |
| Lateral nasal surface (L) | 360 | 50 | −4.64 | <0.001 | |
| Philtrum (C) | 360 | 388 | 6.47 | <0.001 | |
| Nasal ala (L) | 360 | 160 | 8.44 | <0.001 | |
| Nasal tip (C) | 360 | 430 | 13.23 | <0.001 | |
| Nasal dorsum (C) | Mental region (C) | 118 | 430 | 6.89 | <0.001 |
| Lateral forehead (L) | 118 | 350 | 4.46 | 0.001 | |
| Upper forehead region (C) | 118 | 450 | 4.54 | <0.001 | |
| Superolateral forehead region (L) | 118 | 360 | 4.63 | <0.001 | |
| Frontal region (C) | 118 | 360 | 4.23 | 0.002 | |
| Supraorbital region (L) | 118 | 350 | 3.71 | 0.013 | |
| Temporal region (L) | 118 | 390 | −4.4 | 0.001 | |
| Lower eyelid (L) | 118 | 430 | −11.23 | <0.001 | |
| Upper eyelid (L) | 118 | 430 | −13.73 | <0.001 | |
| Philtrum (C) | 118 | 388 | 8.86 | <0.001 | |
| Nasal ala (L) | 118 | 160 | 10.38 | <0.001 | |
| Nasal tip (C) | 118 | 430 | 13.5 | <0.001 | |
| Mental region (C) | Lateral forehead (L) | 430 | 350 | −3.36 | 0.042 |
| Upper forehead region (C) | 430 | 450 | −3.67 | 0.015 | |
| Frontal region (C) | 430 | 360 | −3.74 | 0.011 | |
| Zygomatic region (L) | 430 | 418 | −9.3 | <0.001 | |
| Oral commissure region (L) | 430 | 390 | −12.06 | <0.001 | |
| Mandibular angle region (L) | 430 | 126 | −10.2 | <0.001 | |
| Supraorbital region (L) | 430 | 350 | −4.47 | 0.001 | |
| Infraorbital region (L) | 430 | 440 | −7.26 | <0.001 | |
| Buccal region (L) | 430 | 390 | −6.38 | <0.001 | |
| Temporal region (L) | 430 | 390 | −16.86 | <0.001 | |
| Lower eyelid (L) | 430 | 430 | −27.62 | <0.001 | |
| Upper eyelid (L) | 430 | 430 | −31.42 | <0.001 | |
| Lateral nasal surface (L) | 430 | 50 | −6.42 | <0.001 | |
| Nasal ala (L) | 430 | 160 | 5.87 | <0.001 | |
| Nasal tip (C) | 430 | 430 | 10.07 | <0.001 | |
| Frontal region (lateral) (L) | Zygomatic region (L) | 350 | 418 | −5.48 | <0.001 |
| Oral commissure region (L) | 350 | 390 | −8.16 | <0.001 | |
| Mandibular angle region (L) | 350 | 126 | −7.61 | <0.001 | |
| Infraorbital region (L) | 350 | 440 | −3.49 | 0.027 | |
| Temporal region (L) | 350 | 390 | −12.73 | <0.001 | |
| Lower eyelid (L) | 350 | 430 | −22.8 | <0.001 | |
| Upper eyelid (L) | 350 | 430 | −26.41 | <0.001 | |
| Lateral nasal surface (L) | 350 | 50 | −4.74 | <0.001 | |
| Philtrum (C) | 350 | 388 | 6.2 | <0.001 | |
| Nasal ala (L) | 350 | 160 | 8.23 | <0.001 | |
| Nasal tip (C) | 350 | 430 | 12.9 | <0.001 | |
| Frontal region (upper) (C) | Zygomatic region (L) | 450 | 418 | −5.77 | <0.001 |
| Oral commissure region (L) | 450 | 390 | −8.61 | <0.001 | |
| Mandibular angle region (L) | 450 | 126 | −7.8 | <0.001 | |
| Infraorbital region (L) | 450 | 440 | −3.65 | 0.015 | |
| Temporal region (L) | 450 | 390 | −13.47 | <0.001 | |
| Lower eyelid (L) | 450 | 430 | −24.26 | <0.001 | |
| Upper eyelid (L) | 450 | 430 | −28.11 | <0.001 | |
| Lateral nasal surface (L) | 450 | 50 | −4.77 | <0.001 | |
| Philtrum (C) | 450 | 388 | 6.67 | <0.001 | |
| Nasal ala (L) | 450 | 160 | 8.59 | <0.001 | |
| Nasal tip (C) | 450 | 430 | 13.84 | <0.001 | |
| Frontal region (superolateral) (L) | Zygomatic region (L) | 360 | 418 | −5.76 | <0.001 |
| Oral commissure region (L) | 360 | 390 | −8.45 | <0.001 | |
| Mandibular angle region (L) | 360 | 126 | −7.8 | <0.001 | |
| Infraorbital region (L) | 360 | 440 | −3.75 | 0.011 | |
| Temporal region (L) | 360 | 390 | −13.05 | <0.001 | |
| Lower eyelid (L) | 360 | 430 | −23.21 | <0.001 | |
| Upper eyelid (L) | 360 | 430 | −26.84 | <0.001 | |
| Lateral nasal surface (L) | 360 | 50 | −4.86 | <0.001 | |
| Philtrum (C) | 360 | 388 | 6.01 | <0.001 | |
| Nasal ala (L) | 360 | 160 | 8.09 | <0.001 | |
| Nasal tip (C) | 360 | 430 | 12.76 | <0.001 | |
| Frontal region (C) | Zygomatic region (L) | 360 | 418 | −5.17 | <0.001 |
| Oral commissure region (L) | 360 | 390 | −7.88 | <0.001 | |
| Mandibular angle region (L) | 360 | 126 | −7.4 | <0.001 | |
| Temporal region (L) | 360 | 390 | −12.47 | <0.001 | |
| Lower eyelid (L) | 360 | 430 | −22.62 | <0.001 | |
| Upper eyelid (L) | 360 | 430 | −26.25 | <0.001 | |
| Lateral nasal surface (L) | 360 | 50 | −4.58 | <0.001 | |
| Philtrum (C) | 360 | 388 | 6.59 | <0.001 | |
| Nasal ala (L) | 360 | 160 | 8.54 | <0.001 | |
| Nasal tip (C) | 360 | 430 | 13.35 | <0.001 | |
| Zygomatic region (L) | Mandibular angle region (L) | 418 | 126 | −3.87 | 0.007 |
| Supraorbital region (L) | 418 | 350 | 4.38 | 0.001 | |
| Temporal region (L) | 418 | 390 | −7.67 | <0.001 | |
| Lower eyelid (L) | 418 | 430 | −18.12 | <0.001 | |
| Upper eyelid (L) | 418 | 430 | −21.89 | <0.001 | |
| Philtrum (C) | 418 | 388 | 12.12 | <0.001 | |
| Nasal ala (L) | 418 | 160 | 12.72 | <0.001 | |
| Nasal tip (C) | 418 | 430 | 19.3 | <0.001 | |
| Oral commissure region (L) | Supraorbital region (L) | 390 | 350 | 7.08 | <0.001 |
| Infraorbital region (L) | 390 | 440 | 5.05 | <0.001 | |
| Buccal region (L) | 390 | 390 | 5.54 | <0.001 | |
| Temporal region (L) | 390 | 390 | −4.69 | <0.001 | |
| Lower eyelid (L) | 390 | 430 | −14.88 | <0.001 | |
| Upper eyelid (L) | 390 | 430 | −18.59 | <0.001 | |
| Philtrum (C) | 390 | 388 | 14.75 | <0.001 | |
| Nasal ala (L) | 390 | 160 | 14.77 | <0.001 | |
| Nasal tip (C) | 390 | 430 | 21.87 | <0.001 | |
| Mandibular angle region (L) | Supraorbital region (L) | 126 | 350 | 6.85 | <0.001 |
| Infraorbital region (L) | 126 | 440 | 5.35 | <0.001 | |
| Buccal region (L) | 126 | 390 | 5.72 | <0.001 | |
| Lower eyelid (L) | 126 | 430 | −8.4 | <0.001 | |
| Upper eyelid (L) | 126 | 430 | −10.96 | <0.001 | |
| Philtrum (C) | 126 | 388 | 12.17 | <0.001 | |
| Nasal ala (L) | 126 | 160 | 13.24 | <0.001 | |
| Nasal tip (C) | 126 | 430 | 16.97 | <0.001 | |
| Supraorbital region (L) | Temporal region (L) | 350 | 390 | −11.64 | <0.001 |
| Lower eyelid (L) | 350 | 430 | −21.7 | <0.001 | |
| Upper eyelid (L) | 350 | 430 | −25.3 | <0.001 | |
| Lateral nasal surface (L) | 350 | 50 | −4.22 | 0.002 | |
| Philtrum (C) | 350 | 388 | 7.28 | <0.001 | |
| Nasal ala (L) | 350 | 160 | 9.07 | <0.001 | |
| Nasal tip (C) | 350 | 430 | 14 | <0.001 | |
| Infraorbital region (L) | Temporal region (L) | 440 | 390 | −9.88 | <0.001 |
| Lower eyelid (L) | 440 | 430 | −20.52 | <0.001 | |
| Upper eyelid (L) | 440 | 430 | −24.34 | <0.001 | |
| Philtrum (C) | 440 | 388 | 10.15 | <0.001 | |
| Nasal ala (L) | 440 | 160 | 11.22 | <0.001 | |
| Nasal tip (C) | 440 | 430 | 17.38 | <0.001 | |
| Buccal region (L) | Temporal region (L) | 390 | 390 | −10.23 | <0.001 |
| Lower eyelid (L) | 390 | 430 | −20.55 | <0.001 | |
| Upper eyelid (L) | 390 | 430 | −24.26 | <0.001 | |
| Lateral nasal surface (L) | 390 | 50 | −3.41 | 0.035 | |
| Philtrum (C) | 390 | 388 | 9.22 | <0.001 | |
| Nasal ala (L) | 390 | 160 | 10.54 | <0.001 | |
| Nasal tip (C) | 390 | 430 | 16.2 | <0.001 | |
| Temporal region (L) | Lower eyelid (L) | 390 | 430 | −10.08 | <0.001 |
| Upper eyelid (L) | 390 | 430 | −13.79 | <0.001 | |
| Philtrum (C) | 390 | 388 | 19.44 | <0.001 | |
| Nasal ala (L) | 390 | 160 | 18.35 | <0.001 | |
| Nasal tip (C) | 390 | 430 | 26.68 | <0.001 | |
| Lower eyelid (L) | Upper eyelid (L) | 430 | 430 | −3.8 | 0.009 |
| Lateral nasal surface (L) | 430 | 50 | 6.19 | <0.001 | |
| Philtrum (C) | 430 | 388 | 29.97 | <0.001 | |
| Nasal ala (L) | 430 | 160 | 26.21 | <0.001 | |
| Nasal tip (C) | 430 | 430 | 37.68 | <0.001 | |
| Upper eyelid (L) | Nasal sidewall (L) | 430 | 50 | 7.92 | <0.001 |
| Philtrum (C) | 430 | 388 | 33.67 | <0.001 | |
| Nasal ala (L) | 430 | 160 | 29.01 | <0.001 | |
| Nasal tip (C) | 430 | 430 | 41.49 | <0.001 | |
| Nasal sidewall (L) | Philtrum (C) | 50 | 388 | 7.81 | <0.001 |
| Nasal ala (L) | 50 | 160 | 9.27 | <0.001 | |
| Nasal tip (C) | 50 | 430 | 11.01 | <0.001 | |
| Philtral ridge (C) | Nasal ala (L) | 388 | 160 | 3.5 | 0.027 |
| Nasal tip (C) | 388 | 430 | 6.73 | <0.001 |
| Location | Age | Sample Size | Median | IQR (Interquartile Range) |
|---|---|---|---|---|
| Tear trough (L) | 0–33 | 100 | 1192 | 391.75 |
| 33–40 | 100 | 1294 | 201 | |
| 40–54 | 100 | 1073.5 | 287.75 | |
| 54–73 | 90 | 1304 | 341 | |
| Nasolabial fold (upper) (L) | 0–33 | 100 | 1781 | 492.5 |
| 33–40 | 100 | 1445.5 | 558.5 | |
| 40–54 | 100 | 1820.5 | 473.5 | |
| 54–73 | 90 | 1534.5 | 603 | |
| Upper lip vermilion (C) | 0–33 | 120 | 1093 | 260.75 |
| 33–40 | 100 | 1033.5 | 343.25 | |
| 40–54 | 100 | 1072 | 218.5 | |
| 54–73 | 70 | 1026 | 257.25 | |
| Glabella (C) | 0–33 | 110 | 1559.5 | 522.25 |
| 33–40 | 90 | 1599.5 | 266.5 | |
| 40–54 | 80 | 1199.5 | 592.25 | |
| 54–73 | 80 | 1457 | 436.75 | |
| Nasal dorsum (C) | 0–33 | 30 | 1255 | 232.25 |
| 33–40 | 18 | 1056 | 1272 | |
| 40–54 | 40 | 1289 | 223 | |
| 54–73 | 30 | 1411 | 240.5 | |
| Mental region (C) | 0–33 | 120 | 1609.5 | 692.25 |
| 33–40 | 100 | 1571 | 595.75 | |
| 40–54 | 110 | 1674.5 | 295.75 | |
| 54–73 | 100 | 1605 | 508.25 | |
| Lateral forehead (L) | 0–33 | 110 | 1549 | 514.75 |
| 33–40 | 90 | 1617.5 | 273 | |
| 40–54 | 70 | 1114 | 535 | |
| 54–73 | 80 | 1441 | 518 | |
| Upper forehead region (C) | 0–33 | 130 | 1517 | 464.75 |
| 33–40 | 100 | 1593 | 262.5 | |
| 40–54 | 120 | 1284.5 | 445.5 | |
| 54–73 | 100 | 1538 | 452.25 | |
| Superolateral forehead region (L) | 0–33 | 110 | 1562.5 | 518 |
| 33–40 | 90 | 1600 | 201.25 | |
| 40–54 | 70 | 1107 | 543.25 | |
| 54–73 | 90 | 1449 | 503.75 | |
| Frontal region (C) | 0–33 | 110 | 1509.5 | 520.75 |
| 33–40 | 90 | 1600.5 | 242.25 | |
| 40–54 | 80 | 1187.5 | 526 | |
| 54–73 | 80 | 1443 | 522.75 | |
| Zygomatic region (L) | 0–33 | 110 | 1287.5 | 171 |
| 33–40 | 100 | 1285.5 | 229.75 | |
| 40–54 | 118 | 1354.5 | 219 | |
| 54–73 | 90 | 1307 | 236.25 | |
| Oral commissure region (L) | 0–33 | 100 | 1244.5 | 204 |
| 33–40 | 100 | 1213.5 | 205.5 | |
| 40–54 | 110 | 1240 | 244 | |
| 54–73 | 80 | 1229.5 | 367.75 | |
| Mandibular angle region (L) | 0–33 | 48 | 1244.5 | 239.75 |
| 40–54 | 48 | 1186 | 182.75 | |
| 54–73 | 30 | 1174.5 | 165.25 | |
| Supraorbital region (L) | 0–33 | 110 | 1498.5 | 491.25 |
| 33–40 | 90 | 1556.5 | 277.5 | |
| 40–54 | 70 | 1111.5 | 478.25 | |
| 54–73 | 80 | 1441.5 | 499.25 | |
| Infraorbital region (L) | 0–33 | 130 | 1398 | 320.25 |
| 33–40 | 100 | 1379 | 383.25 | |
| 40–54 | 110 | 1365 | 196 | |
| 54–73 | 100 | 1300 | 269.75 | |
| Buccal region (L) | 0–33 | 120 | 1389.5 | 355 |
| 33–40 | 100 | 1365 | 523.75 | |
| 40–54 | 80 | 1372 | 250.5 | |
| 54–73 | 90 | 1309.5 | 384.5 | |
| Temporal region (L) | 0–33 | 100 | 998.5 | 169.75 |
| 33–40 | 100 | 1175.5 | 266.5 | |
| 40–54 | 100 | 1151 | 188.75 | |
| 54–73 | 90 | 1199.5 | 223.25 | |
| Lower eyelid (L) | 0–33 | 120 | 802.5 | 233.25 |
| 33–40 | 100 | 780 | 196.25 | |
| 40–54 | 110 | 814.5 | 231.25 | |
| 54–73 | 100 | 885.5 | 272 | |
| Upper eyelid (L) | 0–33 | 120 | 569 | 140 |
| 33–40 | 100 | 575 | 136 | |
| 40–54 | 110 | 562 | 105.5 | |
| 54–73 | 100 | 597 | 143.5 | |
| Lateral nasal surface (L) | 0–33 | 10 | 981.5 | 264 |
| 33–40 | 30 | 1197 | 868.75 | |
| 54–73 | 10 | 981.5 | 264 | |
| Philtrum (C) | 0–33 | 110 | 1464 | 276.5 |
| 33–40 | 88 | 1710.5 | 308.5 | |
| 40–54 | 90 | 1607 | 287.25 | |
| 54–73 | 100 | 1729.5 | 393.5 | |
| Nasal ala (L) | 0–33 | 40 | 1751 | 192.25 |
| 33–40 | 40 | 1584 | 550.75 | |
| 40–54 | 40 | 1790.5 | 123.75 | |
| 54–73 | 40 | 1750 | 202.5 | |
| Nasal tip (C) | 0–33 | 120 | 1632 | 327.5 |
| 33–40 | 100 | 1969.5 | 270.5 | |
| 40–54 | 110 | 2013 | 459.25 | |
| 54–73 | 100 | 2045 | 360 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korzekwa, S.; Matusz, K.; Kukulski, M.; Wawrzaszek, P.; Górna, N.; Rosiński, W.; Włosiański, J.; Przystańska, A. Quantitative Analysis of the Human Face Skin Thickness—A High-Frequency Ultrasound Study. J. Clin. Med. 2025, 14, 8401. https://doi.org/10.3390/jcm14238401
Korzekwa S, Matusz K, Kukulski M, Wawrzaszek P, Górna N, Rosiński W, Włosiański J, Przystańska A. Quantitative Analysis of the Human Face Skin Thickness—A High-Frequency Ultrasound Study. Journal of Clinical Medicine. 2025; 14(23):8401. https://doi.org/10.3390/jcm14238401
Chicago/Turabian StyleKorzekwa, Szymon, Krystian Matusz, Michał Kukulski, Paweł Wawrzaszek, Natalie Górna, Włodzimierz Rosiński, Jakub Włosiański, and Agnieszka Przystańska. 2025. "Quantitative Analysis of the Human Face Skin Thickness—A High-Frequency Ultrasound Study" Journal of Clinical Medicine 14, no. 23: 8401. https://doi.org/10.3390/jcm14238401
APA StyleKorzekwa, S., Matusz, K., Kukulski, M., Wawrzaszek, P., Górna, N., Rosiński, W., Włosiański, J., & Przystańska, A. (2025). Quantitative Analysis of the Human Face Skin Thickness—A High-Frequency Ultrasound Study. Journal of Clinical Medicine, 14(23), 8401. https://doi.org/10.3390/jcm14238401






